Therapeutic Potential of Vitamin B Complex in Peripheral Nerve Injury Recovery: An Experimental Rat Model Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedures
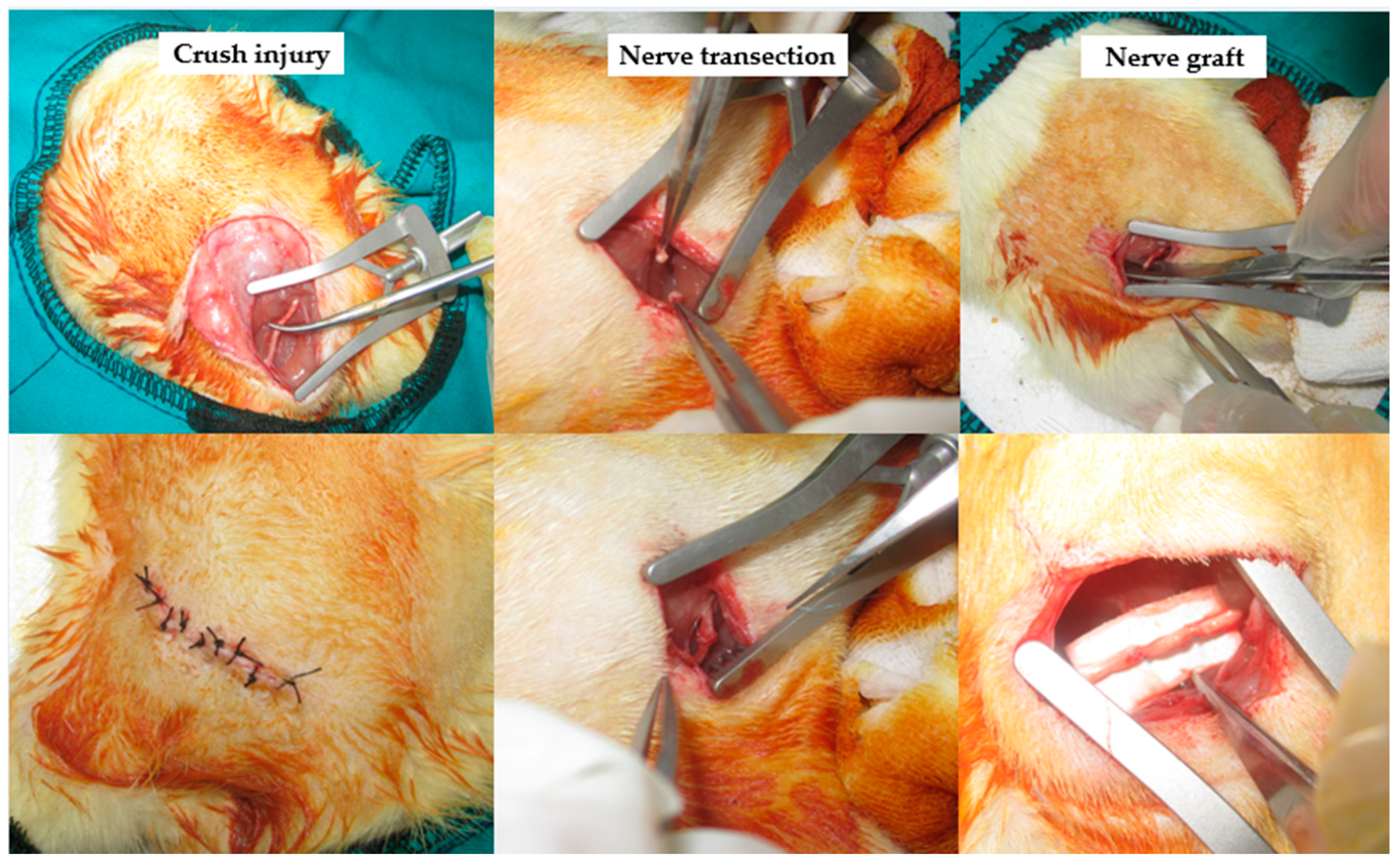
2.2. Surgical Applications
2.3. Groups
2.4. Electrophysiological Evaluation
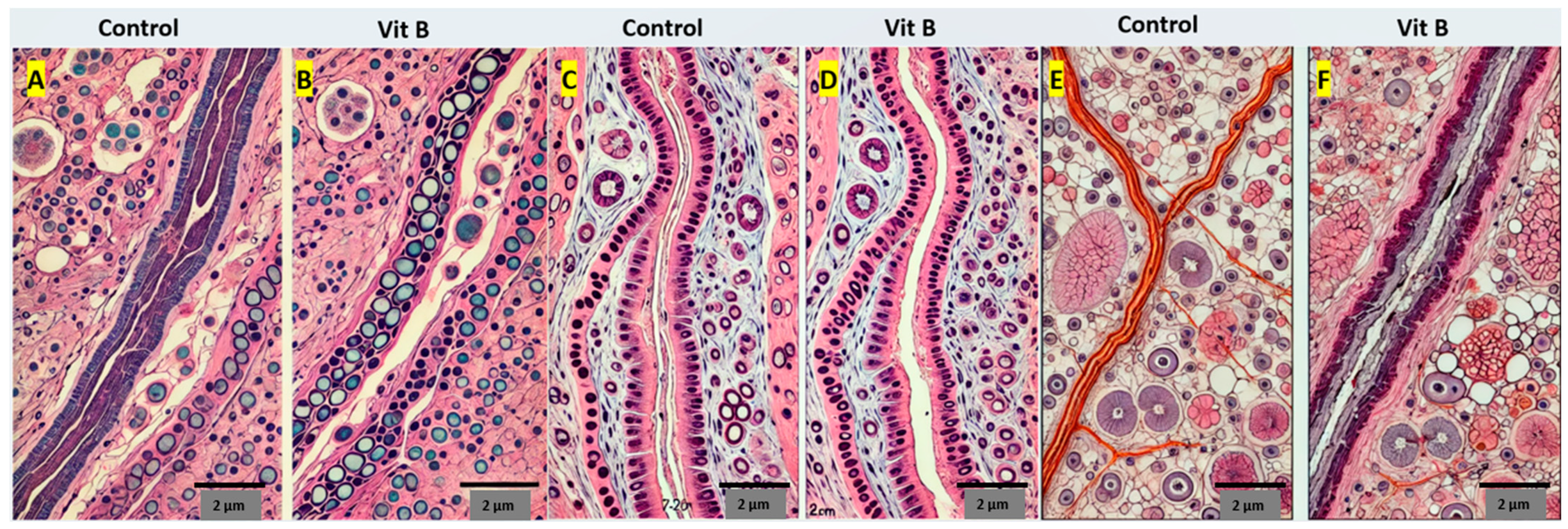
2.5. Histopathological Evaluation
2.6. Walk Test Analysis
2.7. Statistical Analysis
3. Results
3.1. Walk Test Analysis
3.2. Electrophysiological Evaluation
3.3. Histopathological Evaluation
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mekaj, A.; Mekaj, Y. The role of pharmacological agents in nerve regeneration after peripheral nerve repair. Peripher. Nerve Regen. Surg. New Ther. Approaches Incl. Biomater. Cell-Based Ther. Dev. 2017, 10, 151–174. [Google Scholar] [CrossRef]
- Evaniew, N.; Belley-Côté, E.P.; Fallah, N.; Noonan, V.K.; Rivers, C.S.; Dvorak, M.F. Methylprednisolone for the treatment of patients with acute spinal cord injuries: A systematic review and meta-analysis. J. Neurotrauma 2016, 33, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Saez, D.M.; Sasaki, R.T.; Martins, D.O.; Chacur, M.; Kerkis, I.; da Silva, M.C.P. Rat Facial Nerve Regeneration with Human Immature Dental Pulp Stem Cells. Cell Transpl. 2019, 28, 1573–1584. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Reis, R.L.; Oliveira, J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv. Exp. Med. Biol. 2020, 1249, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Samaram, H.; Naghavi, N.; Rassouli, M.B.; Mousavinezhad, M. Types of Short-Duration Electrical Stimulation-Induced Efficiency in the Axonal Regeneration and Recovery: Comparative in Vivo Study in Rat Model of Repaired Sciatic Nerve and its Tibial Branch after Transection Injury. Neurochem. Res. 2024, 49, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.B.; Bora, E.S.; Erbas, O. The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion. Curr. Issues Mol. Biol. 2024, 46, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.M.; Ibrahium, A.M.; Abdelgalil, A.I.; El-Saied, M.A.; El-Bably, S.H. Regenerative Strategies in Treatment of Peripheral Nerve Injuries in Different Animal Models. Tissue Eng. Regen. Med. 2023, 20, 839–877. [Google Scholar] [CrossRef]
- Kutafina, E.; Troglio, A.; de Col, R.; Rohrig, R.; Rossmanith, P.; Namer, B. Decoding Neuropathic Pain: Can We Predict Fluctuations of Propagation Speed in Stimulated Peripheral Nerve? Front. Comput. Neurosci. 2022, 16, 899584. [Google Scholar] [CrossRef]
- Guiotto, M.; Raffoul, W.; Hart, A.M.; Riehle, M.O.; di Summa, P.G. Human Platelet Lysate Acts Synergistically With Laminin to Improve the Neurotrophic Effect of Human Adipose-Derived Stem Cells on Primary Neurons in vitro. Front. Bioeng Biotechnol. 2021, 9, 658176. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Ren, W.; Chen, W.; Chong, Y.X.; Liu, X.; Eisaki, H.; Uchida, S.; Hamidian, M.H.; Davis, J.C.S. On the electron pairing mechanism of copper-oxide high temperature superconductivity. Proc. Natl. Acad. Sci. USA 2022, 119, e2207449119. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, J.M.; Jung, J.; Park, D.C.; Yoo, M.C.; Kim, S.S.; Kim, S.H.; Yeo, S.G. Review of Drug Therapy for Peripheral Facial Nerve Regeneration That Can Be Used in Actual Clinical Practice. Biomedicines 2022, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, L.; Serša, I.; Umek, N.; Cvetko, E.; Snoj, Ž. Correlation between diffusion tensor indices and fascicular morphometric parameters of peripheral nerve. Front. Physiol. 2023, 14, 1070227. [Google Scholar] [CrossRef]
- Pušnik, L.; Lechner, L.; Serša, I.; Cvetko, E.; Haas, P.; Jengojan, S.A.; Snoj, Ž. 3D fascicular reconstruction of median and ulnar nerve: Initial experience and comparison between high-resolution ultrasound and MR microscopy. Eur. Radiol. Exp. 2024, 8, 100. [Google Scholar] [CrossRef]
- Eygi, E.; Gul, R.; Aslan, M.; Tas, Z.A.; Dokuyucu, R. The Promising Effects of Erdosteine and Vitamin B in the Liver Ischemia/Reperfusion Model in Anesthetized Rats. Medicina 2024, 60, 783. [Google Scholar] [CrossRef]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wang, Z.; Wu, Z.; Zhang, P.; Zhang, Y.; Yin, X.; Wong, X.; Qiu, G.; Jiang, B. Epimedium extract promotes peripheral nerve regeneration in rats. Evid Based Complement. Altern. Med. 2013, 2013, 954798. [Google Scholar] [CrossRef] [PubMed]
- Gartner, A.; Pereira, T.; Armada-da-Silva, P.; Amado, S.; Veloso, A.; Amorim, I.; Ribeiro, J.; Santos, J.; Barcia, R.; Cruz, P.; et al. Effects of umbilical cord tissue mesenchymal stem cells (UCX(R)) on rat sciatic nerve regeneration after neurotmesis injuries. J. Stem Cells Regen. Med. 2014, 10, 14–26. [Google Scholar] [CrossRef]
- Raimondo, S.; Fornaro, M.; Di Scipio, F.; Ronchi, G.; Giacobini-Robecchi, M.G.; Geuna, S. Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: Part II-morphological techniques. Int. Rev. Neurobiol. 2009, 87, 81–103. [Google Scholar] [CrossRef]
- de Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Heinzel, J.; Langle, G.; Oberhauser, V.; Hausner, T.; Kolbenschlag, J.; Prahm, C.; Grillari, J.; Hercher, D. Use of the CatWalk gait analysis system to assess functional recovery in rodent models of peripheral nerve injury—A systematic review. J. Neurosci. Methods 2020, 345, 108889. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Wang, L.; Xing, C.; Guo, J.; Li, B.; Ma, H.; Zhong, H.; Zhou, M.; Zhu, S.; et al. Coordination function index: A novel indicator for assessing hindlimb locomotor recovery in spinal cord injury rats based on catwalk gait parameters. Behav. Brain Res. 2024, 459, 114765. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ito, A.; Aoyama, T.; Nakahara, R.; Nakahata, A.; Ji, X.; Zhang, J.; Kawai, H.; Kuroki, H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: A comparison between sciatic functional index and kinematic analysis. PLoS ONE 2018, 13, e0208985. [Google Scholar] [CrossRef]
- Byun, S.H.; Ahn, K.M. Functional and electron-microscopic changes after differential traction injury in the sciatic nerve of a rat. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 12. [Google Scholar] [CrossRef]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic Pavlovic, B.; Vucevic, D.; Pekovic, S.; Bozic Nedeljkovic, B. Vitamin B Complex Treatment Attenuates Local Inflammation after Peripheral Nerve Injury. Molecules 2019, 24, 4615. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Cao, X.C.; Luo, D.Q.; Lian, K.J. Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion. Neural Regen. Res. 2016, 11, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.Y.; Morina, A.A.; Bytyqi, C.I.; Mekaj, Y.H.; Duci, S.B. Application of topical pharmacological agents at the site of peripheral nerve injury and methods used for evaluating the success of the regenerative process. J. Orthop. Surg Res. 2014, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Daltaban, I.S.; Misir, S.; Turksoy, V.A.; Ak, H.; Cakir, E. The effects of barnidipine on an experimental ischemia reperfusion model of spinal cord injury and comparison with methyl prednisolone. North. Clin. Istanb. 2019, 6, 103–109. [Google Scholar] [CrossRef]
- Khuong, T.M.; Hamoudi, Z.; Manion, J.; Loo, L.; Muralidharan, A.; Neely, G.G. Peripheral straightjacket (alpha2delta Ca2+ channel subunit) expression is required for neuropathic sensitization in Drosophila. Philos. Trans. R Soc. Lond B Biol. Sci. 2019, 374, 20190287. [Google Scholar] [CrossRef]
- Mei, H.; Tan, J.; Hu, Y.; Shi, X.; Liu, Y.; Jia, F.; Xu, F. Developing a trans-multisynaptic tracer to map the neural circuit of recovered sciatic nerve after treatment with nerve growth factor. IBRO Neurosci. Rep. 2023, 15, 235–241. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Pajer, K.; Calcagno, D.; Pajenda, G.; Nogradi, A. The Role of Metals in the Neuroregenerative Action of BDNF, GDNF, NGF and Other Neurotrophic Factors. Biomolecules 2022, 12, 1015. [Google Scholar] [CrossRef]
- Zavala, A.; Martinez, P.C.; Gutierrez, G.G.; Vara, M.D.; Pawlikowski, W. The Combined Use of Curcumin and Platelet-Rich Plasma Enhances Axonal Regeneration in Acute Nerve Injuries: An Experimental Study in a Rat Model. J. Hand Microsurg. 2023, 15, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.A.; Onger, M.E.; Kaplan, S. The effects of curcumin and blueberry on axonal regeneration after peripheral nerve injury. J. Chem. Neuroanat. 2023, 130, 102260. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, X.; Liang, Q.; Wang, N.; Zheng, X.; Zhang, Q.; Yu, D. Curcumin-loaded keratin-chitosan hydrogels for enhanced peripheral nerve regeneration. Int. J. Biol. Macromol. 2024, 272, 132448. [Google Scholar] [CrossRef] [PubMed]
- Ehmedah, A.; Nedeljkovic, P.; Dacic, S.; Repac, J.; Draskovic-Pavlovic, B.; Vucevic, D.; Pekovic, S.; Nedeljkovic, B.B. Effect of Vitamin B Complex Treatment on Macrophages to Schwann Cells Association during Neuroinflammation after Peripheral Nerve Injury. Molecules 2020, 25, 5426. [Google Scholar] [CrossRef] [PubMed]
- Radcliff, A.B.; Heidari, M.; Field, A.S.; Duncan, I.D. Feline irradiated diet-induced demyelination; a model of the neuropathology of sub-acute combined degeneration? PLoS ONE 2020, 15, e0228109. [Google Scholar] [CrossRef]
- Wu, F.; Xu, K.; Liu, L.; Zhang, K.; Xia, L.; Zhang, M.; Teng, C.; Tong, H.; He, Y.; Xue, Y.; et al. Vitamin B12 Enhances Nerve Repair and Improves Functional Recovery After Traumatic Brain Injury by Inhibiting ER Stress-Induced Neuron Injury. Front. Pharmacol. 2019, 10, 406. [Google Scholar] [CrossRef]

| Latency (ms) | Velocity (m/s) | Peak-to-Peak (mV) | |
|---|---|---|---|
| Control (Nerve graft) | 3.5 ± 0.4 | 45 ± 5 | 0.8 ± 0.1 |
| Vit B (Nerve graft) | 3.0 ± 0.3 * | 55 ± 6 * | 1.2 ± 0.1 * |
| Control (Nerve transection) | 4.0 ± 0.5 | 40 ± 4 | 0.5 ± 0.1 |
| Vit B (Nerve transection) | 3.3 ± 0.4 * | 50 ± 5 * | 1.0 ± 0.2 * |
| Control (Crush injury) | 4.2 ± 0.6 | 38 ± 4 | 0.6 ± 0.1 |
| Vit B (Crush injury) | 3.6 ± 0.4 * | 48 ± 5 * | 1.1 ± 0.2 * |
| Myelination | Fibrosis | Edema | Mast Cells | |
|---|---|---|---|---|
| Control (nerve graft) | 5.2 ± 0.6 | 1.4 ± 0.3 | 2.3 ± 0.4 | 3.1 ± 0.5 |
| Vit B (nerve graft) | 6.8 ± 0.7 | 1.0 ± 0.2 | 1.8 ± 0.3 | 2.5 ± 0.4 |
| Control (nerve transection) | 4.5 ± 0.5 | 2.0 ± 0.4 | 3.0 ± 0.5 | 3.6 ± 0.6 |
| Vit B (nerve transection) | 6.0 ± 0.6 | 1.2 ± 0.3 | 2.2 ± 0.4 | 2.8 ± 0.5 |
| Control (crush injury) | 4.8 ± 0.6 | 2.1 ± 0.4 | 2.9 ± 0.5 | 3.3 ± 0.5 |
| Vit B (crush injury) | 6.3 ± 0.7 | 1.3 ± 0.3 | 2.1 ± 0.4 | 2.9 ± 0.5 |
| p-values | ||||
| Nerve graft | 0.010 | 0.030 | 0.023 | 0.036 |
| Nerve transection | 0.022 | 0.041 | 0.034 | 0.045 |
| Crush injury | 0.013 | 0.034 | 0.027 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, A.; Temel, M.; Atilgan, N.; Saray, A.; Dokuyucu, R. Therapeutic Potential of Vitamin B Complex in Peripheral Nerve Injury Recovery: An Experimental Rat Model Study. Medicina 2024, 60, 1556. https://doi.org/10.3390/medicina60091556
Kahraman A, Temel M, Atilgan N, Saray A, Dokuyucu R. Therapeutic Potential of Vitamin B Complex in Peripheral Nerve Injury Recovery: An Experimental Rat Model Study. Medicina. 2024; 60(9):1556. https://doi.org/10.3390/medicina60091556
Chicago/Turabian StyleKahraman, Ahmet, Metin Temel, Numan Atilgan, Ahmet Saray, and Recep Dokuyucu. 2024. "Therapeutic Potential of Vitamin B Complex in Peripheral Nerve Injury Recovery: An Experimental Rat Model Study" Medicina 60, no. 9: 1556. https://doi.org/10.3390/medicina60091556






