MIL-101 (Fe) @Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of MIL-101(Fe) and MIL-101(Fe)@Ag
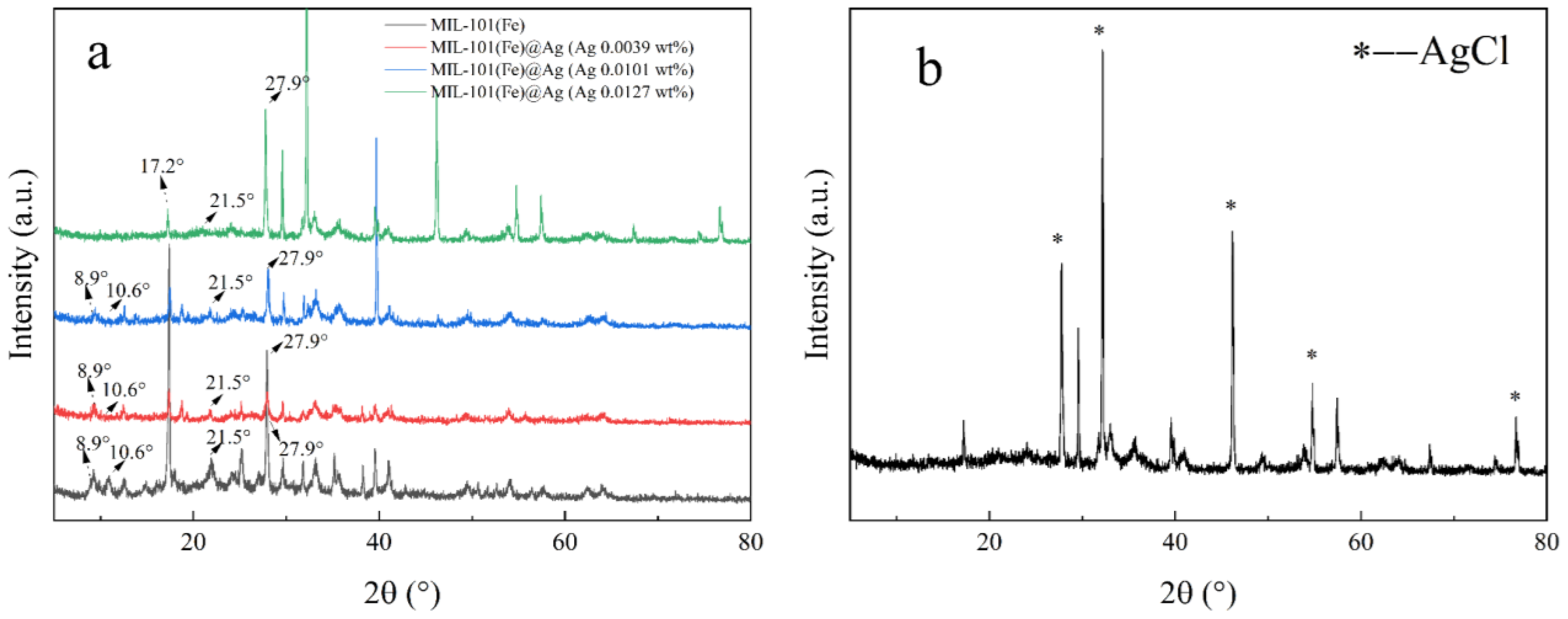
2.1.1. PXRD
2.1.2. FTIR Spectroscopy
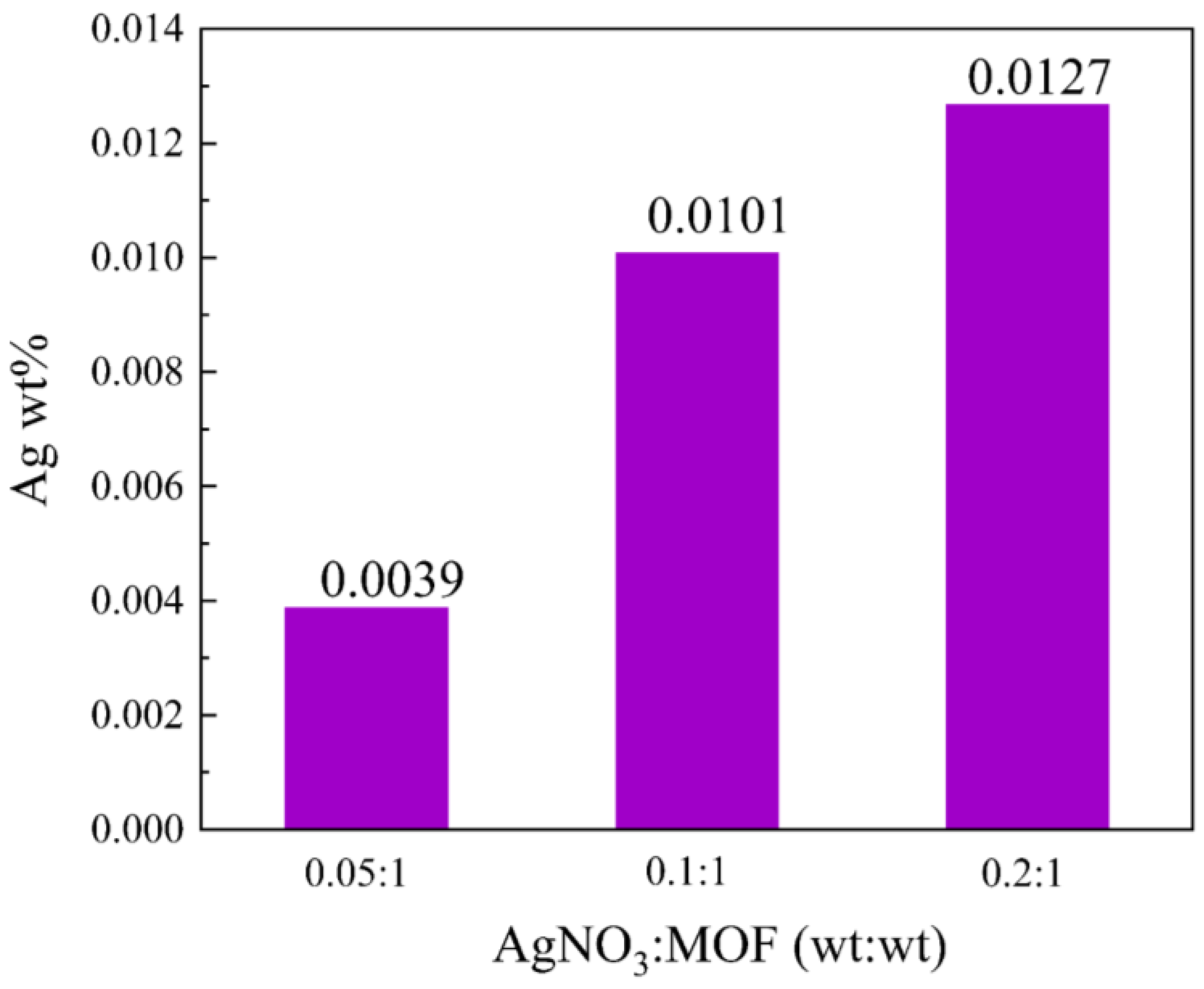
2.1.3. ICP Analysis
2.1.4. UV–vis Spectroscopy
2.1.5. ζ-Potential, Nanoparticle Size, and TG analysis of MIL-101(Fe)@Ag (Ag 0.0127 wt%)
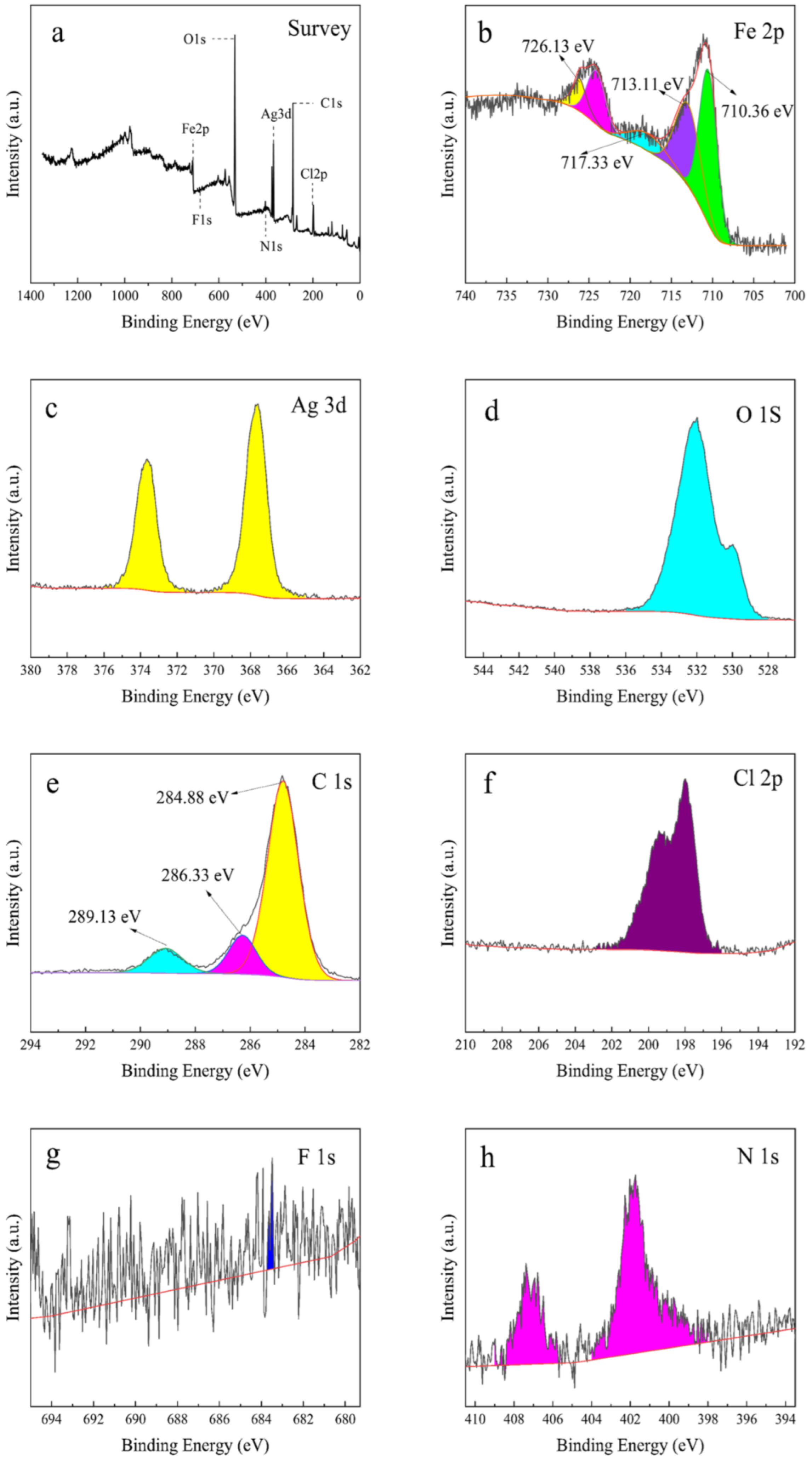
2.1.6. XPS Analysis
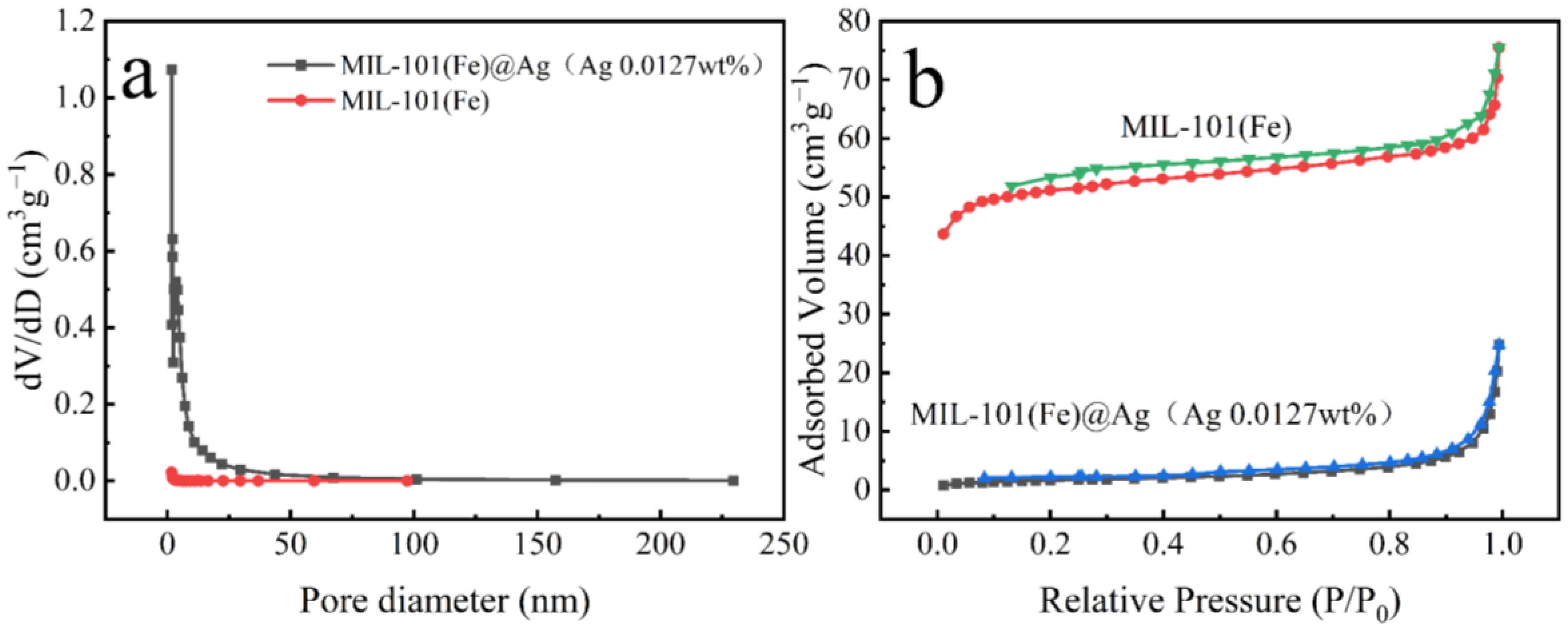
2.1.7. BET Analysis
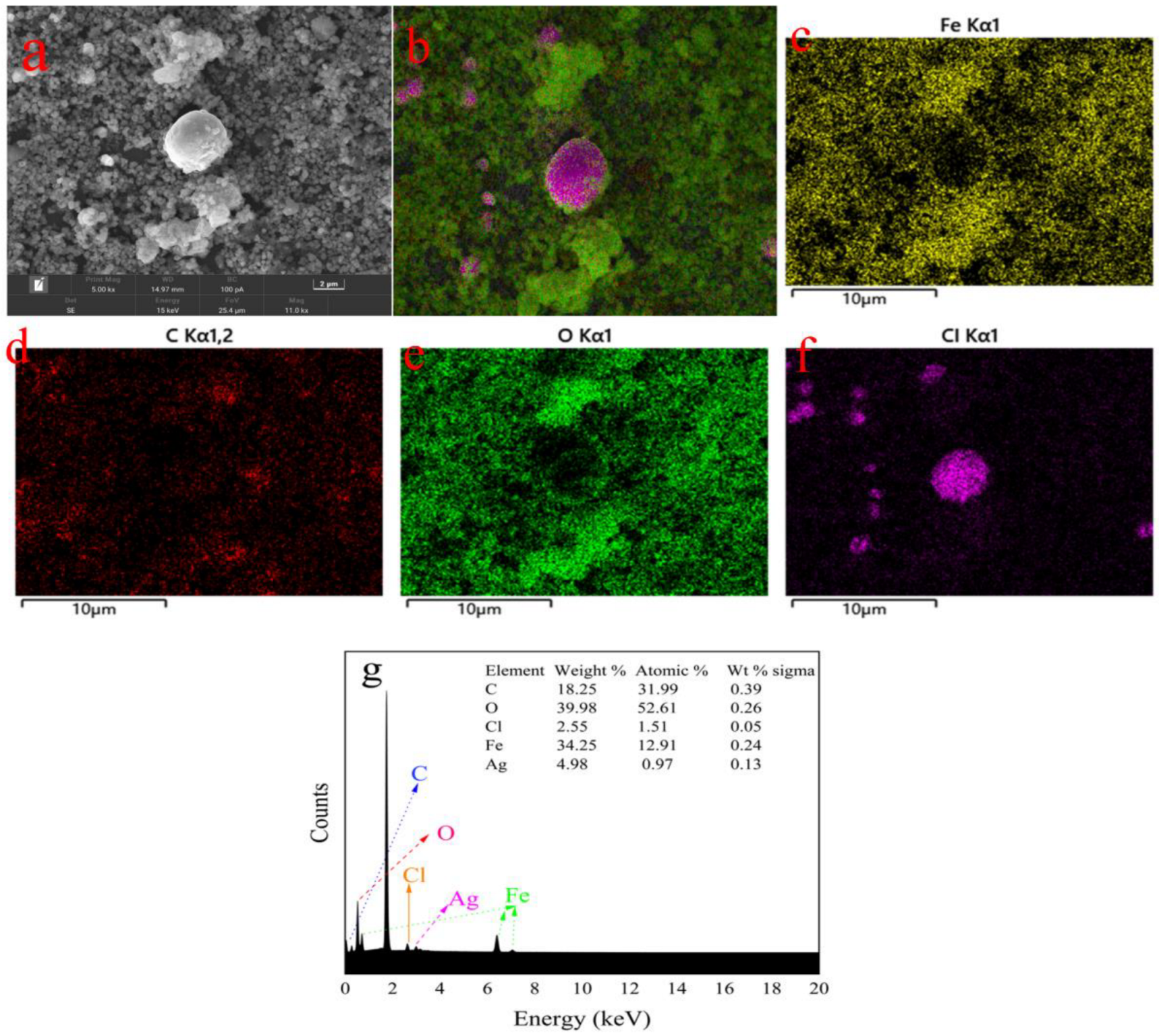
2.1.8. SEM and EDS Analysis
2.2. Effect of MIL-101(Fe)@Ag (Ag 0.0127 wt%) on Bacterial Growth
2.2.1. Inhibition-Zone Experiment
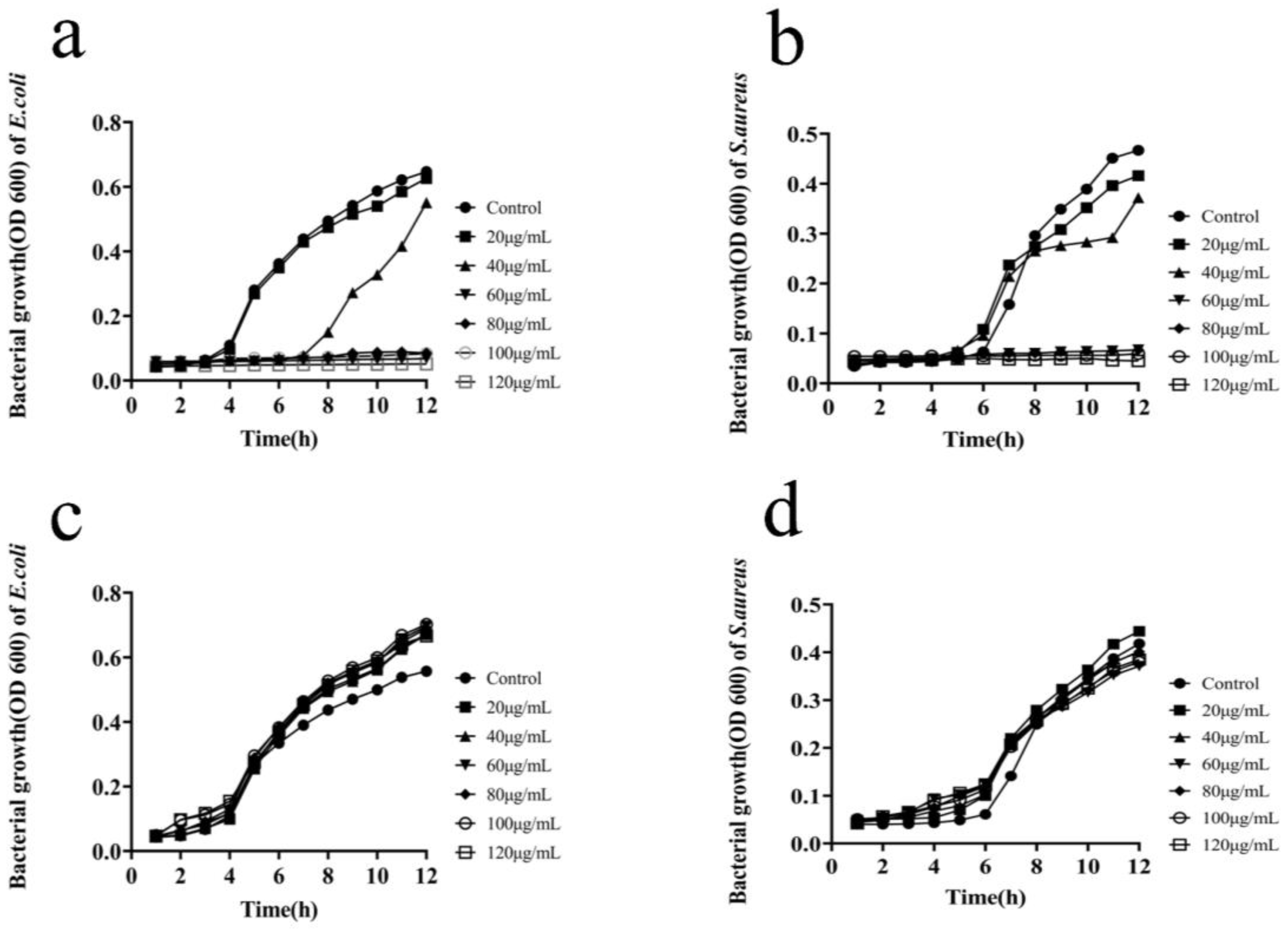
2.2.2. Determination of the Bacterial Growth Curves
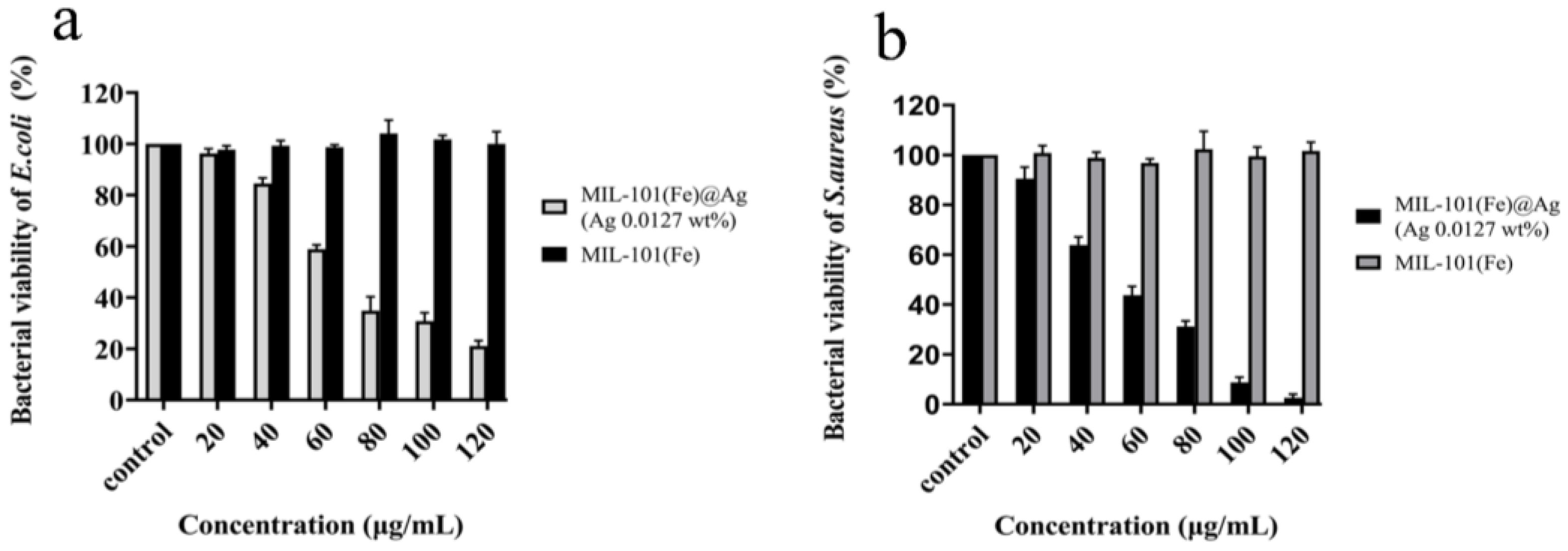
2.2.3. Determination of the Bacterial Survival Rate
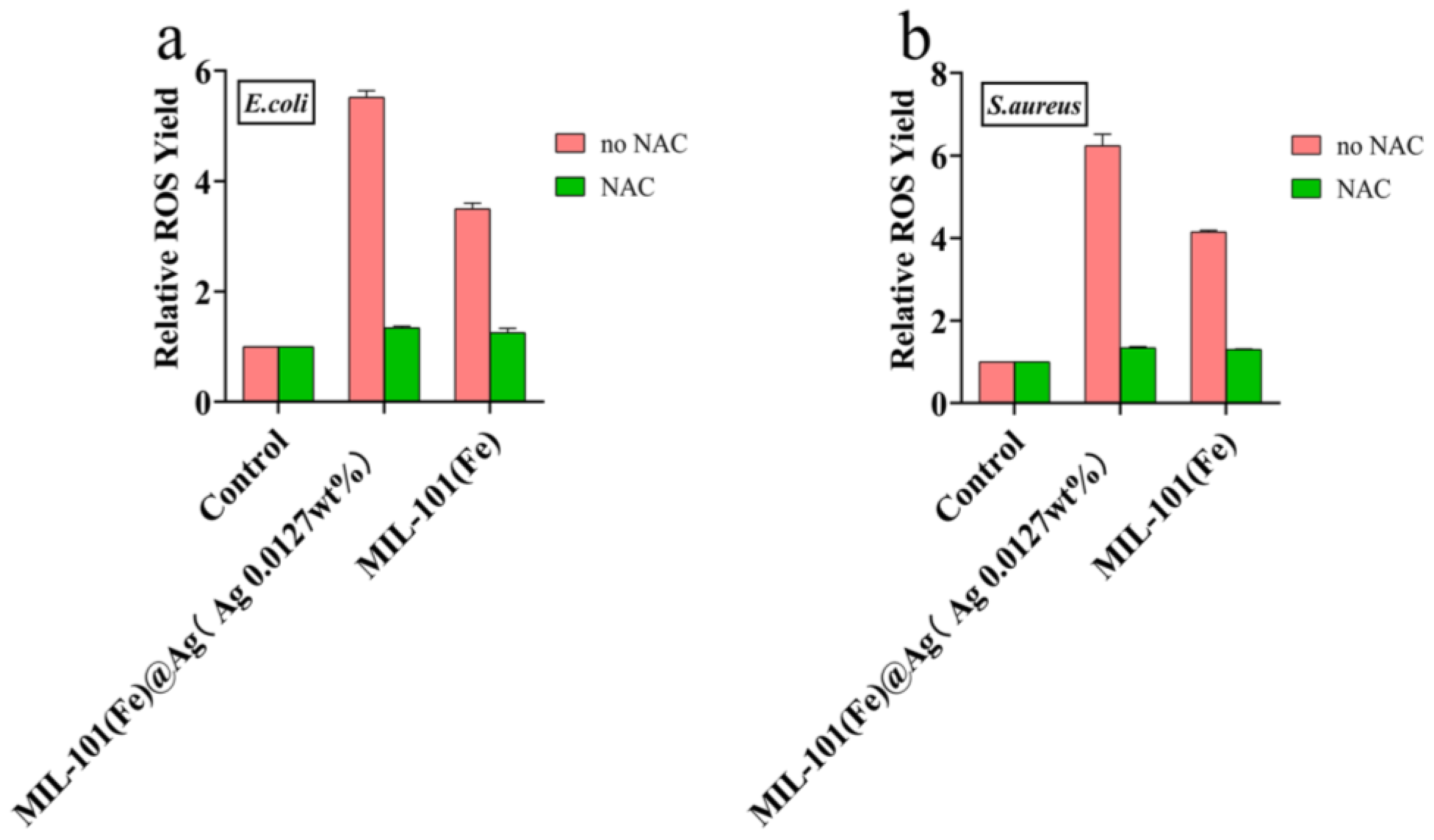
2.3. Antibacterial Mechanism of MIL-101(Fe)@Ag (Ag 0.0127 wt% Ag)
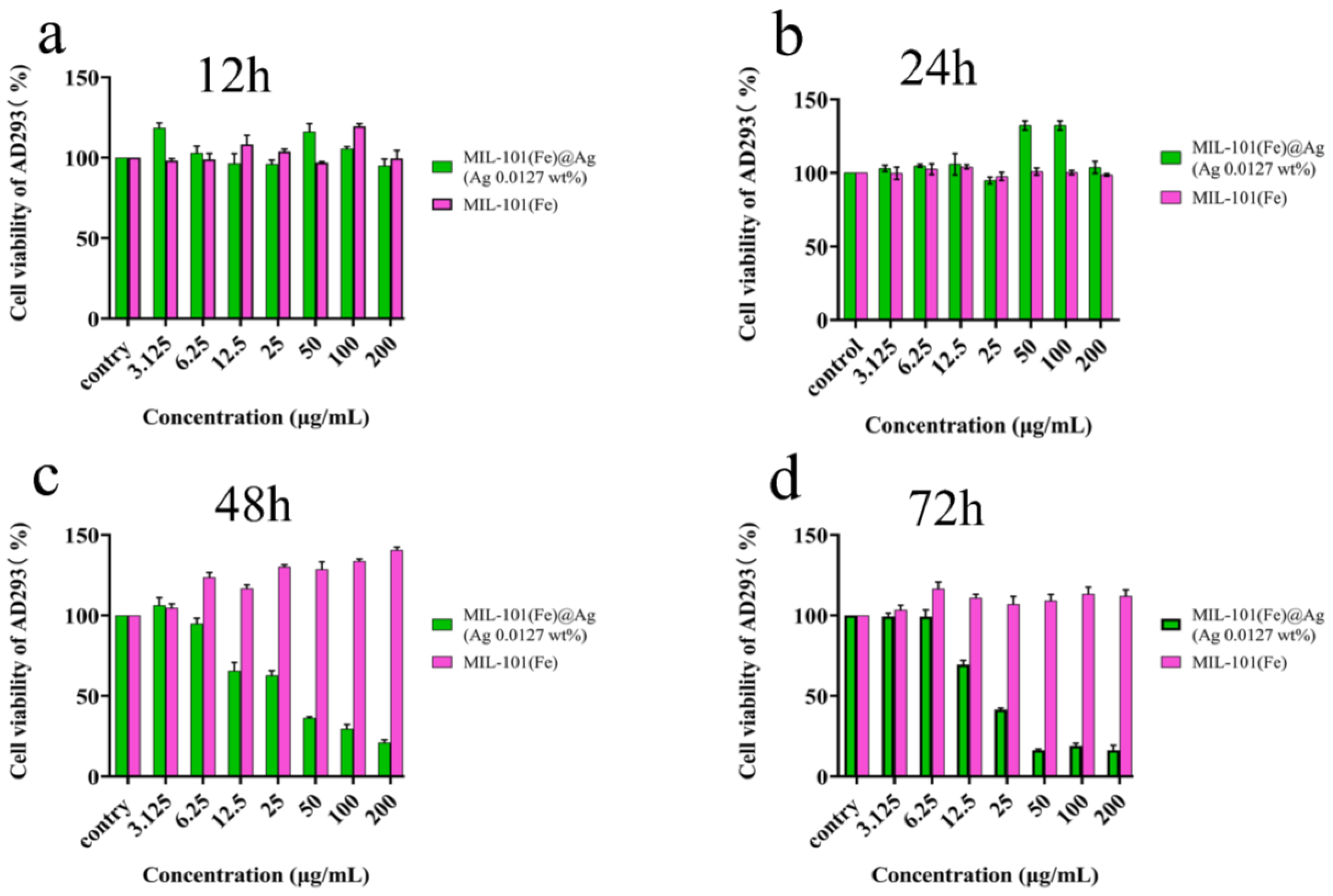
2.4. Cytotoxicity Test
3. Experimental
3.1. Materials
3.2. Preparation of MIL-101(Fe) and MIL-101(Fe)@Ag
3.3. Nanomaterial Characterization
3.4. Antimicrobial Activity
3.4.1. Inhibition-Zone Experiment
3.4.2. Determination of the Bacterial Growth Curve
3.4.3. Determination of the Bacterial Survival Rate
3.5. Investigation of the Antibacterial Mechanism
3.6. Cytotoxicity Test
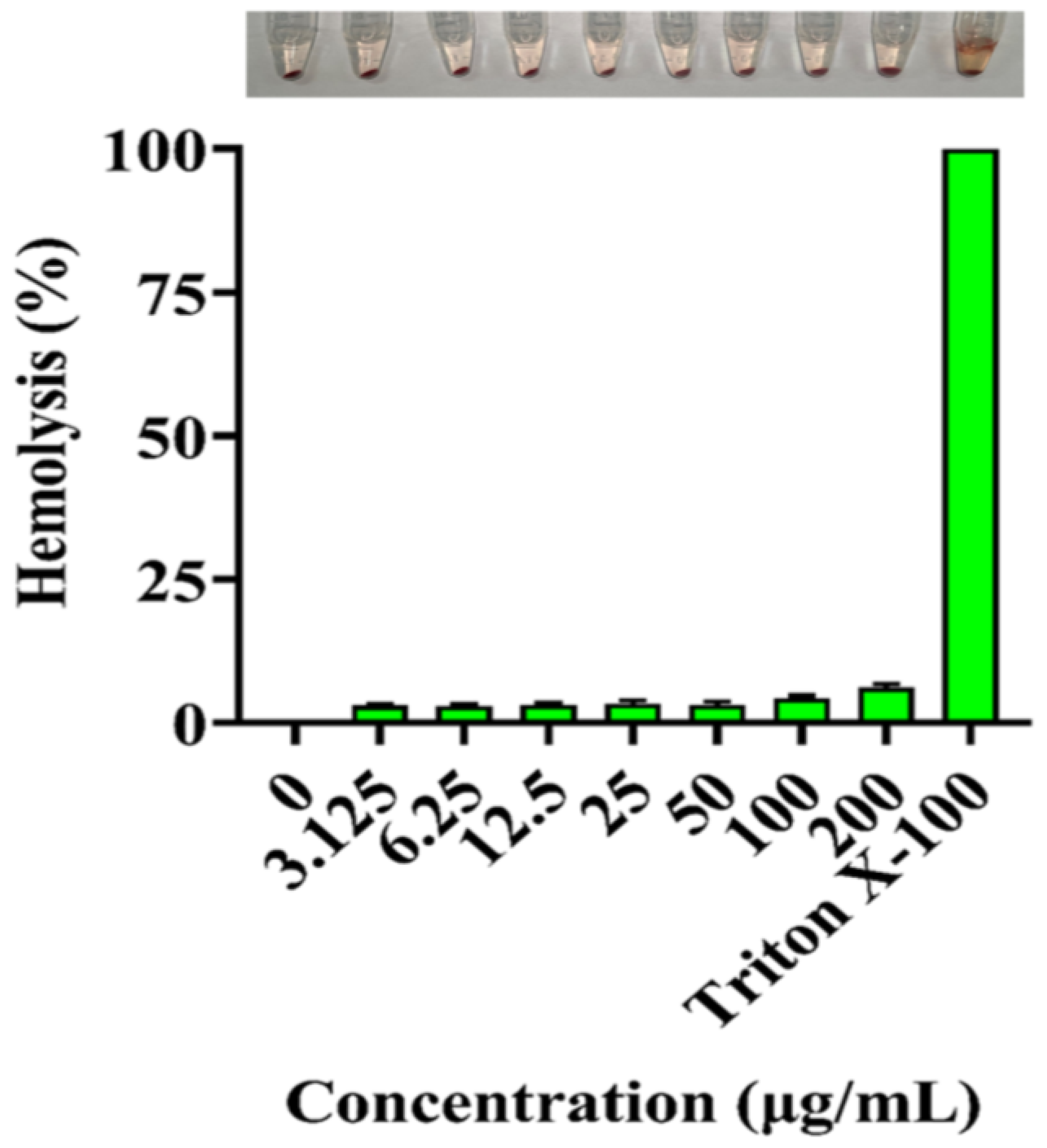
3.7. Hemolytic Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2021, 14, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendaus, M.A.; Jomha, F.A. COVID-19 induced superimposed bacterial infection. J. Biomol. Struct. Dyn. 2021, 39, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Buder, S.; Schöfer, H.; Meyer, T.; Bremer, V.; Kohl, P.K.; Skaletz-Rorowski, A.; Brockmeyer, N. Bacterial sexually transmitted infections. J. Dtsch. Dermatol. Ges. 2019, 17, 287–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettlinger, R.; Lächelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.E.; Wuttke, S. Toxicity of metal-organic framework nanoparticles: From essential analyses to potential applications. Chem. Soc. Rev. 2022, 51, 464–484. [Google Scholar] [CrossRef]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A. Unit of Antibiotic Resistance and Special Pathogens; Unit of Antibiotic Resistance and Special Pathogens of the Department of Infectious Diseases, Istituto Superiore di Sanità, Rome. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Ist. Super. Sanita. 2020, 56, 359–364. [Google Scholar]
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Rodrigues, M.X.; Silva, N.C.C. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: A review. Braz. J. Microbiol. 2020, 51, 347–356. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mahmoud, G.A.; Sharmouk, W. A cerium-based MOFzyme with multi-enzyme-like activity for the disruption and inhibition of fungal recolonization. J. Mater. Chem. B 2020, 8, 7548–7556. [Google Scholar] [CrossRef]
- Armando, R.A.M.; Abuçafy, M.P.; Graminha, A.E.; da Silva, R.S.; Frem, R.C.G. Ru-90@bio-MOF-1: A ruthenium(II) metallodrug occluded in porous Zn-based MOF as a strategy to develop anticancer agents. J. Solid State Chem. 2021, 297, 122081. [Google Scholar] [CrossRef]
- Neal, A.L. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef]
- Inoue, Y.; Hoshino, M.; Takahashi, H.; Noguchi, T.; Murata, T.; Kanzaki, Y.; Hamashima, H.; Sasatsu, M. Bactericidal activity of Ag-zeolite mediated by reactive oxygen species under aerated conditions. J. Inorg. Biochem. 2002, 92, 37–42. [Google Scholar] [CrossRef]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yu, J.; Hu, M.Y.; Zhang, K.H. In-situ reaction precipitation of AgAl alloy powder and oxygen adsorption performance. Joumal Alloy. Compd. 2018, 747, 966–971. [Google Scholar] [CrossRef]
- Kalankesh, L.R.; Rodríguez-Couto, S.; Zazouli, M.A.; Shahamat, Y.D.; Ali Dianati, R.; Arghiani, M. Synthesis and characterization of nanoparticles and composites as bactericides. J. Microbiol. Methods 2019, 167, 105736. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A.; Amiri, O.; Goli, H.R.; Rafiei, A.; Kardan, M.; Salavati-Niasari, M. Facile green synthesis and characterization of Crataegus microphylla extract-capped silver nanoparticles (CME@Ag-NPs) and its potential antibacterial and anticancer activities against AGS and MCF-7 human cancer cells. J. Alloy. Compd. 2020, 820, 153186. [Google Scholar] [CrossRef]
- Teymourinia, H.; Omid Amiri, O.; Salavati-Niasari, M. Synthesis and characterization of cotton-silver-graphene quantum dots (cotton/Ag/GQDs) nanocomposite as a new antibacterial nanopad. Chemosphere 2021, 267, 129293. [Google Scholar] [CrossRef]
- Razavi, R.; Amiri, M.; Alshamsi, H.A.; Eslaminejad, T.; Salavati-Niasari, M. Green synthesis of Ag nanoparticles in oil-in-water nano-emulsion and evaluation of their antibacterial and cytotoxic properties as well as molecular docking. Arab. J. Chem. 2021, 14, 103323. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C. Nanoscale metal-organic frameworks as key players in the context of drug delivery: Evolution toward theranostic platforms. Anal. Bioanal. Chem. 2020, 412, 37–54. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Z.; Guo, Z. Theoretical Investigation on the Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions Performances of Two-Dimensional Metal-Organic Frameworks Fe3(C2X)12 (X = NH, O, S). Molecules 2022, 27, 1528. [Google Scholar] [CrossRef]
- Napolitano-Tabares, P.I.; Gutiérrez-Serpa, A.; Jiménez-Abizanda, A.I.; Jiménez-Moreno, F.; Pasán, J.; Pino, V. Hybrid Materials Formed with Green Metal-Organic Frameworks and Polystyrene as Sorbents in Dispersive Micro-Solid-Phase Extraction for Determining Personal Care Products in Micellar Cosmetics. Molecules 2022, 27, 813. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Liu, Y.; Liu, D.; Chen, C.; Lu, C.; Zhuang, S.; Kumar, A.; Liu, J. Recent advances in Cu(II)/Cu(I)-MOFs based nano-platforms for developing new nano-medicines. J. Inorg. Biochem. 2021, 225, 111599. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, D.; Li, X.; Fu, Q.; Yin, L.; Yang, Q.; Chen, H. Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere 2021, 284, 131386. [Google Scholar] [CrossRef] [PubMed]
- Soomro, N.A.; Wu, Q.; Amur, S.A.; Liang, H.; Ur Rahman, A.; Yuan, Q.; Wei, Y. Natural drug physcion encapsulated zeolitic imidazolate framework, and their application as antimicrobial agent. Colloids Surf. B Biointerfaces 2019, 182, 110364. [Google Scholar]
- Ali Noman, E.; Al-Gheethi, A.; Saphira Radin Mohamed, R.M.; Talip, B.A.; Hossain, M.S.; Ali Hamood Altowayti, W.; Ismail, N. Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: A critical review. J. Hazard. Mater. 2021, 417, 126040. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, H.; Wang, R.; Duan, M. Fabricating Ag@MOF-5 nanoplates by the template of MOF-5 and evaluating its antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 127093. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Lin, W.; Yao, X.; Zhang, M.; He, Q.; Fu, F.; Zhu, H.; Chen, J. A metal-organic framework MIL-53(Fe) containing sliver ions with antibacterial property. J. Solid State Chem. 2021, 302, 122442. [Google Scholar] [CrossRef]
- Jaros, S.W.; Król, J.; Bażanów, B.; Poradowski, D.; Chrószcz, A.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid. Molecules 2020, 25, 2119. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fe-based metal organic framework/graphene oxide composite as an efficient catalyst for Fenton-like degradation of methyl orange. RSC Adv. 2017, 7, 50829–50837. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Zhuang, L.; She, B.; Deng, Y.; Chen, D.; Tang, J. In-situ reduction of monodisperse nanosilver on hierarchical wrinkled mesoporous silica with radial pore channels and its antibacterial performance. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Alzamely, K.O.; Hajizadeh, F.; Heydari, M.; Ghaderi Sede, M.J.; Asl, S.H.; Peydaveisi, M.; Masjedi, A.; Izadi, S.; Nikkhoo, A.; Atyabi, F.; et al. Combined inhibition of CD73 and ZEB1 by Arg-Gly-Asp (RGD)-targeted nanoparticles inhibits tumor growth. Colloids Surf. B Biointerfaces 2021, 197, 111421. [Google Scholar] [CrossRef]
- Jacobson, L. Listeriosis. Pediatr. Rev. 2008, 29, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silveira, Z.; Macêdo, N.S.; Sampaio Dos Santos, J.F.; Sampaio de Freitas, T.; Rodrigues Dos Santos Barbosa, C.; Júnior, D.L.S.; Muniz, D.F.; Castro de Oliveira, L.C.; Júnior, J.P.S.; Cunha, F.A.B.D.; et al. Evaluation of the Antibacterial Activity and Efflux Pump Reversal of Thymol and Carvacrol against Staphylococcus aureus and Their Toxicity in Drosophila melanogaster. Molecules 2020, 25, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Nowak, A.; Zakłos-Szyda, M.; Szczuka, D.; Szymańska, G.; Motyl, I. Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 2262. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Parivar, K.; Baharara, J.; Kerachian, M.A.; Asili, J. Hemolytic and cytotoxic properties of saponin purified from Holothuria leucospilota sea cucumber. Rep. Biochem. Mol. Biol. 2014, 3, 43–50. [Google Scholar] [PubMed]
- Diaz, T.M.; Moscoso, I.; Centeno, A.; Lopez-Pelaez, E.; Ortega, D.; Doménech, N. Flow cytometry complement-mediated cytotoxicity assay detects baboon xenoantibodies directed to porcine epitopes undetected by hemolytic assay. Transpl. Immunol. 2004, 13, 313–317. [Google Scholar] [CrossRef]
- Karimi Alavijeh, R.; Akhbari, K. Biocompatible MIL-101(Fe) as a Smart Carrier with High Loading Potential and Sustained Release of Curcumin. Inorg Chem. 2020, 59, 3570–3578. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Bakhtari, M.F.; Luo, J. Preparation of GO/MIL-101(Fe,Cu) composite and its adsorption mechanisms for phosphate in aqueous solution. Environ. Sci. Pollut. Res. Int. 2021, 28, 51391–51403. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.; Chen, Y.; Ou, H. Enhanced photocatalysis using metal-organic framework MIL-101(Fe) for organophosphate degradation in water. Environ. Sci. Pollut. Res. Int. 2019, 26, 24720–24732. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Zhang, Q.; Shapour, H.; Bakhtari, M.F. Preparation of a GO/MIL-101(Fe) Composite for the Removal of Methyl Orange from Aqueous Solution. ACS Omega 2021, 6, 4597–4608. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Lin, J.; Xiao, L.; Wang, X. The preparation of nano-MIL-101(Fe)@chitosan hybrid sponge and its rapid and efficient adsorption to anionic dyes. Int. J. Biol. Macromol. 2020, 165, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, L.; Zhuo, K.; Zhou, H.; Zhang, Y. Simultaneous catalytic reduction of p-nitrophenol and hydrogen production on MIL-101(Fe)-based composites. New J. Chem. 2021, 45, 3120–3127. [Google Scholar] [CrossRef]
- Veerasamy, N.; Takamasa, A.; Murugan, R.; Kasar, S.; Aono, T.; Inoue, K.; Fukushi, M.; Sahoo, S.K. Chemical Separation of Uranium and Precise Measurement of 234U/238U and 235U/238U Ratios in Soil Samples Using Multi Collector Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 2138. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A. Eremenko IL. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. [Google Scholar] [CrossRef]
- Ehman, N.V.; Ita-Nagy, D.; Felissia, F.E.; Vallejos, M.E.; Quispe, I.; Area, M.C.; Chinga-Carrasco, G. Biocomposites of Bio-Polyethylene Reinforced with a Hydrothermal-Alkaline Sugarcane Bagasse Pulp and Coupled with a Bio-Based Compatibilizer. Molecules 2020, 25, 2158. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.-H.; Kim, D.-J.; Lee, Y.-J.; Jhee, K.H.; Sohn, Y.; Jang, E.-S. Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: Insight into an ROS-mediated antibacterial mechanism under UV light. J. Solid State Chem. 2018, 267, 124–133. [Google Scholar] [CrossRef]
- Shah, J.A.; Butt, T.A.; Mirza, C.R.; Shaikh, A.J.; Khan, M.S.; Arshad, M.; Riaz, N.; Haroon, H.; Gardazi, S.M.H.; Yaqoob, K.; et al. Phosphoric Acid Activated Carbon from Melia azedarach Waste Sawdust for Adsorptive Removal of Reactive Orange 16: Equilibrium Modelling and Thermodynamic Analysis. Molecules 2020, 25, 2118. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Liu, Y.; Zeng, P.; Wang, Y.; Zhang, Y. Removal of Berberine from Wastewater by MIL-101(Fe): Performance and Mechanism. ACS Omega 2020, 5, 27962–27971. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef]
- Lee, B.; Hwang, J.S.; Lee, D.G. Antibacterial action of lactoferricin B like peptide against Escherichia coli: Reactive oxygen species-induced apoptosis-like death. J. Appl. Microbiol. 2020, 129, 287–295. [Google Scholar] [CrossRef]
- Verma, A.; Shivalkar, S.; Sk, M.P.; Samanta, S.K.; Sahoo, A.K. Nanocomposite of Ag nanoparticles and catalytic fluorescent carbon dots for synergistic bactericidal activity through enhanced reactive oxygen species generation. Nanotechnology 2020, 31, 405704. [Google Scholar] [CrossRef] [PubMed]
- Gecgel, C.; Simsek, U.B.; Gozmen, B.; Turabik, M. Comparison of MIL-101(Fe) and amine-functionalized MIL-101(Fe) as photocatalysts for the removal of imidacloprid in aqueous solution. J. Iran. Chem. Soc. 2019, 16, 1735–1748. [Google Scholar] [CrossRef]
- Krishnamurthi, V.R.; Niyonshuti, I.I.; Chen, J.; Wang, Y. A new analysis method for evaluating bacterial growth with microplate readers. PLoS ONE 2021, 16, e0245205. [Google Scholar] [CrossRef] [PubMed]
- Amdeha, E.; Mohamed, R.S. A green synthesized recyclable ZnO/MIL-101(Fe) for Rhodamine B dye removal via adsorption and photo-degradation under UV and visible light irradiation. Environ. Technol. 2021, 42, 842–859. [Google Scholar] [CrossRef]
- Rajabi, S.K.; Sohrabnezhad, S.; Ghafourian, S. Fabrication of Fe3O4@CuO core-shell from MOF based materials and its antibacterial activity. J. Solid State Chem. 2016, 244, 160–163. [Google Scholar] [CrossRef]
- Azizabadi, O.; Akbarzadeh, F.; Danshina, S.; Chauhan, N.P.S.; Sargazi, G. An efficient ultrasonic assisted reverse micelle synthesis route for Fe3O4@Cu-MOF/core-shell nanostructures and its antibacterial activities. J. Solid State Chem. 2021, 294, 121897. [Google Scholar] [CrossRef]
- Beheshti, A.; Panahi, F.; Mayer, P.; Motamedi, H.; Parisi, E.; Centore, R. Synthesis, structural characterization, antibacterial activity and selective dye adsorption of silver (I)-based coordination polymers by tuning spacer length and binding mode of chromate anion. J. Solid State Chem. 2020, 287, 121322. [Google Scholar] [CrossRef]
- Ali, A.; Ovais, M.; Zhou, H.; Rui, Y.; Chen, C. Tailoring metal-organic frameworks-based nanozymes for bacterial theranostics. Biomaterials 2021, 275, 120951. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, H.C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, D.; Dong, X.; Zhang, X.; Liu, J.; Peng, L.; Meng, B.; Hua, Q.; Pei, X.; Zhao, L.; et al. LncDACH1 promotes mitochondrial oxidative stress of cardiomyocytes by interacting with sirtuin3 and aggravates diabetic cardiomyopathy. Sci. China Life Sci. 2021; ahead of print. [Google Scholar] [CrossRef]
- Bosch, M.; Yuan, S.; Rutledge, W.; Zhou, H.C. Stepwise Synthesis of Metal-Organic Frameworks. Acc. Chem. Res. 2017, 50, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Batul, R.; Bhave, M.; JMahon, P.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Roman, S.; MacNeil, S. Improving the biocompatibility of biomaterial constructs and constructs delivering cells for the pelvic floor. Curr. Opin. Urol. 2019, 29, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, X.; Zheng, H.; Chen, J.; Ye, X.; Liu, T. Anti-Influenza Virus Study of Composite Material with MIL-101(Fe)-Adsorbed Favipiravir. Molecules 2022, 27, 2288. [Google Scholar] [CrossRef]






| Types of Bacteria | MIL-101(Fe)@Ag (Ag 0.0127 wt%) | MIL-101(Fe) |
|---|---|---|
| Escherichia coli | 12 | 9 |
| Staphylococcus aureus | 12.3 | 9 |
| Staphylococcus epidermidis | 10 | 9 |
| Acinetobacter cereus | 11 | 9 |
| Acinetobacter jungii | 11 | 9 |
| Pseudomonas aeruginosa | 11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zheng, H.; Chen, J.; Xu, M.; Bai, Y.; Liu, T. MIL-101 (Fe) @Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials. Molecules 2022, 27, 3497. https://doi.org/10.3390/molecules27113497
Li X, Zheng H, Chen J, Xu M, Bai Y, Liu T. MIL-101 (Fe) @Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials. Molecules. 2022; 27(11):3497. https://doi.org/10.3390/molecules27113497
Chicago/Turabian StyleLi, Xi, Huiying Zheng, Jiehan Chen, Mengyuan Xu, Yan Bai, and Tiantian Liu. 2022. "MIL-101 (Fe) @Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials" Molecules 27, no. 11: 3497. https://doi.org/10.3390/molecules27113497
APA StyleLi, X., Zheng, H., Chen, J., Xu, M., Bai, Y., & Liu, T. (2022). MIL-101 (Fe) @Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials. Molecules, 27(11), 3497. https://doi.org/10.3390/molecules27113497






