Effects of Chitin and Sepia Ink Hybrid Hemostatic Sponge on the Blood Parameters of Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of Chitin and Sepia Ink Sponge
| Color | Water absorption (/time) | Ash content (%) | Deacetylation degree (%) |
|---|---|---|---|
| gray | 25 | 1.224 | 30.700 |
2.2. Effects of CTSH Sponge on the Coagulation Parameters

2.3. Effects of CTSH Sponge on the Anticoagulation Parameters

2.4. Effects of CTSH Sponge on the Fibrinolytic Parameters

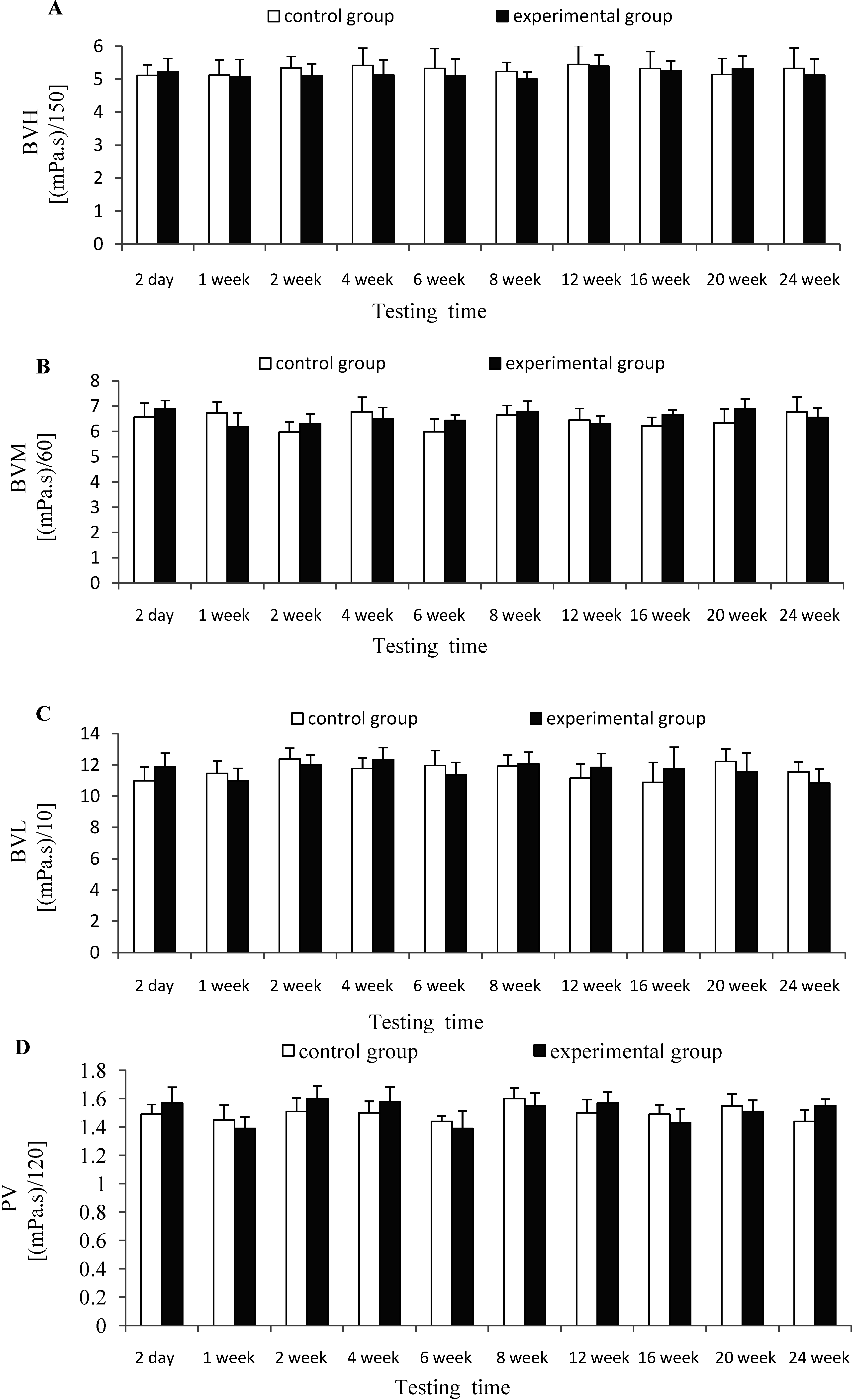
2.5. Effects of CTSH Sponge on the Hemorheology Parameters
3. Experimental Section
3.1. Materials and Regents
3.2. Preparation of CTSH Sponge
3.3. Animal Experiments
3.4. Collection of Blood Sample
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hyun, S.W.; Wolff, K.; Yong, H.Z. Hemostatic agents derived from chitin and chitosan. J. Macromol. Sci. Part C Polym. Rev. 2005, 45, 309–323. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable application of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Arthur, F.P.; Panda, T. Studies on applications of chitin and its derivatives. Bioprocess Eng. 1999, 20, 505–512. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. Carbohydr. Polym. 2000, 46, 1–27. [Google Scholar]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Harish, K.V.P.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potentialdan overview. Trends Food. Sci. Tech. 2007, 18, 117–131. [Google Scholar]
- Entsar, S.A.; Khaled, S.A.N.; Maher, Z.E. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Shi, Z.L.; Neoh, K.G.; Kang, E.T.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral. Maxil. Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Fox, D.L. Melanins. In Physiology of Mollusca; Academic Press: New York, NY, USA, 1966; pp. 257–261. [Google Scholar]
- Takaya, Y. Biological activities of natural resources around us are now in the limelight. Yakugaku Zasshi 2000, 120, 1075–1089. [Google Scholar]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Guo, X.; Chen, S.G.; Hu, Y.Q.; Li, G.Y.; Liao, N.B.; Ye, X.Q.; Liu, D.H.; Xue, C.H. Preparation of water-soluble melanin from squid ink using ultrasoundassisted degradation and its anti-oxidant activity. J. Food. Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Zhong, J.P.; Wang, G.; Shang, J.H.; Pan, J.Q.; Li, K.; Huang, Y.; Liu, H.Z. Protective effects of Squid ink extract towards hemopoietic injuries induced by cyclo- phosphamine. Mar. Drugs 2009, 7, 9–18. [Google Scholar] [CrossRef]
- Wang, R.; Sun, D.Y.; Meng, Z.H. Squid ink—A new systemic hemostatic. Chin. J. Zool. 1975, 1, 20–21. [Google Scholar]
- Wang, X.H.; Yan, Y.N.; Zhang, R.J.A. Comparison of chitosan and collagen sponges as hemostatic dressings. J. Bioact. Compat. Pol. 2006, 21, 39–54. [Google Scholar] [CrossRef]
- Broekema, F.I.; van Oeveren, W.; Selten, M.H.A.; Meijer, R.J.H.; de Wolf, J.T.M.; Bos, R.R.M. In vivo hemostatic efficacy of polyurethane foam compared to collagen and gelatin. Clin. Oral. Invest. 2013, 17, 1273–1278. [Google Scholar] [CrossRef]
- Spector, D.; Perry, Z.; Konobeck, T.; Mooradian, D.; Shikora, S. Comparison of hemostatic properties between collagen and synthetic buttress materials used in staple line reinforcement in a swine splenic hemorrhage model. Surg. Endosc. 2011, 25, 1148–1152. [Google Scholar] [CrossRef]
- Otani, Y.; Tabata, Y.; Ikada, Y. A new biological glue from gelatin and poly(L-glutamic acid). J. Biomed. Mater. Res. 1996, 31, 157–166. [Google Scholar] [CrossRef]
- The United States Pharmacopeia: The National Formulary, 27th ed.The United States Pharmacopeial Convention: Rockville, MD, USA, 2008.
- Ohshima, Y.; Nishino, K.; Okuda, R.; Minami, A.; Kihune, K. Clinical application of new chitin non-woven fabric and new chitin sponge sheet as wound dressing. Eur. J. Plast. Surg. 1991, 14, 202–211. [Google Scholar]
- Jang, D.H.; Weaver, M.D.; Pizon, A.F. In vitro study of N-acetylcysteine on coagulation factors in plasma samples from healthy subjects. J. Med. Toxicol. 2013, 9, 49–53. [Google Scholar] [CrossRef]
- Tibbs, R.F.; Elghetany, M.T.; Tran, L.T.; Bonn, W.V.; Romano, T.; Cowan, D.F. Characterization of the coagulation system in healthy dolphins: The coagulation factors, natural anticoagulants, and fibrinolytic products. Comp. Clin. Path. 2005, 14, 95–98. [Google Scholar] [CrossRef]
- Xiong, L.F.; Li, S.R. Basis of Clinical Examination, 3rd ed.People’s Medical Publishing House: Beijing, China, 2003; pp. 88–93. (In Chinese) [Google Scholar]
- Jaeger, B.R.; Labarrere, C.A. Fibrinogen and at herothrombosis: Vulnerable plaque or vulnerable patient? Herz 2003, 28, 530–538. [Google Scholar] [CrossRef]
- Artjoms, S.; Inta, J.; Simona, D.; Ainis, P.; Jelena, G.U.; Irina, S.; Aurika, B.; Dainis, K. Dynamics of CXC group chemokine plateletfactor 4 (PF4) plasma levels in non-small cell lung cancer (NSCLC). J. Transl. Med. 2012, 10, 28–30. [Google Scholar] [CrossRef]
- Zheng, M.Q.; Lin, Y.; Li, B.Q.; Yu, L.L. Changes of plasma levels of vWF, PLG, D-D in patients with cerebral infraction and their clinical significance. Mod. Prac. Med. 2006, 18, 618–620. [Google Scholar]
- Modern Experimental Methods in Pharmacology. Zhang, J.T. (Ed.) Medical University and China Concordance University Combined Press: Beijing, China, 1997; p. 1209. (In Chinese)
- Yokoyama, S.; Takayama, K.; Murakami, T.; Ishikawa, Y.; Fujiwara, N.; Ganaha, H. Surgical treatment of spontaneous intracerebral hemorrhage in a full-term infant with coagulopathy case report. Neurol. Med. Chir. 2003, 43, 85–87. [Google Scholar] [CrossRef]
- Rodrigues, T.C.; Snell, J.K.; Maahs, D.M.; Kinney, G.L.; Rewers, M. Higher fibrinogen levels predict progression of coronary artery calcification in adults with type1 diabetes. Atherosclerosis 2010, 210, 671–673. [Google Scholar] [CrossRef]
- Yang, L.; Manithody, C.; Qureshi, S.H.; Rezaie, A.R. Contribution of exosite occupancy by heparin to the regulation of coagulation proteases by antithrombin. Thromb. Haemost. 2010, 103, 277–283. [Google Scholar]
- Turczyński, B.; Michalska-Małecka, K.; Słowińska, L.; Szczesny, S.; Romaniuk, W. Correlations between the severity of retinopathy in diabetic patients and whole blood and plasma viscosity. Clin. Hemorheol. Microcirc. 2003, 29, 129–137. [Google Scholar]
- Experimental Hemorheology. Zhang, Y. (Ed.) Guangxi Normal University Press: Nanning, China, 2009; pp. 9–25. (In Chinese)
- Wei, W.; Wu, X.M.; Li, Y.J. Experimental Methodology of Pharmacology, 4th ed.; People’s Medical Publishing House: Beijing, China, 2010; p. 1698. (In Chinese) [Google Scholar]
- Darren, J.; Costain, B.S.; Renee, K.M.D.; Curtis, C.M.D.; Vivian, C.; McAlister, M.D.; Timothy, D.G.L. Prevention of postsurgical adhesions with N,O-carboxymethyl chitosan: Examination of the most efficacious preparation and the effect of N,O-carboxymethyl chitosan on postsurgical healing. Surgery 1997, 121, 314–319. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Sun, Y.-L.; Chen, D.-H. Effects of Chitin and Sepia Ink Hybrid Hemostatic Sponge on the Blood Parameters of Mice. Mar. Drugs 2014, 12, 2269-2281. https://doi.org/10.3390/md12042269
Zhang W, Sun Y-L, Chen D-H. Effects of Chitin and Sepia Ink Hybrid Hemostatic Sponge on the Blood Parameters of Mice. Marine Drugs. 2014; 12(4):2269-2281. https://doi.org/10.3390/md12042269
Chicago/Turabian StyleZhang, Wei, Yu-Lin Sun, and Dao-Hai Chen. 2014. "Effects of Chitin and Sepia Ink Hybrid Hemostatic Sponge on the Blood Parameters of Mice" Marine Drugs 12, no. 4: 2269-2281. https://doi.org/10.3390/md12042269




