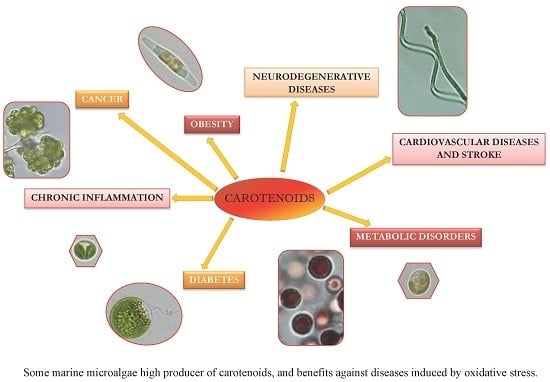
Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases
Abstract
:1. Introduction
2. Carotenoids from Marine Microalgae
| Main Carotenoid | Chemical IUPAC Name/Chemical Structure | Concentration | Microalga | Other Carotenoids | Remarks | References |
|---|---|---|---|---|---|---|
| β-carotene | β,β-carotene | 10%–13% DW | Dunaliella salina | zeaxanthin, lutein, α-carotene | occur mostly as a mixture of 9-cis and all-trans, but also other cis isomers | [31,32,33,34,35,36,37] |
 | 50% TC (TC = 0.9% DW) | Chlorella zofingiensis | canthaxanthin (25% TC or 97% DW), astaxanthin (0.7% DW) | |||
| 80% TC | Arthrospira | astaxanthin, lutein β-cryptoxanthin, zeaxanthin, echinenone, oscillaxanthin, myxoxanthophyll | ||||
| astaxanthin(as 3S,3′S isomer) | 3,3′-dihydroxy-β,β-carotene-4,4′-dione | up to 7% DW; 75% TC | Haematococcus pluvialis | β-carotene, lutein, canthaxanthin, neoxanthin, violaxanthin, zeaxanthin, echinenone | occur as a racemic mixture of mainly mono- and diesters (ca. 73% TC), but also as freeastaxanthin | [21,38,39,40,41,42] |
 | ||||||
| lutein | β,ε-carotene-3,3′-diol | 0.2%–0.4% DW | C. pyrenoidosa | violaxanthin, loroxanthin, α- and β-carotene | [43] | |
 | ||||||
| canthaxanthin | β,β-carotene-4,4′-dione | 4.75% DW | Coelastrella striolata var. multistriata | astaxanthin 0.15% DW, β-carotene 0.7% DW | [37] | |
 | ||||||
| canthaxanthin lutein | β,β-carotene-4,4′-dione β,ε-carotene-3,3′-diol | 45% TC | C. vulgaris | astaxanthin 12.5% TCviolaxanthin | [44,45] | |
| fucoxanthin | acetic acid [(1S,3R)-3-hydroxy-4-[(3E,5E,7E,9E,11E,13E,15E)-18-[(1S,4S,6R)-4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl]-3,7,12,16-tetramethyl-17-oxooctadeca-1,3,5,7,9,11,13,15-octaenylidene]-3,5,5-trimethylcyclohexyl] ester | 1.65%DW | P. tricornutum | diadinoxanthin, eaxanthin, neoxanthin, violaxanthin, β-carotene | occur mainly as all-trans but also as cis isomers | [46,47,48] |
 | 1.8% DW | Isochrysis aff. galbana | [49] | |||
| 0.52% DW | Cylindrotheca closterium | [50] | ||||
| up to 2.2% DW | Odontella aurita | diadinoxanthin, β-carotene | [51] | |||
| zeaxanthin | β,β-carotene-4,4′-diol | 97.4% TC | P. cruentum | β-carotene | [52] | |
 | ||||||
| echinenone (extracelular) | β,β-carotene-4-one | 0.17% DW | B. braunii | botryoxanthins A and B—0.03% DW braunixanthins 1 and 2—0.06% DW | extracellular pigments are produced and secreted into the intercellular matrix | [53] |
 | ||||||
| Lutein (intracelular) | β,ε-carotene-3,3′-diol | up to 0.16% DW | neox/loroxanthin 0.042% DW α- and β-carotene 0.031% DW violaxanthin 0.02% DW | |||
| violaxanthin | (1S,4S,6R)-1-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4S,6R)-4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,2,6-trimethyl-7-oxabicyclo[4.1.0] heptan-4-ol | C. ellipsodea | antheraxanthin, zeaxanthin | [45] | ||
 | ||||||

3. Oxidative Stress—The Importance of Reactive Species in Aging and Age-Related Diseases

| Groups of Reactive Species | Examples |
|---|---|
| ROS | O2•−, H2O2, HO•, 1O2, O3 |
| RNS | NO•, ONOO•, NO2− |
| Lipid hydroperoxides | LO•, LOO• |
| RCS | HOCl, HOBr |
| Glycoxidative species | AGE, ALE |
| Others: | Carbonyl radicals, RSS, GS• |
| Biomarkers | Remarks | References |
|---|---|---|
| MDA | reactive aldehyde; lipid peroxidation of ω-3 and ω-6 FA | [81] |
| 4-HNE | reactive aldehyde; lipid peroxidation of ω-3 and ω-6 FA | [81] |
| acrolein | reactive carbonyl | [5] |
| LOOH | lipid hydroperoxides | [82] |
| Glycoxidation products: | carbonyl-derived from protein changes | [8,76,83] |
| pentosidine (AGE) | marker for diabetes-associated complications | |
| Nε-(carboxymethyl)lysine (AGE) | (CML); marker for diabetes-associated complications | |
| Nε-(carboxymethyl)hydroxylysine | (CMhL) (AGE) | |
| o-, m-, and dityrosine | ||
| fructoselysine | marker for diabetes | |
| fructosehydroxylysine | ||
| ketoimine and ketoamine adducts | to protein | |
| ALE | advanced lipoxidation end products | |
| Dopaminergic markers: tyrosine hydrolase dopamine transporter | [84,85] | |
| Cell apoptosis markers (hallmarkers): DNA fragmentation condensation of cell nuclei | [86] | |
| PARP breaking | poly ADP ribose polymerase | |
| Markers for PD’s substantia nigra: 8-OHdG MDA LOOH protein carbonyls | [74] | |
| Markers for oxidative stress in tissues: 8-OHdG* 8-nitroguanine* 4-HNE | [22,73,74,76,78,79,87,88,89,90,91] | |
| GSH | Glutathione | |
| isoprostanes (e.g., IPF2α-I) | useful for atherosclerosis plaques; markers for lipid peroxidation in vivo | |
| LOOH & thiobarbituric acid reactive species (TBARS) | ||
| enediol radical anion | marker for diabetic patients; results from autoxidation of glucose |
4. Carotenoids from Marine Microalgae against Oxidative Stress
4.1. Uptake and Bioavailability
4.2. Antioxidant Protection
| Carotenoid | AO activity | Reactive Species | References |
|---|---|---|---|
| astaxanthin | 1O2 quencher, | 1O2, | [6,86,119,120,121,122,123] |
| radical scavenging, | O2•−, H2O2, HO•, | ||
| ROS and RNS quencher, | NO, LOOH, ONOO−, HOCl | ||
| chain-breaking AO, lipid peroxidation inhibitor, inhibits hallmarkers | |||
| β-carotene | 1O2 quencher; | 1O2, | [6,124,125,126] |
| radical scavenger; | NO2, ONOOH and ONOO− | ||
| inhibits Na+K+-ATPase, stimulates catalase and GS transferase | |||
| canthaxanthin | ROS and RNS quencher; chain-breaking AO | 1O2 | [119,121] |
| fucoxanthin | 1O2 quencher, | 1O2, | [6,103,124,127,128,129,130,131,132,133] |
| radical scavenger; | O2•−, HO•,ONOO−, HOCl, | ||
| inhibits Na+K+-ATPase, stimulates catalase and glutathione transferase | DPPH•, 12-DS•, NB•-L, AAPH, ABTS, ABAP, AIBN |
4.3. Role of Carotenoids against Reactive Species and Diseases
5. Why Is There Limited Success of Carotenoids as Anti-Oxidant Agents in Studies/Clinical Trials?
6. Conclusions/Final Remarks
Acknowledgments
Conflicts of Interest
References
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflam. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Gul, M.Z.; Singh, R.; Ankati, S. Free radicals: Implications in etiology of chronic diseases and their amelioration through nutraceuticals. Pharmacologia 2015, 6, 11–20. [Google Scholar]
- Kirkham, P. Oxidative stress and macrophage function: A failure to resolve the inflammatory response. Biochem. Soc. Trans. 2007, 35, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Marintti, L.R.B.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Arnlöv, J.; Larsson, A.; Basu, S. Low dietary intake of β-carotene, α-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Br. J. Nutr. 2009, 101, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tanq, G.; Sparrow, J.R.; Gierhart, D.; Shanq, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscler. Rep. 2009, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L. Anti-inflammatory agents and antioxidants as a possible “third great wave” in cardiovascular secondary prevention. Am. J. Cardiol. 2008, 101, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Hazen, S.L.; Lockwood, S.F. Seven day oral supplementation with Cardax (disodium disuccinate astaxanthin) provides significant cardioprotection and reduces oxidative stress in rats. Mol. Cell. Biochem. 2006, 283, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Pesce, M.; Patruno, A.; Fransceschelli, S.; de Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [PubMed]
- Lavy, A.; Naveh, Y.; Coleman, R.; Mokady, S.; Werman, M.J. Dietary Dunaliella bardawil, a β-carotene-rich alga, protects against acetic acid-induced small bowel inflammation in rats. Inflamm. Bowel Dis. 2003, 9, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Chidambara-Murthy, K.N.; Vanitha, A.; Rajesha, J.; Mahadeva-Swamy, M.; Sowmya, P.R.; Ravishankar, G.A. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Fucoxanthin restrains oxidstive stress induced by retinol deficiency through modulation of Na+K+-ATPase and antioxidant enzyme activities in rats. Eur. J. Nutr. 2008, 47, 432–441. [Google Scholar]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Gesaghy, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs. placebo on arterial stiffness oxidative stress and inflammation in renal transplant patients (Xanthin): A randomized controlled trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 5, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, Z.; Tracton, I.; Taunton, K.; Mathews, M. Effectiveness of whole dried Dunaliella salina marine microalgae in the chelating and detoxification of toxic minerals and heavy metals. Available online: http://www.interclinical.com.au/publications/archived%20publications/DetoxPaper100308.pdf (assessed on 5 August 2014).
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic live disease and normal liver fat. Diabetes Obes. Metable. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Bi, Y.-H.; Zhou, Z.-G. Microalgae as the production platform for carotenoids. In Recent Advances in Microalgal Biotechnology; Liu, J., Sun, Z., Gerken, H., Eds.; Omics Group eBooks: Foster City, CA, USA, 2014; pp. 1–17. [Google Scholar]
- Takaichi, S. Carotenoids in algae: Distribution, biosynthesis and function. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Abdoul-Enein, A.M.; El-Baroty, G.-S.; Youssef, A.M.; El-Baky, H.H.A. Accumulation of antioxidant vitamins in Dunaliella salina. J. Biol. Sci. 2002, 2, 220–223. [Google Scholar]
- Bar, E.; Rise, M.; Vishkautsan, M.; Arad, S. Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J. Plant Physiol. 1995, 146, 527–534. [Google Scholar] [CrossRef]
- Patel, A.; Mishr, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Express. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.S.; Cintra, R.G.; Barros, S.B.; Mancini-Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, H.H.A.; El-Baz, F.K.; El-Baroty, G.-S. Spirulina species as a source of carotenoids and α-tocopherol and its anticarcinoma factors. Biotechnology 2003, 2, 222–240. [Google Scholar]
- Jaime, L.; Mendiola, J.A.; Herrero, M.; Soler-Rivas, C.; Santoyo, S.; Señorans, F.J.; Cifuentes, A.; Ibáñez, E. Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 2005, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, J.; Linden, H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydrolase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol. 2001, 125, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Cerón, M.C.; García-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernandez, J.M.; del Rio, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, S.; Shi, X. Supercritical fluid extraction and determination of lutein in heterotrophically cultivated Chlorella pyrenoidosa. J. Food Proc. Eng. 2007, 30, 174–185. [Google Scholar] [CrossRef]
- Mendes, R.L.; Fernandes, H.L.; Coelho, J.P.; Reis, E.C.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem. 1995, 53, 99–103. [Google Scholar]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; d’Alcalá, M.R. Circadian variability in the photobiology of Phaeodactylum tricornutum: Pigment content. J. Plankton Res. 2007, 29, 141–156. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, S.-W.; Kwon, O.N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Rijstenbil, J.W. Effects of UVB radiation and salt stress on growth, pigments and oxidative defence of the marine diatom Cylindrotheca closterium. Mar. Ecol. Prog. Ser. 2003, 254, 37–48. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Tonegawa, I.; Okada, S.; Murakami, M.; Yamagushi, K. Pigment composition of the green microalga Botryococcus braunii Kawagushi-1. Fish. Sci. 1998, 64, 305–308. [Google Scholar]
- Lichtenthaler, T.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.; Schwender, J.; Müller, C.; Lichtenthaler, H.K.; Rohmer, M. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC 6714. Biochem. J. 1998, 333, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Miziorko, H.M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, K.; Eckert, M.; Hirschberg, J.; Hagen, C. Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol. 2000, 122, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, J.; Linden, H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: Regulation of photosynthetic redox control. Plant Mol. Biol. 2003, 52, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Pogson, B.; Sun, Z.; McDonald, K.A.; Della Penna, D.; Gantt, E. Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 1996, 8, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M. Carotenoid biosynthesis in diatoms. Photosynth. Res. 2010, 106, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Rettori, D.; Augusto, O.; Medeiros, M.H.G.; Di Mascio, P. linoleic hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen. Proc. Natl. Acad. Sci. USA 2006, 103, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Ronsein, G.E.; Prado, F.M.; Uemi, M.; Corrêa, T.C.; Toma, I.N.; Bertolucci, A.; Oliveira, M.C.B.; Motta, F.D.; Medeiros, M.H.G.; et al. Biological hydroperoxides and singlet molecular oxygen generation. IUBMB Life 2007, 59, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Wink, D.A.; Kasprak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.W.; Allen, J.S.; et al. DNA deaminating activity and genotoxicity of nitric oxide and its progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochem. Pol. 2013, 60, 1–16. [Google Scholar]
- Kanner, J.; German, J.B.; Kinsella, J.E. Initiation of lipid peroxidation in biological systems. Crit. Rev. Food Sci. Nutr. 1987, 25, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Guglielmi, M.D.; Pierdomenico, S.D.; Costantini, F.; Cipollone, F.; De Cesare, D.; Bucciarelli, T.; Ucchino, S.; Chiarelli, F.; Cuccurullo, F.; et al. Increased systemic oxidative stress after elective endarterectomy. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, I.; Hagihara, M.; Tsunekawa, H.; Maseki, M.; Yagi, K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem. Med. 1981, 25, 373–378. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Cipollone, F.; Cuccurullo, F. Oxidative stress and cardiovascular complications in diabetes: Isoprostanes as new markers on an old paradigm. Cardiovasc. Res. 2000, 47, 475–488. [Google Scholar] [CrossRef]
- Praticò, D.; FitzGerald, G.A. Generation of 8-epi prostaglandin F2α by human monocytes: Discriminate production by reactive oxygen species and prostaglandin endoperoxide synthase-2. J. Biol. Chem. 1996, 271, 8919–8924. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification: Their potential role of autoxidative glycosylation in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; McCarthy, S.; Betteridge, D.J.; Wolff, S.P. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes 1995, 44, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Poljak, K.B.; Milisav, I. Aging, oxidative stress and antioxidants. InTech 2013, 14, 331–353. [Google Scholar]
- Giera, M.; Lingeman, H.; Niessen, W.M.A. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): A brief overview. Chromatographia 2012, 75, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, S.; García, S.; Maldonado, M.; Machado, A.; Ayala, A. Do the serum oxidative stress biomarkers provide a reasonable index of the general oxidative stress status? Biochim. Biophys. Acta 2004, 1674, 254–259. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Dyer, D.G.; Dunn, J.A.; Bailie, K.E.; Thorpe, S.R.; Baynes, J.W.; Lyons, T.J. Maillard reaction products and their relation to the complications of diabetes. J. Clin. Invest. 1993, 91, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Yen, J.C.; Yin, P.H.; Chi, C.W.; Lee, H.C. Involvement of oxidative stress-activated JNK signalling in the methamphetamine-induced cell death of human SH-SY5Y cells. Toxicology 2008, 246, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Matsuzaki, M.; Takeda, A.; Kikuchi, A.; Furukawa, K.; Shibahara, S.; Itoyama, Y. Increased dopamine and its metabolites in SH-SY5Y neuroblastoma cells that express tyrosinase. J. Neurochem. 2003, 87, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Sakama, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokumi, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid. Redox Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010, 30, 2721–2725. [Google Scholar] [PubMed]
- Praticò, D.; Iuliano, L.; Spagnoli, L.; Mauriello, A.; Lawson, J.A.; Rokach, J.; Maclouf, J.; Violi, F.; FitzGerald, G.A. Localization of distinct F2 isoprostanes in human atherosclerotic lesions. J. Clin. Invest. 1997, 100, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Barry, O.P.; Lawson, J.A.; Adiyaman, M.; Hwang, S.W.; Khanapure, S.P.; Iuliano, L.; Rokach, J.; FitzGerald, G.A. IPF2 α-I: An index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA 1998, 95, 3449–3454. [Google Scholar] [CrossRef] [PubMed]
- Guiwotta, C.; Morrow, J.D.; Roberts, L.J.I.I.; Kuhn, H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3236–3241. [Google Scholar]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [PubMed]
- Olson, J.A. Absorption, transport, and metabolism of carotenoids in humans. Pure Appl. Chem. 1994, 66, 1011–1106. [Google Scholar] [CrossRef]
- Parker, R.S. Absorption, metabolism, and of transport carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [PubMed]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Elliot, R. Mechanisms of genomic and non-genomic actions of carotenoids. Biochim. Biophys. Acta 2005, 1740, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Paetau, I.; Chen, H.; Goh, N.M.-Y.; White, W.S. Interactions of the postprandial appearance of β-carotene and canthaxanthin in plasma triacylglycerol-rich lipoproteins in humans. Am. J. Clin. Nutr. 1997, 66, 1133–1143. [Google Scholar] [PubMed]
- Stahl, W.; Schwarz, W.; van Laar, J.; Sies, H. All-trans-β-carotene preferentially accumulates in human chylomicron and very low density lipoproteins compared with 9-cis geometrical isomer. J. Nutr. 1995, 125, 2128–2133. [Google Scholar] [PubMed]
- Krinsky, N.I.; Cornwell, D.G.; Oncley, J.L. The transport of vitamin A and carotenoids in human plasma. Arch. Biochem. Biophys. 1958, 73, 233–246. [Google Scholar] [CrossRef]
- Goulinet, S.; Chapman, M.J. Plasma LDL and HDL subspecies are heterogeneous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Arterioscl. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Østerlie, M.; Bjerkeng, B.; Liaan-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Gärtner, C.; Stahl, W.; Sies, H. Preferential increase in chylomicron levels of xanthophylls lutein and zeaxanthin compared to β-carotene in the human. Int. J. Vitamin Nutr. Res. 1996, 66, 119–125. [Google Scholar]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.M.; Lignell, A.; Petterson, A.; Hoglung, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Manabe, E.; Handa, O.; Naito, Y.; Mizushima, K.; Akagiri, S.; Adachi, S.; Takagi, T.; Kokura, S.; Maoka, T.; Yoshikawa, T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2008, 103, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Kobayashi, M.; Terasaki, M.; Nagao, A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 2010, 140, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Herstad, O.; Liaaen-Jensen, S. Fucoxanthin metabolites in egg yolks of laying hens. Comp. Biochem. Phys. A Mol. Integr. Physiol. 1998, 119, 963–974. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and Hep G2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Tsushima, M.; Matsuno, T.; Tanaka, Y.; Okuzumi, J.; Murakoshi, M.; Satomi, Y.; Takatasu, Y.; Tokuda, H.; Nishino, A. Anti-neoplastic effect of halocynthiaxanthin, a metabolite of fucoxanthin. Anticancer Drugs 1992, 3, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ren, R.D.; Hashimoto, T.; Kanazawa, K. Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J. Agric. Food Chem. 2010, 58, 6090–6095. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass—A repeated dose study. Food Res. Int. 2013, 54, 711–717. [Google Scholar]
- Ben-Amotz, A.; Levy, Y. Bioavailability of natural isomers mixture compared with synthetic all-trans β-carotene in human serum. Am. J. Clin. Nutr. 1996, 63, 729–734. [Google Scholar] [PubMed]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Coral-Hinostroza, G.; Ytrestoyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acids diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.J.; Morais, A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 297, 291–295. [Google Scholar] [CrossRef]
- Krinsky, N.I. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989, 7, 617–635. [Google Scholar] [CrossRef]
- Khan, S.K.; Malinski, T.; Mason, R.P.; Kubant, R.; Jacob, R.F.; Fujioka, K.; Denstadet, S.J.; King, T.J.; Jackson, H.L.; Hieber, A.D.; et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb. Res. 2010, 126, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, anti-carcinogenesis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Ingold, K.U. β-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Hiramoto, K.; Tomiyama, S.; Assano, Y. β-Carotene effectively scavenges toxic nitrogen dioxide and peroxynitrous acid. FEBS Lett. 1997, 404, 175–178. [Google Scholar] [CrossRef]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Mármol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus. vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Chin, Y.T.; Hu, M.-L. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL.2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J. Agric. Food Chem. 2011, 59, 11344–11351. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Etoh, H.; Kato, K.; Nakatugawa, H.; Kato, H.; Maejima, Y.; Matsumoto, G.; Mori, H.; Hosokawa, M.; Miyashita, K.; et al. Nitrocapsanthin and nitrofucoxanthin, respective products of capsanthin and fucoxanthin reaction with peroxynitrite. J. Agric. Food Chem. 2011, 59, 10572–10578. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hizikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M. Risk factors associated with chronic obstructive lung disease. Ann. N. Y. Acad. Sci. 1991, 624, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Hirano, A.; Kunito, S.; Kawakami, Y. Fucoxanthin, an antioxidative substance from marine diatom Phaeodactylum tricornutum. J. Mar. Biotechnol. 1995, 3, 132–135. [Google Scholar]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical inplications and therapeutic possibilities. Diabetes Care 2008, 31, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kim, O.K.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leucocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar] [PubMed]
- Scheidegger, R.; Pande, A.K.; Bounds, P.L.; Koppenol, W.H. The reaction of peroxynitrite with zeaxanthin. Nitric Oxide 1998, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. Function of marine carotenoids. Forum Nutr. 2009, 61, 136–146. [Google Scholar] [PubMed]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, J. Man versus beast: Pharmacokinetic scalling in mammals. J. Pharm. Sci. 1986, 75, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo Sci. 2007, 56, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Shokuhin Eiseigaku Zasshi 2011, 52, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 2010, 5, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hosokawa, M.; Matsukawa, N.; Hagio, M.; Shinoki, A.; Nishimukai, M.; Miyashita, K.; Yajima, T.; Hara, H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr. 2010, 49, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Gemello, E.; Riccioni, G.; D’Orazio, N. Marine bioactives and potential application in sports. Mar. Drugs 2014, 12, 2357–2382. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; de Girolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Tso, M.O.M.; Lam, T.T. Method of Retarding and Ameliorating Central Nervous System and Eye Damage. U.S. Patent 5527533, 18 June 1996. [Google Scholar]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Tomasoni, C.; Jacquot, C.; Kaas, R.; Le Guedes, R.; Cadoret, J.P.; Müller-Feuga, A.; Kontiza, I.; Vagias, C.; Roussis, V.; et al. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ. Toxicol. Pharmacol. 2006, 22, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [PubMed]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsodea in RAW 264.7 macrophages. Biol. Pharmacol. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef]
- Eichler, O.; Sies, H.; Stahl, W. Divergent optimum levels of lycopene, β-carotene and lutein protecting against UVB irradiation in human fibroblast. Photochem. Photobiol. 2002, 75, 503–506. [Google Scholar] [CrossRef]
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; Ranelletti, F.O.; Bartoli, G.M. β-Carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic. Biol. Med. 2001, 30, 1000–1007. [Google Scholar] [CrossRef]
- Lowe, G.M.; Booth, L.A.; Young, A.J.; Bilton, R.F. Lycopene and β-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic. Res. 1999, 30, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Le Tutour, B.; Benslimane, F.; Gouleau, M.P.; Gouygou, J.P.; Saadan, B.; Quemeneur, F. Antioxidant and prooxidant activities of the brown algae, Laminaria digitata, Himanthalia elongata, Fucus vesiculosus, Fucus serratus and Ascophyllum nodosum. J. Appl. Phycol. 1998, 10, 121–129. [Google Scholar] [CrossRef]
- Li, T.L.; King, J.M.; Min, D.B. Quenching mechanisms and kinetics of carotenoids in riboflavin photosensitized singlet oxygen oxidation of vitamin D2. J. Food Biochem. 2000, 24, 477–492. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Jacob, R.F.; Mason, R.P. Biologic activity of carotenoids related to distinct membrane physicochemical interactions. Am. J. Cardiol. 2008, 101, 20–29. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raposo, M.F.d.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128-5155. https://doi.org/10.3390/md13085128
Raposo MFdJ, De Morais AMMB, De Morais RMSC. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Marine Drugs. 2015; 13(8):5128-5155. https://doi.org/10.3390/md13085128
Chicago/Turabian StyleRaposo, Maria Filomena de Jesus, Alcina Maria Miranda Bernardo De Morais, and Rui Manuel Santos Costa De Morais. 2015. "Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases" Marine Drugs 13, no. 8: 5128-5155. https://doi.org/10.3390/md13085128
APA StyleRaposo, M. F. d. J., De Morais, A. M. M. B., & De Morais, R. M. S. C. (2015). Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Marine Drugs, 13(8), 5128-5155. https://doi.org/10.3390/md13085128