Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. Protein Expression
2.2. mRNA Expression of Target Genes
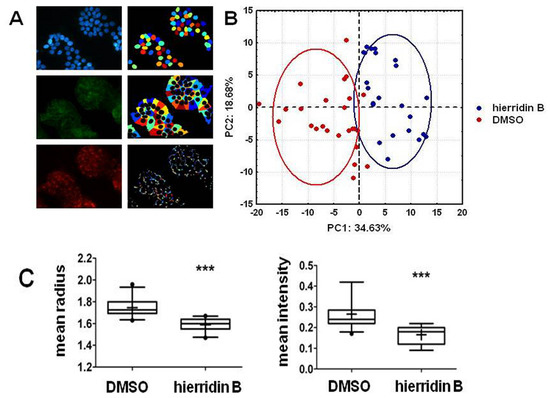
2.3. Fluorescence Microscopy Analysis
3. Discussion
4. Materials and Methods
4.1. Cyanobacteria Culture and Isolation of Hierridin B
4.2. HT-29 Assays
4.3. Protein Extraction and Two-Dimensional Gel Electrophoresis (2DGE)
4.4. Gel Staining, Image Acquisition, and Protein Expression Analysis
4.5. Protein Identification
4.6. Real-Time Analysis
4.7. Fluorescence Microscopy
4.8. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.W.; Hong, S.L.; Lee, G.S.; Yaacob, H.; Malek, S.N.A. Non-aqueous extract of Curcuma manga rhizomes induced cell death in human colorectal adenocarcinoma cell line (HT-29) via induction of apoptosis and cell cycle arrest at G0/G1 phase. Asian Pac. J. Trop. Med. 2016, 9, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.-J.; Zhou, Z.-W.; Zhu, D.-J.; Ju, Y.-L.; Wu, J.-H.; Ouyang, M.-Z.; Chen, X.-W.; Zhou, S.-F. Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Supresses EMT in HT29 and Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [PubMed]
- Kluru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhakuoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Pereira, P.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Toxicity of culturable cyanobacteria strains isolated from the Portuguese coast. Toxicon 2005, 46, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Fernandez, N.; Beiras, R.; Vasconcelos, V. Toxicity assessment of crude and partially purifies extracts of marine Synechocystis and Synechococcus cyanobacterial strains in marine invertebrates. Toxicon 2007, 50, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skærven, K.; Vasconcelos, V.M. Antimicrobial and Cytotoxic Assessment of Marine Cyanobacteria—Synechocystis and Synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine Cyanobacteria Compounds with Anticancer Properties: A Review on the Implication of Apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Garcia, M.; Costa-Rodrigues, J.; Costa, M.; Ribeiro, M.; Fernandes, M.; Barros, P.; Barreiro, A.; Vasconcelos, V.; Martins, R. Exploring bioactive properties of marine cyanobacteria isolated from the Portuguese coast: High potential as a source of anticancer compounds. Mar. Drugs 2014, 12, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS ONE 2013, 8, e69562. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Urbatzka, R.; Galante-Oliveira, S.; Rocha, E.; Castro, L.F.C.; Cunha, I. Normalization strategies for gene expression studies by real-time PCR in a marine fish species, Scophthalmus maximus. Mar. Genom. 2013, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Janik, J.E.; Younes, A. Brentuximab Vedotin (SGN-35). Clin. Cancer Res. 2011, 17, 6428–6436. [Google Scholar] [CrossRef] [PubMed]
- Marine Pharmaceuticals. Available online: http://marinepharmacology.midwestern.edu/clinPipeline.htm (accessed on 16 June 2016).
- Freitas, S.; Martins, R.; Campos, A.; Azevedo, J.; Osório, H.; Costa, M.; Barros, P.; Vasconcelos, V.; Urbatzka, R. Insights into the potential of picoplanktonic marine cyanobacteria strains for cancer therapies—Cytotoxic mechanisms against the RKO colon cancer cell line. Toxicon 2016, 119, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Ben-Hail, D.; Admoni, L.; Krelin, Y.; Tripathi, S.S. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta 2015, 1848, 2547–2575. [Google Scholar] [CrossRef] [PubMed]
- Keinan, N.; Pahima, H.; Ben-Hail, D.; Shoshan-Barmatz, V. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochim. Biophys. Acta 2013, 1833, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Kham, S.K.; Koh, J.S.; Suang Lim, J.Y.; Ariffin, H.; Chew, F.T.; Yeoh, A.E. Identification of prognostic protein biomarkers in childhood acute lymphoblastic leukemia (ALL). J. Proteom. 2011, 74, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Antonioli, P.; Astner, H.; Hamdan, M.; Righetti, S.C.; Perego, P.; Zunino, F.; Righetti, P.G. A proteomic approach to cisplatin resistance in the cervix squamous cell carcinoma cell line A431. Proteomics 2004, 4, 3246–3267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Liu, R.H.; Sheu, J.N.; Chen, S.T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J. Biomed. Sci. 2007, 14, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Weisthal, S.; Keinan, N.; Ben-Hail, D.; Arif, T.; Shoshan-Barmatz, V. Ca2+-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim. Biophys. Acta 2014, 1843, 2270–2281. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Chang, W.T.; Lan, Y.H.; Hour, M.J.; Lee, H.Z. Application of proteomics to identify the target molecules involved in Lonicera japonica-induced photokilling in human lung cancer CH27 cells. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Zhang, X.; Yu, Z. Synergistic effect of retinoic acid and vitamin D analog EB1089-induced apoptosis of hepatocellular cancer cells. Cytotechnology 2013, 65, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Le, S.B.; Holmuhamedov, E.L.; Narayanan, V.L.; Sausville, E.A.; Kaufmann, S.A. Adaphostin and other anticancer drugs quench the fluorescence of mitochondrial potential probes. Cell Death Differ. 2006, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiaoyan, X.; Xuan, Y.; Xiangke, L.; Zichang, Y.; Ran, Z.; Liuxing, W.; Qingxia, F. Silencing stathmin-modulating efficiency of chemotherapy for esophageal squamous cell cancer with paclitaxel. Cancer Gene Ther. 2015, 22, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Akhtar, J.; Wang, Z. Anti-STMN1 therapy improves sensitivity to antimicrotubule drugs in esophageal squamous cell carcinoma. Tumour Biol. 2015, 36, 7797–7806. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, S.; Bellido, J.; Gonçalves, J.; Zabala, J.C.; Soares, H. Tubulin cofactor A gene silencing in mammalian cells induces changes in microtubule cytoskeleton, cell cycle arrest and cell death. FEBS Lett. 2005, 579, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Choia, H.K.; Ryub, H.; Sonb, A.; Seob, B.; Hwangb, S.G.; Songb, J.Y.; Ahnb, J. The novel anthraquinone derivative IMP1338 induces death of human cancer cells by p53-independent S and G2/M cell cycle arrest. Biomed. Pharmacother. 2016, 79, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa. Molecules 2016, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Zia, F.; Draoui, M.; Brenneman, D.E.; Fridkin, M.; Davidson, A.; Gozes, I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc. Natl. Acad. Sci. USA 1993, 90, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- LEGE CC. Culture Collection of Microorganisms at the University of Porto (CIIMAR). Available online: http://www.ciimar.up.pt/legecc/ (accessed on 16 June 2016).
- Ferreira, R.; Vitorino, R.; Alves, R.M.; Appell, H.J.; Powers, S.K.; Duarte, J.A.; Amado, F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 2010, 10, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 97, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Cell Profiler. Cell Image Analysis Software. Available online: http://cellprofiler.org/ (accessed on 16 June 2016).






| SPP | Protein Name | DMSO (Mean ± SD) | Hierridin B (Mean ± SD) | Fold (×) | Acession Number | Gene | Protein Score | Matched Peptides MS MS/MS | |
|---|---|---|---|---|---|---|---|---|---|
| 506 | Calreticulin | 3316 ± 504 | 1951 ± 172 | 0.6 | CALR_HUMAN | CALR | 215 | 15 | 2 |
| 1513 | Diablo homolog, mitochondria | 717 ± 23 | 502 ± 12 | 0.7 | DBLOH_HUMAN | DIABLO | 105 | 7 | 2 |
| 2602 | Tumor protein D52 | 946 ± 96 | 633 ± 146 | 0.7 | TPD52_HUMAN | TPD52 | 133 | 8 | 2 |
| 2713 | - | 281 ± 80 | 76 ± 70 | 0.3 | - | - | - | - | - |
| 3101 | Tubulin-specific chaperone A | 730 ± 313 | 88 ± 18 | 0.1 | TBCA_HUMAN | TBCA | 130 | 6 | 3 |
| 3113 | - | 1504 ± 226 | 148 ± 142 | 0.1 | - | - | - | - | - |
| 3304 | Stathmin | 700 ± 179 | 95 ± 67 | 0.1 | STMN1_HUMAN | STMN1 | 85 | 5 | 1 |
| 3507 | - | 2247 ± 768 | 6225 ± 2081 | 2.8 | - | - | - | - | - |
| 3514 | UMP-CMP kinase | 721 ± 148 | 240 ± 243 | 0.3 | KCY_HUMAN | CMPK1 | 100 | 5 | 2 |
| 4704 | Serum albumin | 6705 ± 611 | 3282 ± 1123 | 0.5 | ALBU_HUMAN | ALB | 74 | 4 | 1 |
| 4802 | Gelsolin | 743 ± 239 | 355 ± 192 | 0.5 | GELS_HUMAN | GSN | 178 | 17 | 2 |
| 4901 | Neutral alpha-glucosidase AB | 577 ± 178 | 153 ± 69 | 0.3 | GANAB_HUMAN | GANAB | 304 | 23 | 3 |
| 5601 | Heat-shock protein beta-1 | 680 ± 70 | 517 ± 75 | 0.8 | HSPB1_HUMAN | HSPB1 | 128 | 3 | 1 |
| 5801 | Delta(3,5)-delta(2,4)-dienoyl-CoA isomerase, mitochondrial | 980 ± 40 | 1368 ± 230 | 1.4 | ECH1_HUMAN | ECH1 | 200 | 10 | 3 |
| 6105 | Ubiquitin-like protein ISG15 | 2519 ± 237 | 884 ± 473 | 0.4 | ISG15_HUMAN | ISG15 | 80 | 4 | 1 |
| 6202 | Alpha-enolase | 9861 ± 410 | 7341 ± 1801 | 0.7 | ENOA_HUMAN | ENO1 | 499 | 23 | 3 |
| 7112 | - | 1293 ± 155 | 232 ± 131 | 0.2 | - | - | - | - | - |
| 7305 | Serine hydroxymethyl transferase, mitochondrial | 718 ± 113 | 308 ± 93 | 0.4 | GLYM_HUMAN | SHMT2 | 277 | 17 | 2 |
| 7803 | Elongation factor 2 | 511 ± 90 | 181 ± 92 | 0.4 | EF2_HUMAN | EEF2 | 304 | 22 | 3 |
| 7804 | Elongation factor 2 | 811 ± 236 | 405 ± 128 | 0.5 | EF2_HUMAN | EEF2 | 307 | 26 | 3 |
| 8404 | t-complex protein 1 subunit delta | 543 ± 163 | 272 ± 112 | 0.5 | TCPD_HUMAN | CCT4 | 158 | 12 | 3 |
| 8802 | Voltage-dependent anion-selective channel protein 1 | 2319 ± 695 | 3487 ± 162 | 1.5 | VDAC1_HUMAN | VDAC1 | 111 | 8 | 1 |
| Gene | Name | Primer Sequence | Efficiency | R2 | Anneling Temperature |
|---|---|---|---|---|---|
| VDAC | Voltage-dependent anion channel 1 | F: CGGAAGGCAGAAGATGGC | 101.6% | 0.936 | 57 °C |
| R: TTGGTGGTCTCAGTGTTGG | |||||
| SHMT2 | Serine Hydroxymethyl transferase 2 | F: CTGCGACTTCCGAGTTGCGATG | 101.6% | 0.963 | 57 °C |
| R: GGCTGCGTTGCTGTGCTGAG | |||||
| TRAIL | Tumor Necrosis factor superfamily, member 10 | F: TCTCTCTGTGTGGCTGTAAC | 97.9% | 0.992 | 57 °C |
| R: TCATACTCTCTTCGTCATTGGG | |||||
| VIPR | Vasoactive Intestinal Peptide receptor 1 | F: CACCATCAACTCCTCACTG | 105.7% | 0.992 | 57 °C |
| R: CTGCTGTCACTCTTCCTG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, S.; Martins, R.; Costa, M.; Leão, P.N.; Vitorino, R.; Vasconcelos, V.; Urbatzka, R. Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells. Mar. Drugs 2016, 14, 158. https://doi.org/10.3390/md14090158
Freitas S, Martins R, Costa M, Leão PN, Vitorino R, Vasconcelos V, Urbatzka R. Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells. Marine Drugs. 2016; 14(9):158. https://doi.org/10.3390/md14090158
Chicago/Turabian StyleFreitas, Sara, Rosário Martins, Margarida Costa, Pedro N. Leão, Rui Vitorino, Vitor Vasconcelos, and Ralph Urbatzka. 2016. "Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells" Marine Drugs 14, no. 9: 158. https://doi.org/10.3390/md14090158
APA StyleFreitas, S., Martins, R., Costa, M., Leão, P. N., Vitorino, R., Vasconcelos, V., & Urbatzka, R. (2016). Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells. Marine Drugs, 14(9), 158. https://doi.org/10.3390/md14090158