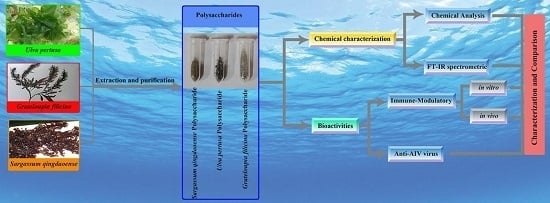
Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides
Abstract
:1. Introduction
| Author | Phylum | Species | Bioactivities |
|---|---|---|---|
| Qi, et al., 2005 [14] | Chlorophyta | Ulva pertusa | Antioxidant activity |
| Zhang et al., 2010 [2] | Ulva pertusa | Antioxidant activity | |
| Enteromorpha linza | |||
| Bryopsis plumose | |||
| Cho et al., 2010 [15] | Enteromorpha prolifera | Antitumor and immunomodulating activities | |
| Jiao et al., 2010 [16] | Enteromorpha intestinalis | Antitumor and immunomodulating activities | |
| Tabarsa et al., 2012 [17] | Ulva pertusa | Immunomodulatory, anticancer activities | |
| Zhang et al., 2013 [18] | Enteromorpha linza | Immunological and antioxidant activities | |
| Aguilar-Briseño et al., 2015 [19] | Ulva clathrata | Antiviral activity | |
| Zhang et al., 2010 [2] | Ochrophyta | Laminaria japonica | Antioxidant activity |
| Ye et al., 2008 [20] | Sargassum pallidum | Antitumor and antioxidant activities | |
| Wang et al., 2011 [21] | Laminaria japonica | Anticoagulant activity | |
| Li et al., 2012 [22] | Sargassum pallidum | Immune responses | |
| Dore et al., 2013 [10] | Sargassum vulgare | Anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects | |
| Suresh et al., 2013 [23] | Sargassum plagiophyllum | Anticancer and antioxidant activities | |
| Imbs et al., 2014 [24] | Fucus evanescens | Antioxidant activity | |
| Hwang et al., 2015 [25] | Sargassum hemiphyllum | Anti-inflammatory | |
| Wen et al., 2014 [26] | Sargassum horneri | Antioxidant activity | |
| Shao et al., 2014 [27] | Sargassum horneri | Antioxidant and antitumor activities | |
| Shobharani et al., 2014 [28] | Sargassum sp. | Antioxidant and anticoagulant activities | |
| Aguilar-Briseño et al., 2015 [19] | Cladosiphon okamuranus | Antiviral activity | |
| Zhang et al., 2014 [29] | Ascophyllum nodosum | Induces Th1 and Tc1 Immune Responses | |
| Yuan et al., 2015 [30] | Ascophyllum nodosum | Antioxidant activity | |
| Ammar et al., 2015 [31] | Cystoseira sedoides, | Anti-radical, anti-inflammatory and gastroprotective activities | |
| Cystoseira compressa, | |||
| Cystoseira crinita | |||
| Shao et al., 2015 [32] | Sargassum horneri | Antioxidant and moisture-preserving activities | |
| Athukorala et al., 2005 [33] | Rhodophyta | Grateloupia filicina | Antioxidant activity, protecting ability for H2O2-induced DNA damage |
| Wang et al., 2007 [34] | Grateloupia longifolia | Anti-virus activity | |
| Grateloupia filicina | |||
| Zhang et al., 2010 [2] | Porphyra haitanensis | Antioxidant activity | |
| Yu et al., 2012 [35] | Eucheuma denticulatum | Anti-virus activity | |
| Shi et al., 2014 [36] | Porphyra haitanensis | Anti-allergic activity | |
| Chen et al., 2015 [37] | Grateloupia filicina | Anticoagulant activity | |
| Fleita et al., 2015 [38] | Pterocladia capillacea | Antioxidant activity |
2. Results
2.1. Chemical Characterization of Three Sulfated Polysaccharides
2.1.1. Chemical Analysis
| Sample | Yield (%) | Total Sugar (%) | Sulfate (%) | Monosaccharides Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | Glc A | Glc | Gal | Xyl | Fuc | ||||
| UPP | 12.1 | 53.13 | 13.54 | 0.06 | 1 | 0.53 | 0.19 | 0.09 | 0.39 | 0.02 |
| GFP | 19.7 | 40.9 | 19.89 | 0.01 | - | 0.02 | 0.07 | 1 | 0.1 | 0.05 |
| SQP | 7.2 | 20.81 | 5.64 | 0.56 | - | 0.13 | 0.37 | 0.6 | - | 1 |
2.1.2. FT-IR Spectrometric Characterization

2.2. Cytotoxic Activity of the Polysaccharides
2.3. Immunologic Modulation of Three Sulfated Polysaccharides in Vitro

2.4. Immune-Modulation of Three Sulfated Polysaccharides in Vivo
2.4.1. H9N2-Specific Antibody Titer
2.4.2. Effect on Cytokine Production Stimulation


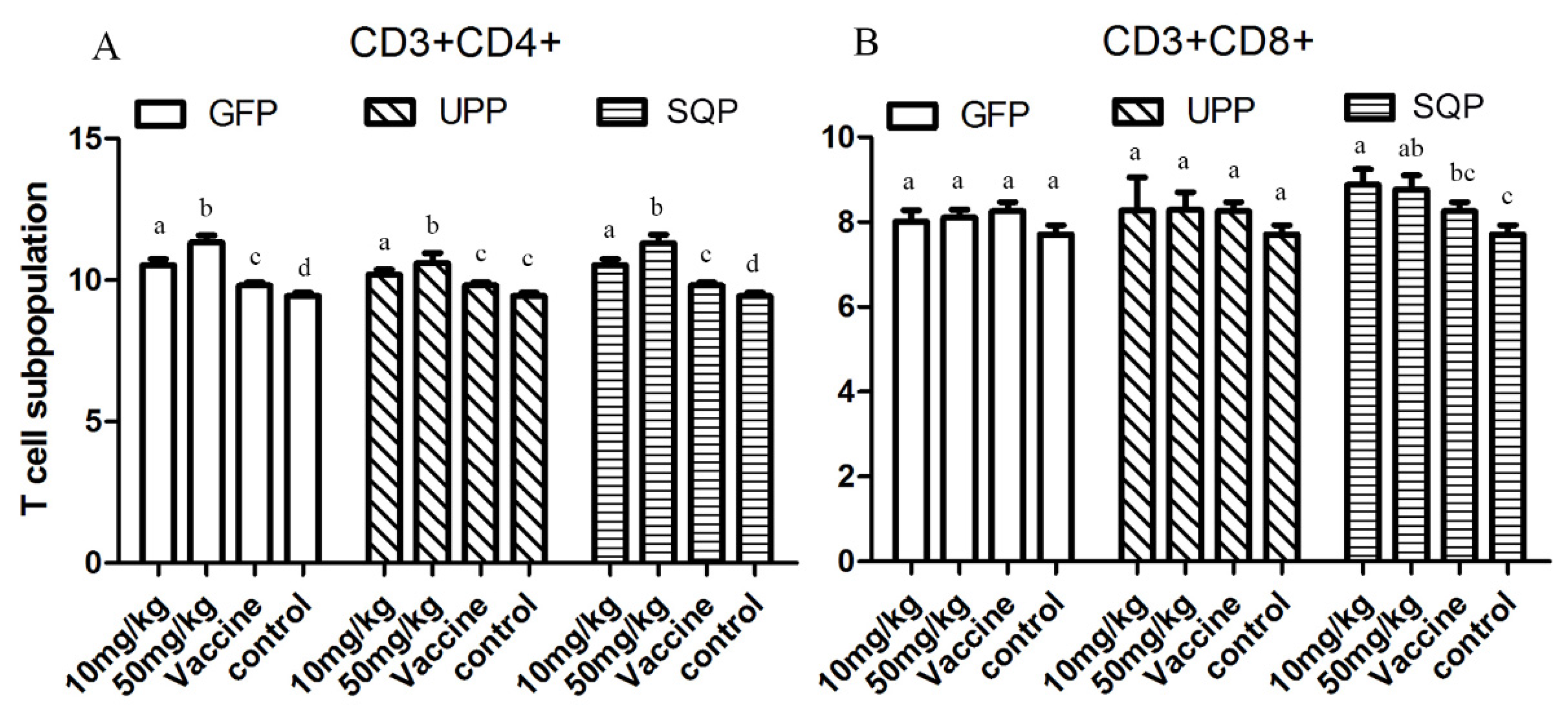
2.4.3. T-Cell Subpopulation
2.5. Anti-H9N2 Effect of Three Sulfated Polysaccharides in Vitro

3. Discussion
4. Materials and Methods
4.1. Algal Samples
4.2. Extraction of Water-Soluble Sulfated Algal Polysaccharide
4.3. Chemical Characterization
4.4. Animals and Maintenance
4.5. Cell Lines, Virus, and Tissue Culture
4.6. Cytotoxic Activity Evaluation
4.7. Immuno-Modulatory Effect
4.7.1. Mouse Splenic Lymphocyte Proliferation Assay
4.7.2. Animals Grouping and Treatment
4.7.3 AIV-Specific Antibody Titer Detection
4.7.4. Cytokines Production
4.7.5. T-Cell Subpopulation and Flow Cytometry
4.8. Anti-AIV Effect in Vitro
4.8.1. Virus Titers Assay
4.8.2. Relative Expression of Viruses
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Conc (mg/mL) | 10 | 5 | 2.5 | 1.25 | 0.625 | 0.3125 | 0.156 | 0.078 |
|---|---|---|---|---|---|---|---|---|
| UPP | 0.82 | 1.03 | 1.16 | 1.09 | 1.05 | 1.08 | 1.06 | 1.02 |
| GFP | 0.74 | 0.81 | 0.89 | 0.9 | 0.95 | 0.92 | 1.01 | 1.13 |
| SQP | 0.78 | 0.96 | 1.01 | 1 | 0.97 | 1.05 | 0.99 | 1.03 |
References
- Bohn, J.A.; Bemiller, J.N. (1→3)-β-d-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the Polysaccharides from Five Algae and Their Potential Antioxidant Activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Nikapitiya, C.; De Zoysa, Mahanama; Jeon, Y.-J.; Lee, J.; Jee, Y.H. Isolation of Sulfated Anticoagulant Compound from Fermented Red Seaweed Grateloupia Filicina. J. World Aquac. Soc. 2007, 38, 407–417. [Google Scholar] [CrossRef]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spanò, A.; Morabito, M.; Maugeri, T.L. In vitro Evaluation of Antibacterial Activity of Asparagopsis Taxiformis from the Straits of Messina Against Pathogens Relevant in Aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Saari, F.A.N. High-value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Fucanomics and Galactanomics: Marine Distribution, Medicinal Impact, Conceptions, and Challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Morabito, M.; Minicante, S.A.; Piano, G.L.; Pagano, M.; Genovese, G. Potential Use of Polysaccharides from the Brown Alga Undaria Pinnatifida as Anticoagulants. Braz. Arch. Biol. Technol. 2015, 58. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; Lin, C. Chemical Characteristic of an Anticoagulant-Active Sulfated Polysaccharide from Enteromorpha Clathrata. Carbohydr. Polym. 2012, 90, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Dore, C.M.; das, C.F.A.M.G.; Will, L.S.; Costa, T.G.; Sabry, D.A.; de Souza Rego, L.A.; Accardo, C.M.; Rocha, H.A.; Filgueira, L.G.; Leite, E.L. A Sulfated Polysaccharide, Fucans, Isolated from Brown Algae Sargassum Vulgare with Anticoagulant, Antithrombotic, Antioxidant and Anti-Inflammatory Effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kang, S.H.; Lee, H.J.; You, A.K.; Youn, H.J.; Lee, B.J.; Chung, H. In vitro Screening of Seaweed Extract on the Proliferation of Mouse Spleen and Thymus Cell. Biotechnol. Bioprocess Eng. 2006, 11, 160–163. [Google Scholar] [CrossRef]
- Ping, S.; Jia, L.; Chen, X.; Fang, Z.; Sun, P. Structural Features and Antitumor Activity of a Purified Polysaccharide Extracted from Sargassum Horneri. Int. J. Biol. Macromol. 2015, 73, 124–130. [Google Scholar]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal Sulfated Carrageenan Inhibits Proliferation of Mda-Mb-231 Cells via Apoptosis Regulatory Genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Rong, C.; Hong, Z.; Niu, X.; Li, Z. Antioxidant Activity of Different Sulfate Content Derivatives of Polysaccharide Extracted from Ulva Pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.L.; Yang, C.; Sang, M.K.; You, S.G. Molecular Characterization and Biological Activities of Water-Soluble Sulfated Polysaccharides from Enteromorpha Prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Jiao, L.; Xia, L.; Li, T.; Peng, J.; Zhang, L.; Wu, M.; Zhang, L. Characterization and Anti-Tumor Activity of Alkali-Extracted Polysaccharide from Enteromorpha Intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular Characteristics and Immunomodulatory Activities of Water-Soluble Sulfated Polysaccharides from Ulva Pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhao, M.; Yu, S.; Qi, H. The Immunological and Antioxidant Activities of Polysaccharides Extracted from Enteromorpha Linza. Int. J. Biol. Macromol. 2013, 57, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.-F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva Clathrata and Cladosiphon Okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, Antitumor and Antioxidant Activities in vitro of Polysaccharides from the Brown Seaweed Sargassum Pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Hou, Y.; Zhang, H. In-vitro Anticoagulant Activity of Fucoidan Derivatives from Brown Seaweed Laminaria Japonica. Chin. J. Oceanol. Limn. 2011, 29, 679–685. [Google Scholar] [CrossRef]
- Li, L.J.; Li, M.Y.; Li, Y.T.; Feng, J.J.; Hao, F.Q.; Lun, Z. Adjuvant Activity of Sargassum Pallidum Polysaccharides Against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines. Mar. Drugs 2012, 10, 2648–2660. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, Purification and Preliminary Characterization of Sulfated Polysaccharides from Sargassum Plagiophyllum and its in vitro Anticancer and Antioxidant Activity. Process Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant Activity of Fucose-Containing Sulfated Polysaccharides Obtained from Fucus Evanescens by Different Extraction Methods. J. Appl. Phycol. 2014, 27, 1–9. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Chien, S.Y. Inhibitory Activity of Sargassum Hemiphyllum Sulfated Polysaccharide in Arachidonic Acid-Induced Animal Models of Inflammation. J. Food Drug Anal. 2015, 23, 49–56. [Google Scholar] [CrossRef]
- Wen, Z.S.; Liu, L.J.; Ouyang, X.K.; Qu, Y.L.; Yin, C.; Ding, G.F. Protective Effect of Polysaccharides from Sargassum Horneri Against Oxidative Stress in Raw264.7 Cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ping, S.; Chen, X.; Sun, P. Chemical Characterization, Antioxidant and Antitumor Activity of Sulfated Polysaccharide from Sargassum Horneri. Carbohyd. Polym. 2014, 105, 260–269. [Google Scholar]
- Shobharani, P.; Nanishankar, V.H.; Halami, P.M.; Sachindra, N.M. Antioxidant and Anticoagulant Activity of Polyphenol and Polysaccharides from Fermented Sargassum Sp. Int. J. Biol. Macromol. 2014, 65, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, J.Y.; Jiang, Z.D.; Okimura, T.; Oda, T.; Yu, Q.; Jin, J.-O. Ascophyllan Purified from Ascophyllum Nodosum Induces Th1 and Tc1 Immune Responses by Promoting Dendritic Cell Maturation. Mar. Drugs 2014, 12, 4148–4164. [Google Scholar]
- Yuan, Y.; Macquarrie, D. Microwave Assisted Extraction of Sulfated Polysaccharides (fucoidan) from Ascophyllum Nodosum and its Antioxidant Activity. Carbohyd. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.H.; Lajili, S.; Said, R.B.; Cerf, D.L.; Bouraoui, A.; Majdoub, H. Physico-Chemical Characterization and Pharmacological Evaluation of Sulfated Polysaccharides from Three Species of Mediterranean Brown Algae of the Genus Cystoseira. Daru J. Pharm. Sci. 2015, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Improvement of Antioxidant and Moisture-Preserving Activities of Sargassum Horneri Polysaccharide Enzymatic Hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Lee, K.W.; Park, E.J.; Heo, M.S.; Yeo, I.K.; Lee, Y.D.; Jeon, Y.J. Reduction of Lipid Peroxidation and H2O2-Mediated DNA Damage by a Red Alga (Grateloupia Filicina) Methanolic Extract. J. Sci. Food Agr. 2005, 85, 2341–2348. [Google Scholar] [CrossRef]
- Wang, S.C.; Bligh, S.W.; Shi, S.S.; Wang, Z.T.; Hu, Z.B.; Crowder, J.; Branford-White, C.; Vella, C. Structural Features and Anti-HIV-1 Activity of Novel Polysaccharides from Red Algae Grateloupia Longifolia and Grateloupia Filicina. Int. J. Biol. Macromol. 2007, 41, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, M.; Wang, W.; Liu, X.; Zhao, X.; Lv, Y.; Li, G.; Jiao, G.; Zhao, X. Structure and Anti-Influenza A (H1N1) Virus Activity of Three Polysaccharides from Eucheuma Denticulatum. J. Ocean U. China 2012, 11, 527–532. [Google Scholar] [CrossRef]
- Shi, C.; Pan, T.; Cao, M.J.; Liu, Q.M.; Zhang, L.J.; Liu, G.M. Suppression of Th2 Immune Responses by the Sulfated Polysaccharide from Porphyra Haitanensis in Tropomyosin-Sensitized Mice. Int. Immunopharmacol. 2015, 24, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, S.; Wang, J.; Song, L.; Xing, R.; Liu, S.; Yu, H.; Li, P. Sulfated Polysaccharides Isolated from Cloned Grateloupia Filicina and their Anticoagulant Activity. Biomed Res. Int. 2015, 2015, 1–5. [Google Scholar]
- Fleita, D.; El-Sayed, M.; Rifaat, D. Evaluation of the Antioxidant Activity of Enzymatically-Hydrolyzed Sulfated Polysaccharides Extracted from Red Algae; Pterocladia Capillacea. LWT—Food Sci. Technol. 2015, 63, 1236–1244. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; You, S.G. Molecular Characteristics of Sulfated Polysaccharides from Monostroma Nitidum and their in vitro Anticancer and Immunomodulatory Activities. Int. J. Biol. Macromol. 2011, 48, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Kido, N.; Sugiyama, T.; Yokochi, T. Antiviral Activity of Acidic Polysaccharides from Coccomyxa Gloeobotrydiformi, a Green Alga, Against An in vitro Human Influenza A Virus Infection. Immunopharmacology Immunotoxicology 2012, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Lee, H.J.; Lee, D.H.; Lee, Y.J.; Mo, I.P.; Nahm, S.S.; Kim, M.J.; Lee, J.B.; Park, S.Y.; Choi, I.S. Immune Responses and Pathogenesis in Immunocompromised Chickens in Response to Infection with the H9N2 Low Pathogenic Avian Influenza Virus. Virus Res. 2008, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.K.; Lee, Y.N.; Song, J.M.; Kang, S.M.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. H9N2 Avian Influenza Virus-Like Particle Vaccine Provides Protective Immunity and a Strategy for the Differentiation of Infected from Vaccinated Animals. Vaccine 2011, 29, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, L.; Bao, L.; Yao, Y.; Deng, W.; Li, F.; Lv, Q.; Gu, S.; Wei, Q.; Qin, C. Characterization of an H9N2 Avian Influenza Virus from a Fringilla Montifringilla Brambling in Northern China. Virology 2015, 476, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kallon, S.; Li, X.; Ji, J.; Chen, C.; Xi, Q.; Shuang, C.; Xue, C.; Ma, J.; Xie, Q.; Zhang, Y. Astragalus Polysaccharide Enhances Immunity and Inhibits H9N2 Avian Influenza Virus in vitro and in vivo. J. Anim. Sci. Biotechnol. 2013, 4, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wei, B.; Yang, Y.; Yao, M.; Cai, Y.; Gao, Y.; Xia, X.; Zhao, X.; Liu, Z.; Li, X.; et al. Experimental Transmission in Guinea Pigs of H9N2 Avian Influenza Viruses from Indoor Air of Chicken Houses. Virus Res. 2012, 170, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Shaw, M.; Gregory, V.; Cameron, K.; Lim, W.; Klimov, A.; Subbarao, K.; Guan, Y.; Krauss, S.; Shortridge, K. Avian-to-human Transmission of H9N2 Subtype Influenza a Viruses: Relationship Between H9N2 and H5N1 Human Isolates. Proc. Natl. Acad. Sci. 2000, 97, 9654–9658. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, W.; Wei, R.; Zhao, H. Isolation and Identification of Swine Influenza Recombinant a/Swine/Shandong/1/2003(H9N2) Virus. Microbes Infect. 2004, 6, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sorrell, E.M.; Song, H.; Hossain, M.J.; Ramirez-Nieto, G.; Monne, I.; Stevens, J.; Cattoli, G.; Capua, I.; Chen, L.-M.; et al. Replication and transmission of H9N2 Influenza Viruses in Ferrets: Evaluation of Pandemic Potential. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Schrauwen, E.J.A.; de Graaf, M.; Bestebroer, T.M.; Spronken, M.I.J.; van Boheemen, S.; de Meulder, D.; Lexmond, P.; Linster, M.; Herfst, S. Limited Airborne Transmission of H7N9 Influenza a Virus Between Ferrets. Nature 2013, 501, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Dalby, A.R.; Iqbal, M. A Global Phylogenetic Analysis in Order to Determine the Host Species and Geography Dependent Features Present in the Evolution of Avian H9N2 Influenza Hemagglutinin. PeerJ 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.-Y.; Wang, J.; Shen, Y.Y.; Zhou, B.P.; Duan, L.; Cheung, C.-L.; Ma, C.; Lycett, S.J.; Leung, Y.H.; Chen, X.C.; et al. The Genesis and Source of the H7N9 Influenza Viruses Causing Human Infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.; Yuen, K.Y.; Leung, C.W.; Chan, K.H.; lp, P.L.; Lai, R.W.; Orr, W.K.; Shortridge, K.F. Human Infection with Influenza H9N2. Lancet 1999, 354, 916–917. [Google Scholar] [CrossRef]
- Westenius, V.; Mäkelä, S.M.; Ziegler, T.; Julkunen, I.; Osterlund, P. Efficient Replication and Strong Induction of Innate Immune Responses by H9N2 Avian Influenza Virus in Human Dendritic Cells. Virology 2014, 471, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Haslin, C.; Chermann, J.C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus Coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella Thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In vitro Inhibition of Influenza a Virus Infection by Marine Microalga-Derived Sulfated Polysaccharide P-Kg03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral Activity Against Dengue Virus of Diverse Classes of Algal Sulfated Polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.B.; Chen, X.E. Structure Elucidation and Immunological Activity of a Novel Pectic Polysaccharide from the Stems of Avicennia Marina. Eur. Food Res. Technol. 2013, 236, 243–248. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.; Li, P.; Li, X.; Chen, X.; Qiu, S.; Gao, Z.; et al. The Comparison of Antioxidative and Hepatoprotective Activities of Codonopsis Pilosula Polysaccharide (CP) and Sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Lee, S.J.; You, S. Structural Analysis of Immunostimulating Sulfated Polysaccharides from Ulva Pertusa. Carbohydr. Res. 2012, 361, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Wang, J.; Liu, Z.; Zhao, S. Antitumor Activity of a Sulfated Polysaccharide from Enteromorpha Intestinalis Targeted Against Hepatoma through Mitochondrial Pathway. Tumor Biol. 2013, 35, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yan, J.; Wang, S.; Ji, L.; Ding, K.; Vella, C.; Wang, Z.; Hu, Z. Antiangiogenic Effects of GFP08, an Agaran-Type Polysaccharide Isolated from Grateloupia Filicina. Glycobiology 2012, 22, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of Anticoagulant Activity of Two Algal Polysaccharides. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, M.; Sun, R.; Pan, L. Extraction, Characterization of a Ginseng Fruits Polysaccharide and its Immune Modulating Activities in Rats with Lewis Lung Carcinoma. Carbohydr. Polym. 2015, 127, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Turan, K.; Nagata, K.; Kuru, A. Antiviral effect of Sanicula Europaea L. Leaves Extract on Influenza Virus-Infected Cells. Biochem. Biophys. Res. Commun. 1996, 225, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Xue, M.Y.; Wang, C.; Wang, J.B.; Chen, P.Y. Bursopentine as a Novel Immunoadjuvant Enhances Both Humoral and Cell-Mediated Immune Responses to Inactivated H9N2 Avian Influenza Virus in Chickens. Clin. Vaccine Immunol. CVI 2011, 18, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhang, Q.B.; Wang, J.; Shi, X.L.; Zhang, Z.S. Analysis of the Monosaccharide Composition of Fucoidan by Precolumn Derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis Pyrifera has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Thelen, T.; Hao, Y.; Medeiros, A.I.; Curtis, J.L.; Serezani, C.H.; Kobzik, L.; Harris, L.H.; Aronoff, D.M. The Class A Scavenger Receptor, Macrophage Receptor with Collagenous Structure, is the Major Phagocytic Receptor for Clostridium Sordellii Expressed by Human Decidual Macrophages. J. Immunol. 2010, 185, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Zhang, W.; Du, J.-Y.; Wong, K.-W.; Oda, T.; Yu, Q. Fucoidan can Function as an Adjuvant in vivo to Enhance Dendritic Cell Maturation and Function and Promote Antigen-Specific T Cell Immune Responses. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- de Godoi, A. M.; Faccin-Galhardi, L.C.; Lopes, N.; Rechenchoski, D.Z.; de Almeida, R.R.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Antiviral Activity of Sulfated Polysaccharide of Adenanthera Pavonina Against Poliovirus in Hep-2 Cells. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, W.; Zeng, L.; Wang, D.; Liu, J.; Wu, Y.; Hu, Y. Comparison of Bush Sophora Root Polysaccharide and its Sulfate's Anti-Duck Hepatitis A Virus Activity and Mechanism. Carbohydr. Polym. 2014, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, M.; Wang, Y.; Xiong, W.; Zeng, L.; Zhang, S.; Xu, M.; Du, H.; Liu, J.; Wang, D.; et al. The Anti-DHAV Activities of Astragalus Polysaccharide and its Sulfate Compared with those of BSRPs and its Sulfate. Carbohydr. Polym. 2015, 117, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.F.; Liang, J.P.; Na, Z.Y.; Yang, H.J.; Lu, Y.; Hua, L.Y.; Guo, W.Z.; Cui, Y.; Wang, L. In vivo Inhibition of NAS Preparation on H9N2 Subtype Aiv. Virol. Sin. 2010, 25, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method For Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L.L. Characterization of a Heteropolysaccharide Isolated from Diploid Gynostemma Pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Seno, N.; Anno, K. A Modified Method for Chondrosulfatase Assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Cardozo, F.T.; Camelini, C.M.; Cordeiro, M.N.; Mascarello, A.; Malagoli, B.G.; Larsen, I.V.; Rossi, M.J.; Nunes, R.J.; Braga, F.C.; Brandt, C.R.; et al. Characterization and Cytotoxic Activity of Sulfated Derivatives of Polysaccharides from Agaricus Brasiliensis. Int. J. Biol. Macromol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Mao, X.; Pei, R.; Miao, S.; Xiang, C.; Lv, Y.; Yang, X.; Sun, J.; Jia, S.; Liu, Y. Antitumor Activity of Polysaccharides from Lepista Sordida Against Laryngocarcinoma in vitro and in vivo. Int. J. Biol. Macromol. 2013, 60, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Faggio, C. The Use of Erythrocyte Fragility to Assess Xenobiotic Cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.X.; Yang, W.J.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q.H. Purification, Characterization and Antitumor Activity of Polysaccharides from Pleurotus Eryngii Residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.W.; Lee, H.J.; Kim, Y.A.; Youn, H.J.; Lee, B.-J. Effects of Several Salt Marsh Plants on Mouse Spleen and Thymus Cell Proliferation Using MTT Assay. Ocean Sci. J. 2005, 40, 209–212. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, C.; Hu, Y.; Zhao, X.; Shi, C.; Yu, Y.; Liu, C.; Tao, Y.; Pan, H.; Feng, Y.; et al. Immunoenhancement Effect of Rehmannia Glutinosa Polysaccharide on Lymphocyte Proliferation and Dendritic Cell. Carbohydr. Polym. 2013, 96, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Su, X.; Wang, F.; Wei, J.; Wang, F.; Cao, R.; Zhou, B.; Mao, X.; Zheng, Q.; Chen, P. Isolation and Potential Immunological Characterization of Tpsglvy, a Novel Bursal Septpeptide Isolated from the Bursa of Fabricius. Peptides 2010, 31, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Feng, X.L.; Zhou, B.; Cao, R.B.; Li, X.F.; Ma, Z.Y.; Chen, P.Y. Isolation, Modulatory Functions on Murine B Cell Development and Antigen-Specific Immune Responses of Bp11, a Novel Peptide from the Chicken Bursa of Fabricius. Peptides 2012, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Byankina, A.O.; Kalitnik, A.A.; Kim, Y.H.; Bogdanovich, L.N.; Solov'eva, T.F.; Yermak, I.M. Influence of Red Algal Sulfated Polysaccharides on Blood Coagulation and Platelets Activation in vitro. J. Biomed. Mater. Res. Part A 2014, 102, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Mar. Drugs 2016, 14, 4. https://doi.org/10.3390/md14010004
Song L, Chen X, Liu X, Zhang F, Hu L, Yue Y, Li K, Li P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Marine Drugs. 2016; 14(1):4. https://doi.org/10.3390/md14010004
Chicago/Turabian StyleSong, Lin, Xiaolin Chen, Xiaodong Liu, Fubo Zhang, Linfeng Hu, Yang Yue, Kecheng Li, and Pengcheng Li. 2016. "Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides" Marine Drugs 14, no. 1: 4. https://doi.org/10.3390/md14010004
APA StyleSong, L., Chen, X., Liu, X., Zhang, F., Hu, L., Yue, Y., Li, K., & Li, P. (2016). Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Marine Drugs, 14(1), 4. https://doi.org/10.3390/md14010004