Abstract
Four new compounds (1–4), including two brevianamides and two mycochromenic acid derivatives along with six known compounds were isolated from the deep-sea-derived fungus Penicillium brevicompactum DFFSCS025. Their structures were elucidated by spectroscopic analysis. Moreover, the absolute configurations of 1 and 2 were determined by quantum chemical calculations of the electronic circular dichroism (ECD) spectra. Compound 9 showed moderate cytotoxicity against human colon cancer HCT116 cell line with IC50 value of 15.6 μM. In addition, 3 and 5 had significant antifouling activity against Bugula neritina larval settlement with EC50 values of 13.7 and 22.6 μM, respectively. The NMR data of 6, 8, and 9 were assigned for the first time.
1. Introduction
Deep-sea-derived microorganisms are new potential resources for discovery of bioactive secondary metabolites [1,2,3]. In our ongoing search for active compounds from marine fungi, four brevianamides and five mycochromenic acid derivatives were obtained from the deep-sea-derived fungus Penicillium brevicompactum DFFSCS025. Brevianamides, a class of indole alkaloids, were isolated from P. brevicompactum in 1969 for the first time [4]. Their unique bicyclo[2.2.2]diazaoctane skeleton and multiple bio-activities were attractive to scientists. Most of them exhibited anti-bacterial, anti-insect pests and antitubercular potentials [5,6]. Several brevianamides have been totally synthesized [7,8,9]. Mycophenolic acid, a phenyl derivative, was found from Penicillium sp. in 1893 for the first time [10]. It was an inhibitor of human inosine 5′-monophosphate dehydrogenase (IMPDH), a target for immunosuppressive chemotherapy [11]. Mycophenolic acid and its derivative mycophenolate mofetil have been used as immunosuppressant drugs in the management of auto-immune disorders since the 1990s [12]. Because of instrument limitations, some brevianamides and mycophenolate acid derivatives are short of reliable spectral data including NMR data [4,10,13]. Herein, we describe the separation, structure elucidation, and bioactivities of Compounds 1–10 (Figure 1). The NMR data of 6, 8, and 9 were assigned for the first time.
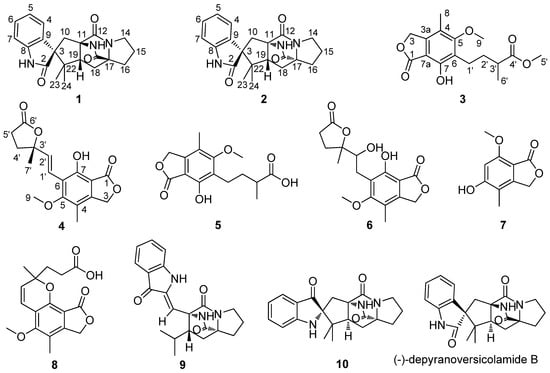
Figure 1.
Structures of Compounds 1–10.
2. Results and Discussion
The deep-sea-derived fungal stain DFFSCS025 was inoculated in liquid medium and fermented in standing situation for 32 days at 28 °C. The culture broths were absorbed with XAD-16 resin; meanwhile, mycelium portions were extracted with 80% acetone. The combined extract (24 g from 30 L) was subjected to silica gel column, ODS column, Sephadex LH-20, and purified with semi-preparation HPLC to yield Compounds 1–10.
Brevianamide X (1) was obtained as yellowish powder. Its molecular formula of C21H23N3O3 was established by HRESIMS (m/z 366.1810 [M + H]+). The 1H NMR spectrum (Table 1) revealed the presence of two methyls at δH 0.72 (3H, s) and 0.74 (3H, s), four aromatic protons at δH 6.83 (1H, d, J = 7.6 Hz), 6.98 (1H, td, J = 0.8, 7.6, 7.6 Hz), 7.20 (1H, td, J = 0.9, 7.6, 7.6 Hz), 7.23 (1H, d, J = 7.5 Hz), and two exchangeable protons at δH 9.13 (1H, br s) and 10.36 (1H, br s). The 13C NMR spectrum (Table 1) exhibited 21 carbons including two methyls (δC 20.2, 23.7), five methylenes (δC 24.8, 29.5, 29.9, 33.6, 43.8), five methines (δC 55.9, 109.6, 121.5, 126.4, 128.3), and nine quaternary carbons (δC 45.6, 61.9, 66.1, 68.5, 131.0, 142.8, 169.8, 173.5, 182.8). These NMR data were similar to those of (−)-depyranoversicolamide B (11) [7] except the little differences of the chemical shifts of C-3/11/19/20/22. Detailed analysis of 2D NMR spectra revealed that 1 had the same planar structure as (−)-depyranoversicolamide B (11) (Figure 2). The relative configuration of 1 was further determined by NOESY spectrum. The NOE correlations between H-10β, H-19, and H-21 established that they were on the same side, while NOE correlation between H-4 and H-10α indicated that they were on the other side (Figure 3). The relative configuration of 1 was therefore proposed to be 3S*, 11R*, 17R*, and 19R*. In order to assign the absolute configuration of 1, we carried out molecular mechanics calculation using DFT method at B3LYP/6-31G (d) level [14,15]. Furthermore, ECD/TDDFT calculations of all low-energy conformer afforded ECD spectra consistent with the experimental spectrum (Figure 4). The results indicated the 3S, 11R, 17R, 19R configuration for 1 on the basis of the relative configuration. Therefore, Compound 1 was inferred to be a diastereomer of (−)-depyranoversicolamide B.

Table 1.
1H NMR data (500 MHz) and 13C NMR data (125 MHz) of 1 and 2 in DMSO-d6.
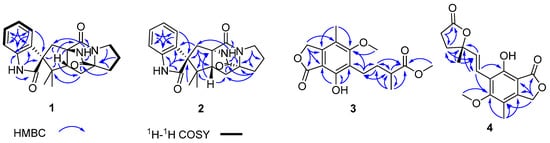
Figure 2.
Key HMBC and COSY correlations of 1–4.
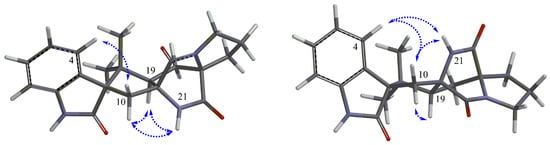
Figure 3.
Key NOESY correlations (dashed arrows) of 1 (left) and 2 (right).
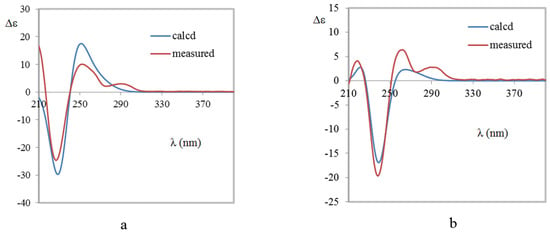
Figure 4.
Comparison of the measured and calculated ECD spectra of 1 (a) and 2 (b). (a) ECD spectra of (3S, 11R, 17R, 19R)-1 in MeOH (σ = 0.3 eV, shift = −3 nm); (b) ECD spectra of (3S, 11S, 17S, 19R)-2 in MeOH (σ = 0.27 eV, shift = −2 nm).
Brevianamide Y (2) had same molecular formula of C21H23N3O3 as 1 according to HRESIMS (m/z 366.1813 [M + H]+). The NMR data of 2 showed great similarity to those of 1 with the only obvious difference of the high-field shift of C-19 (from δC 55.9 in 1 to δC 50.5 in 2) (Table 1). Detailed analysis of 2D NMR spectra revealed that 2 had the same planar structure as 1 (Figure 2). In the NOESY spectrum, NOE correlations between H-4, H-10α, H-21, and H-23 suggested they were on the same side, while NOE correlation between H-19 and H-24 disclosed that they were on the other side (Figure 3). The relative configuration of 2 was suggested as 3S*, 11S*, 17S*, and 19R*. The absolute configuration of 2 was also determined by molecular mechanics calculation and quantum chemical computations [14,15]. The calculated ECD curve of 3S, 11S, 17S, and 19R was consistent with experimental one (Figure 4). The structure of 2 was confirmed as (3S, 11S, 17S, 19R)-brevianamide Y.
The molecular formula of 3 was determined as C16H20O6 by the HRESIMS (m/z 331.1164 [M + Na]+). The NMR data (Table 2) of 3 was closely resembled with known compound 6-(3-carboxybutyl)-7-hydroxy-5-methoxy-4-methylphthalan-1-one (5) [10] except for the presence of an extra methoxy group (δH 3.68 and δC 51.6). The HMBC correlations from H3-5′ to C-4′ suggested that the methoxy group was linked to the C-4’ of mycophenolic acid skeleton (Figure 2). No specific optical rotation ([α] 0 (c 0.1, MeOH)) or circular dichroism spectral data (±0) indicated 3 was isolated as a racemic mixture. We failed to separate the racemic mixture using chiral column by HPLC (CHIRALPAK® IC, Daicel Corporation, Osaka, Japan). Compound 3 was named as 6-(methyl 3-methylbutanoate)-7-hydroxy-5 -methoxy-4-methylphthalan-1-one.

Table 2.
1H NMR data (500 MHz) and 13C NMR data (125 MHz) of 3 and 4.
The molecular formula of 4 was determined as C17H18O6 by HRESIMS (m/z 341.1005 [M + Na]+). The similar NMR data (Table 2) of 4 and 3 indicated they had the same benzofuranone skeleton. The down-field shift of C-6 (from δC 122.5 in 3 to δC 117.9 in 4) was induced by the hyperconjugation of a double bond (C-1′, δC 118.23; C-2′, δC 137.38) with benzofuranone skeleton, which was confirmed by the HMBC correlations between H-1′/H-2′ and C-6 (Figure 3). The E-configured double bond ∆1′2′ was determined by the coupling constant value of approximately 16 Hz between H-1′ and H-2′. The NMR data (δH 2.19, 2.24, 2.56, 2.64; δC 26.4, 28.8, 34.0, 85.8, 177.0) and HMBC correlations from H-7′ to C-3′, C-4′, from H-4 to C-3′, C-5′, C-6′, C-7′, and H-5′ to C-4′, C-6′ elucidated the presence of a methyl-furanone residue [16,17] attached at C-2′ according to the HMBC correlations from H-1′ to C-3′, and from H-2′ to C-3′. Compound 4 had one chiral center at C-3′. The configuration of 4 was determined by molecular mechanics calculation and quantum chemical computations [14,15]. The (3′S)-4 of calculated ECD curve was consistent with experimental one (Figure 5). Compound 4 was named as (3′S)-(E)-7-hydroxy-5-methoxy-4-methyl-6-(2-(2-methyl-5-oxotetrahydrofuran-2-yl)vinyl) isobenzofuran-1(3H)-one.
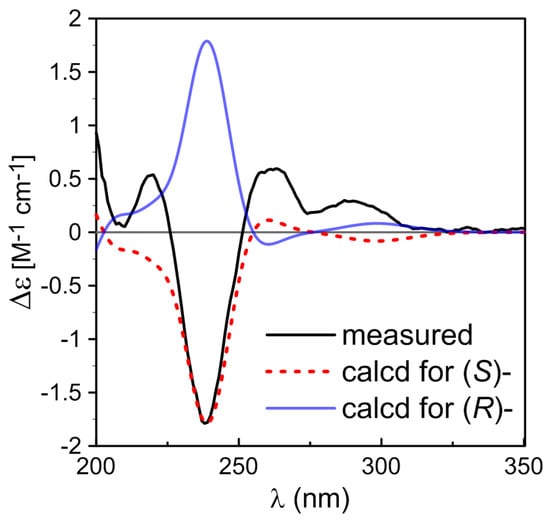
Figure 5.
Comparison of the measured ECD spectrum of 4 with B3LYP/TZVP calculated spectra of (S)- and (R)-4 in MeOH (σ = 0.20 eV, shift = −18 nm).
Compounds 5–10 were identified as 6-(3-carboxybutyl)-7-hydroxy-5-methoxy -4-methylphthalan-1-one [18], 7-hydroxy-6-[2-hydroxy-2-(2-methyl-5-oxotetrahydro-2-furyl) ethyl]-5-methoxy-4-methyl-1-phthalanone [10], 5-hydroxy-7-methoxy-4 –methylphtalide [19], mycochromenic acid [10,20], (−)-brevianamide C [21], and (+)-brevianamide A [22], respectively.
Compounds 1–10 were tested for cytotoxicity against human colon cancer HCT116 cell line using sulforhodamine B (SRB) assay. Only Compound 9 exhibited moderate activity with an IC50 value of 15.6 µM. In addition, antifouling activities of 1, 3, 5, and 8 were also evaluated in settlement inhibition assays with Bugula neritina larvae. The larval settlement bioassay showed that 3 and 5 could strongly inhibit the larvae settlement of B. neritina larvae with EC50 values of 13.7 and 22.6 μM, respectively, and LC50/EC50 > 100, while 1 and 8 showed no antilarval activity. Moreover, 1–10 were evaluated for their anti-bacterial activities against Streptococcus mutans (ATCC35668) and S. sobrinus (ATCC33478), anti-fungal activities against Fusarium oxysporum f. sp. cubense Race 1 and Race 4; however, they did not show any obvious activity at the concentration of 20 µg/mL.
3. Experimental Section
3.1. General Experimental Procedure
Optical rotations were measured with an Anton Paar MCP 500 polarimeter (Anton Paar GmbH, Graz, Austria). UV spectra were measured with a Shimadzu UV-2600 UV–vis spectrophotometer (Shimadzu, Kyoto, Japan) in a MeOH solution. Infrared spectra (IR) were recorded on a Shimadzu IRAffinity-1 Fourier transform infrared spectrophotometer (Shimadzu, Kyoto, Japan). 1H/13C NMR and 2D NMR spectra were recorded on a Bruker AV-500 spectrometer (Bruker, Billerica, MA, USA) with TMS as reference. High-resolution electrospray-ionization (HRESIMS) was performed on a Bruker microTOF-QII mass spectrometer (Bruker, Bremen, Germany). Analysis HPLC was performed on an Angilent 1100 liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA). Semi-preparative reversed-phase HPLC was performed on a Shimadzu LC-20A preparative liquid chromatography (Shimadzu, Kyoto, Japan) with YMC-Pack ODS-A column 250 × 10 mm i.d., S-5 μm × 12 nm, and 250 × 20 mm i.d., S-5 μm × 12 nm. Column chromatography (CC) was performed on silica gel (200–300 mesh, Qingdao Marine Chemical, Qingdao, China), Sephadex LH-20 (GE Healthcare, Barrington, IL, USA), or Rp-18 silica (Pharmacia Co. Ltd., St. Paul, MN, USA).
3.2. Fungal Material
The fungal strain DFFSCS025 (GenBank access number JX156371) was isolated from a deep-sea sediment sample collected in the South China Sea, Sansha City (18°5′ N, 118°31′ E; 3928 m depth), Hainan Province, and identified as Penicillium brevicompactum by ITS rDNA sequence homology (99% similarity with P. brevicompactum). The strain was deposited in the RNAM Center, South China Sea Institute of Oceanology, Chinese Academy of Sciences.
3.3. Fermentation and Extraction
Spores of the fungal strain were inoculated into 1000 mL Erlenmeyer flasks each containing 300 mL of liquid medium (glucose 1%, maltose 2%, monosodium glutamate 1%, KH2PO4 0.05%, MgSO4·7H2O 0.003%, corn steep liquor 0.05%, yeast extract 0.3%, dissolved in sea water, pH 6.5). After 32 days of stationary cultivation at 28 °C, the whole broths (30 L) were filtered through cheesecloth. Sterilized XAD-16 resin (20 g/L) was added to the liquor and shaken at low speed for 30 min to absorb the organic products. The resin was washed with distilled water to remove medium residue then eluted with methanol. The methanol solvent was removed under vacuum to yield a brown residue (18 g). The mycelium portion was smashed and extracted twice with 80% acetone/H2O. The acetone soluble fraction was dried in vacuo to yield 6 g of residue. The residues of liquor and mycelium extracts were combined together according to TLC chromatography detecting.
3.4. Purification
The combined extract (24 g) was subjected to silica gel column (500 g) and eluted with CHCl3/MeOH (100:0–80:20, v/v) to yield ten fractions (Fractions 1–10). Fraction 4 (10.5 g) was separated by silica gel column and eluted with CHCl3/MeOH to give seven sub-fractions (Fractions 4-1–4-7). Fraction 4-4 (0.9 g) was subjected to Develosil ODS column eluting with a decreasing polarity of MeOH/H2O (20:80–70:30) and purified with semi-preparation HPLC (MeOH/H2O, 65:35) at the flow rate of 3 mL/min to yield 6 (tR 68.2 min, 16.5 mg) and 7 (tR 15.3, 2.4 mg). Fraction 6 was isolated with Develosil ODS column eluting with MeOH/H2O (15:85–70:30) to obtain six sub-fractions (Fractions 6-1–6-6). Fraction 6-4 was purified by preparatory HPLC (CH3CN/H2O, 34:66) at the flow rate of 6 mL/min to yield 2 (tR 18.4 min, 4.7 mg), 10 (tR 25.5 min, 1.7 mg), 9 (tR 28.4 min, 2.7 mg), 5 (tR 35.8 min, 4.2 mg), and 4 (tR 53.4 min, 6.0 mg), respectively. Fraction 6-5 was purified by HPLC (MeOH/H2O, 65:35) at the flow rate of 6 mL/min to yield 1 (tR 13.6 min, 3.2 mg), 8 (tR 15.8 min, 55.3 mg), and 3 (tR 27.1 min, 25.5 mg).
Brevianamide X (1): yellowish powder; [α] +8.7 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 209 (4.44), 253 (3.68), 286 (3.08) nm; CD (MeOH) λmax (∆ε) 227 (‒24.6), 252 (+10.1), 290 (+3.0); 1H and 13C NMR data, see Table 1; HRESIMS m/z 366.1810 [M + H]+ (calcd. for 366.1812), 388.1630 [M + Na]+ (calcd. for 388.1632).
Brevianamide Y (2): yellowish powder; [α] +11.5 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 209 (4.64), 252 (3.88), 286 (3.25) nm; CD (MeOH) λmax (∆ε) 207 (‒2.0), 218 (+4.5), 238 (‒21.7), 262 (+7.1), 276 (+2.0), 293 (+3.1); 1H and 13C NMR data, see Table 1; HRESIMS m/z 366.1813 [M + H]+ (calcd. for 366.1812), 388.1636 [M + Na]+ (calcd. for 388.1632).
6-(Methyl 3-methylbutanoate)-7-hydroxy-5-methoxy-4-methylphthalan-1-one (3): white powder; [α] 0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 209 (4.67), 286 (3.31), 313 (2.67) nm; CD (MeOH) λmax (∆ε) ±0; 1H and 13C NMR data, see Table 2; HRESIMS m/z 309.1332 [M + H]+ (calcd. for 309.1333), 331.1164 [M + Na]+ (calcd. for 331.1152).
(3′S)-(E)-7-Hydroxy-5-methoxy-4-methyl-6-(2-(2-methyl-5-oxotetrahydrofuran-2-yl)vinyl)isobenzofuran-1(3H)-one (4): white powder; [α] +2.2 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 209 (4.05), 244 (4.04), 334 (3.22) nm; CD (MeOH) λmax (∆ε) 210 (+0.05), 220 (+0.54), 238 (‒1.79), 261 (+0.59), 274 (+0.17), 290 (+0.29); 1H 13C NMR data, see Table 2; HRESIMS m/z 341.1005 [M + Na]+ (calcd. for 341.0996).
7-Hydroxy-6-[2-hydroxy-2-(2-methyl-5-oxotetrahydro-2-furyl)ethyl]-5-methoxy-4-methyl-1-phthalanone (6): white powder; [α] ‒0.8 (c 0.1, MeOH); CD (MeOH) λmax (∆ε) ±0; 1H NMR (500 MHz, DMSO-d6) δH: 5.24 (2H, s, H-3), 2.09 (3H, s, H-8), 3.75 (3H, s, H-9), 2.78 (1H, dd, J = 2.0, 13.5 Hz, H-1′α), 2.71 (1H, dd, J = 10.1, 13.5 Hz, H-1′β), 3.83 (1H, dd, J = 1.9, 9.8 Hz, H-2′), 2.37 (1H, ddd, J = 7.3, 10.0, 12.3 Hz, H-4′α), 1.87 (1H, ddd, J = 6.3, 10.3, 12.5 Hz, H-4′β), 2.61 (1H, dd, J = 7.3, 10.8 Hz, H-5′α), 2.56 (1H, dd, J = 6.4, 10.0 Hz, H-5′β), 1.41 (3H, s, H-7′); 13C NMR (125 MHz, DMSO-d6) δC: 170.23 (C, C-1), 68.85 (CH2, C-3), 146.84 (C, C-3a), 116.26 (C, C-4), 163.50 (C, C-5), 120.99 (C, C-6), 154.31 (C, C-7), 107.48 (C, C-7a), 11.58 (CH3, C-8), 61.13 (CH3, C-9), 26.65 (CH2, C-1′), 74.61 (CH, C-2′), 88.65 (C, C-3′), 28.58 (CH2, C-4′), 29.35 (CH2, C-5′), 177.13 (C, C-6′), 22.53 (CH3, C-7′); HRESIMS m/z 335.1146 (M − H)− (calcd. for 335.1136).
Mycochromenic acid (8): white powder; [α] ‒3.1 (c 0.15, MeOH); 1H NMR (500 MHz, DMSO-d6) δH: 5.22 (2H, s, H-3), 2.07 (3H, s, H-8), 3.75 (3H, s, H-9), 6.62 (1H, d, J = 10.1 Hz, H-1′), 5.83 (1H, d, J = 10.1 Hz, H-2′), 1.93 (2H, m, H-4′), 2.35 (2H, t, J = 7.8 Hz, H-5′), 1.40 (3H, s, H-7′); 13C NMR (125 MHz, DMSO-d6) δC: 168.22 (C, C-1), 68.73 (CH2, C-3), 148.95 (C, C-3a), 116.98 (C, C-4), 159.36 (C, C-5), 114.70 (C, C-6), 151.03 (C, C-7), 107.95 (C, C-7a), 11.02 (CH3, C-8), 62.04 (CH3, C-9), 117.52 (CH, C-1′), 130.02 (CH, C-2′), 79.19 (C, C-3′), 35.52 (CH2, C-4′), 28.79 (CH2, C-5′), 174.54 (C, C-6′), 26.10 (CH3, C-7′); ESIMS m/z 363 (M + Na)+.
(‒)-Brevianamide C (9): yellowish powder; [α] −60.4 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 202 (4.02), 235 (4.02), 259 (4.13) nm; CD (MeOH) λmax (∆ε) 205 (−16.40), 225 (+10.43), 244 (+1.22), 252 (+1.61), 265 (−2.15), 302 (+0.57); 1H NMR (500 MHz, CDCl3) δH: 7.70 (1H, d, J = 7.5 Hz, H-4), 6.93 (1H, t, J = 6.0 Hz, H-5), 7.45 (1H, td, J = 6.0, 6.0, 1.0 Hz, H-6), 6.91 (1H, d, J = 8.5 Hz, H-7), 5.84 (1H, s, H-10), 3.52 (2H, m, H-14), 2.04 (2H, dt, J = 6.5, 6.5, 12.7 Hz, H-15), 1.87 (1H, d, J = 15.4 Hz, H-16α), 2.80 (1H, dt, J = 6.9, 6.9, 13.4 Hz, H-16β), 1.83 (1H, dd, J = 6.0, 13.3 Hz, H-18α), 1.96 (1H, dd, J = 10.1, 13.3 Hz, H-18β), 2.46 (1H, m, H-19), 2.13 (1H, m, H-22), 0.85 (3H, d, J = 6.9 Hz, H-23), 0.86 (3H, d, J = 6.7 Hz, H-24); 13C NMR (125 MHz, CDCl3) δC: 186.76 (C, C-2), 139.03 (C, C-3), 125.60 (CH, C-4), 120.65 (CH, C-5), 136.80 (CH, C-6), 112.59 (CH, C-7), 154.55 (C, C-8), 121.62 (C, C-9), 104.03 (CH, C-10), 66.89 (C, C-11), 168.83 (C, C-12), 44.48 (CH2, C-14), 24.55 (CH2, C-15), 29.20 (CH2, C-16), 66.21 (C, C-17), 30.83 (CH2, C-18), 46.75 (CH, C-19), 172.07 (C, C-20), 27.67 (CH, C-22), 22.16 (CH3, C-23), 16.17 (CH3, C-24); ESIMS m/z 388 (M + Na)+.
3.5. Computational Methods
Molecular Merck force field (MMFF) and DFT/TDDFT calculations were performed with Spartan′14 software package (Wavefunction Inc., Irvine, CA, USA) and Gaussian09 program package [23], respectively, using default grids and convergence criteria. MMFF conformational search generated low-energy conformers within a 10 kcal/mol energy window were subjected to geometry optimization using the B3LYP/def2-SVP method. Frequency calculations were run with the same method to verify that each optimized conformer was a true minimum and to estimate their relative thermal free energies (∆G) at 298.15 K. Energies of the low-energy conformers in MeOH were calculated at the B3LYP/def2-TZVP level. Solvent effects were taken into account by using a polarizable continuum model (PCM). The TDDFT calculations were performed using the hybrid B3LYP [24,25,26] PBE1PBE [27,28] and/or TPSSh [29] functionals, and Ahlrichs’ basis set TZVP (triple zeta valence plus polarization) [30]. The number of excited states per each molecule was 30. The ECD spectra were generated by the program SpecDis [31] using a Gaussian band shape from dipole-length dipolar and rotational strengths. Equilibrium population of each conformer at 298.15 K was calculated from its relative free energies using Boltzmann statistics. The calculated spectra were generated from the low-energy conformers according to the Boltzmann weighting of each conformer in a MeOH solution.
3.6. Cytotoxicity
All compounds were tested for cytotoxicity against HTC116 cell line with SRB method. Briefly, Cytotoxicity assays involving HCT116 cells were performed using sulforhodamine B based on slightly modified protocols [32]. HCT116 cells were maintained in a DMEM medium with 10% fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA, USA). Tested samples were prepared using 10% aqueous DMSO as solvent. The cell suspension was added into 96-well microliter plates in 190 µL at plating densities of 5000 cells/well. One plate was fixed in situ with TCA to represent a no-growth control at the time of drug addition (Day 0). Then, 10 μL of 10% aqueous DMSO was used as control group. After 72 h incubation, the cells were fixed to plastic substratum by the addition of 50 μL of cold 50% aqueous trichloroacetic acid and washed with water after incubation at 4 °C for 30 min. After staining cells with 100 μL of 0.4% sulforhodamine B in 1% aqueous AcOH for 30 min, unbound dye was removed by washing four times with 1% aqueous AcOH. The plates were allowed to dry at room temperature, then the bound dye was solubilized with 200 μL of 10 mM unbuffered Tris base, pH 10. Shaken for 5 min or until the dye was completely solubilized and the optical density was measured at 515 nm using an ELISA plate reader (Bio-Rad, Hercules, CA, USA). The average data were expressed as a percentage, relative to the control. Percentage growth inhibition was calculated as (OD (cells + samples) − OD (Day 0 only cells))/(OD (cells + 10% DMSO) − OD (Day 0 only cells)) = % survival, cytotoxicity = 1 − % survival. (Graphpad Software, Inc., San Diego, CA, USA).
3.7. Larval Settlement Assays
Larval culture and larval settlement assays matched the method reported in the literature [33]. Briefly, the stock solution of tested samples in DMSO was diluted with autoclaved filtered sea water (FSW) to concentrations ranging from 3.125 to 100 µg/mL. About 20 competent larvae were added to each well in 1 mL of the test solution. Wells containing only FSW with DMSO served as the controls. The plates were incubated at 27 °C for 1 h for B. neritina and 24 h for B. amphitrite. The percentage of larval settlement was determined by counting the settled, live individuals under a dissecting microscope and expressing the result as a proportion of the total number of larvae in the well. EC50 (inhibits 50% of larvae settlement in comparison with the control) and LC50 (lethal concentration, 50%) were calculated by using the Excel software program.
4. Conclusions
In conclusion, four new compounds (1–4), include two brevianamides and two mycochromenic acid derivatives along with six known compounds were isolated from the deep-sea-derived fungus Penicillium brevicompactum DFFSCS025. Their structures were elucidated by spectroscopic analysis and quantum chemical computations. Compound 9 showed moderate cytotoxicity against human colon cancer HCT116 cell line with IC50 values of 15.6 µM. Compounds 3 and 5 had significant antifouling activity against Bugula neritina larval settlement with EC50 values of 13.7 and 22.6 μM, respectively. The NMR data of 6, 8, and 9 were assigned for the first time.
Supplementary Materials
Available online: www.mdpi.com/1660-3397/15/2/43/s1. 1H, 13C, and 2D NMR spectra and HRESIMS of Compounds 1–4 and known Compounds 6, 8, and 9. HPLC spectra of Compounds 3 and 5. Chiral HPLC spectra of Compounds 3 and 6.
Acknowledgments
We thank X.-Y. Wei, South China Botanical Garden, Chinese Academy of Sciences, for quantum chemical computations of 1, 2, and 4. We thank C.-F. Ku, School of Chinese Medicine, Hong Kong Baptist University, for anti-tumor activity determination. We are grateful for the financial support provided by the Regional Innovation Demonstration Project of Guangdong Province Marine Economic Development (GD2012-D01-002), the Natural Science Foundation of China (41606186), the Strategic Leading Special Science and Technology Program of Chinese Academy of Sciences (XDA100304002), the Natural Science Foundation of China (41376160 and 81673326), Guangzhou Science and Technology Research Projects (201607010305) and the National Marine Public Welfare Research Project of China (201305017).
Author Contributions
Shuhua Qi designed and guided the experiment. Xinya Xu purified and conducted the structure elucidation. Xiaoyong Zhang contributed to isolate the fungus from deep-sea sediment. Xuhua Nong had evaluated anti-fouling activity using Bugula neritina larvae. Jie Wang performed anti-bacterial activity and anti-fungal activity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Li, S.; Yang, T.; Zhang, Q.; Ma, L.; Tian, X.; Zhang, H.; Huang, C.; Zhang, S.; et al. Spiroindimicins A–D: New bisindole alkaloids from a deep-sea-derived Actinomycete. Org. Lett. 2012, 14, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.J.; Wright, J.J. The brevianamides: A new class of fungal alkaloid. J. Chem. Soc. D. 1969. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Simmonds, M.J.S.; Kemmelmeier, C.; Blaney, W.M. Effects of brevianamide A, its photolysis product brevianamide D, and ochratoxin A from two Penicillium strains on the insect pests Spodoptera frugiperda and Heliothis virescens. Mycol. Res. 1990, 94, 538–542. [Google Scholar] [CrossRef]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.F.; Xiao, T.; Zhang, D.; Deng, L.F.; Wang, Y.; Qin, Y. Total synthesis of (-)-depyrannoversicolamide B. Chem. Commun. 2015, 51, 16143–16146. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Glinka, T.; Kwast, E.; Coffman, H.; Stille, J.K. Asymmetric, stereocontrolled total synthesis of (-)-brevianamide B. J. Am. Chem. Soc. 1990, 112, 808–821. [Google Scholar] [CrossRef]
- Zhao, L.; May, J.P.; Huang, J.; Perrin, D.M. Stereoselective synthesis of brevianamide E. Org. Lett. 2012, 14, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.F.; Moore, R.H.; Crawley, G.C. Microbial modification of mycophenolic acid. J. Chem. Soc. C. 1970. [Google Scholar] [CrossRef]
- Sintchak, M.D.; Fleming, M.A.; Futer, O.; Raybuck, S.A.; Chambers, S.P.; Caron, P.R.; Murcko, M.A.; Wilson, K.P. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 1996, 85, 921–930. [Google Scholar] [CrossRef]
- Bentley, R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem. Rev. 2000, 100, 3801–3825. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.J.; Wright, J.J. Biosynthesis. XLII. Structural elucidation and same aspects of the biosynthesis of the brevianamides-A and -E. Tetrahedron 1970, 26, 2329–2344. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Morita, H. Circular dichroism calculation for natural products. J. Nat. Prod. 2014, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.P.; Liang, X.R.; Liu, X.H.; Ji, N.Y. Aspewentins A-C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Jakupovic, J.; Bohlmann, F. A new type of sesquiterpene and acorane derivative from Calea prunifolia. J. Nat. Prod. 1984, 47, 802–808. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Pramanik, C.; Chorghade, M.S.; Gurjar, M.K. A short and efficient synthetic strategy for the total syntheses of (S)-(+)- and (R)-(−)-plalolide A. Eur. J. Org. Chem. 2007, 5059–5063. [Google Scholar] [CrossRef]
- Habib, E.; Leon, F.; Bauer, J.D.; Hill, R.A.; Carvalho, P.; Cutler, H.G.; Cutler, S.J. Mycophenolic derivatives from Eupenicillium parvum. J. Nat. Prod. 2008, 71, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.M.M.P.; Ferreira, A.G.; Daolio, C.; Filho, E.R.; Boffo, E.; Souza, A.Q.L.; Sebastianes, F.L.S.; Melo, I.S. Production of 5-hydroxy-7-methoxy-4-methylphthalide in a culture of Penicillium crustosum. An. Acad. Bras. Cienc. 2013, 85, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Nedachi, M.; Maekawa, M.; Murayama, Y.; Miyazawa, M.; Hirai, Y. Synthetic study for two 2H-chromenic acids, 8-chlorocannabiorcichromenic acid and mycochromenic acid. J. Heterocycl. Chem. 2006, 43, 29–41. [Google Scholar] [CrossRef]
- Birch, A.J.; Russell, R.A. Structural elucidations of brevianamides-B, -C, -D and -F. Tetrahedron 1972, 28, 2999–3008. [Google Scholar] [CrossRef]
- Williams, R.M.; Sanz-Cervera, J.F.; Sancenon, F.; Marco, J.A.; Halligan, K.M. Biomimetic Diels-Alder cyclizations for the construction of the brevianamide, paraherquamide, sclerotamide, asperparaline and VM55599 ring systems. Bioorg. Med. Chem. 1998, 6, 1233–1241. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 mode. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Efficient hybrid density functional calculations in solids: Assessment of the Heyd-Scuseria-Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2003, 119, 12129. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Wang, J.; Zhang, J.J.; Song, X.; Ku, C.F.; Zou, J.; Li, J.X.; Rong, L.J.; Pan, L.T.; Zhang, H.J. Henrin A: A new anti-HIV ent-kaurane diterpene from Pteris henryi. Int. J. Mol. Sci. 2015, 16, 27978–27987. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.H.; Xu, Y.; Xiong, H.R.; Qian, P.Y.; Zhang, S. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World J. Microbiol. Biotechnol. 2009, 25, 399–406. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).