Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129
Abstract
:1. Introduction
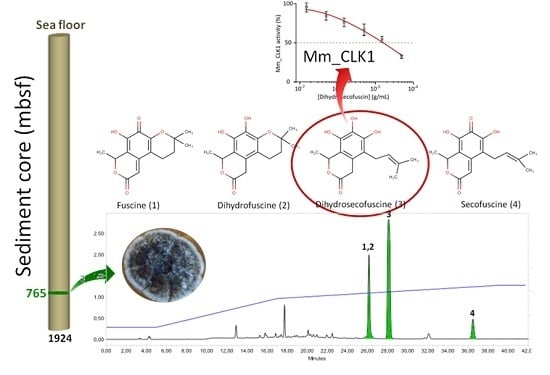
2. Results
2.1. Purification and Characterization of the Bioactive Compounds Produced by Oidiodendron griseum UBOCC-A-114129
2.2. Kinetics of Fuscin Derivative Production
2.3. Biological Activities
2.3.1. Antimicrobial Activity
2.3.2. Disease-Related Kinase Inhibition
3. Discussion
4. Materials and Methods
4.1. Fungal Isolation and Identification
4.2. Cultivation Method and Fermentation
4.3. Kinetics of Biomass and Metabolite Production
4.4. Bio-Guided Isolation
4.5. Purification of Bioactive Compounds
4.6. Characterization/Spectral Data
4.7. Biological Activities
4.7.1. Minimal Inhibitory Concentrations (MICs)
4.7.2. Kinase Assays
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Arnosti, C. Microbial extracellular enzymes and the marine carbon cycle. Annu. Rev. Mar. Sci. 2011, 3, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Rédou, V.; Navarri, M.; Meslet-Cladière, L.; Barbier, G.; Burgaud, G. Species richness and adaptation of marine fungi from deep-subseafloor sediments. Appl. Environ. Microbiol. 2015, 81, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Orsi, W.D.; Edgcomb, V.P.; Christman, G.D.; Biddle, J.F. Gene expression in the deep biosphere. Nature 2013, 499, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Pachiadaki, M.G.; Rédou, V.; Beaudoin, D.J.; Burgaud, G.; Edgcomb, V.P. Fungal and prokaryotic activities in the marine subsurface biosphere at Peru margin and Canterbury basin inferred from RNA-Based analyses and microscopy. Front. Microbiol. 2016, 7, 846. [Google Scholar] [CrossRef] [PubMed]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Culturability and secondary metabolite diversity of extreme microbes: Expanding contribution of deep sea and deep-sea vent microbes to natural product discovery. Mar. Biotechnol. 2011, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Navarri, M.; Jégou, C.; Meslet-Cladière, L.; Brillet, B.; Barbier, G.; Burgaud, G.; Fleury, Y. Deep subseafloor fungi as an untapped reservoir of amphipathic antimicrobial compounds. Mar. Drugs 2016, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.E. Studies in the biochemistry of micro-organisms. 79. Fuscin, a metabolic product of Oidiodendron fuscum Robak. Part 1. Preparation, properties and antibacterial activity. Biochem. J. 1948, 43, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Boulet, C.A.; Poulton, G.A. Pentaketide metabolites from Potebniamyces gallicola n. sp. Can. J. Chem. 1983, 61, 2285–2286. [Google Scholar] [CrossRef]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev. Anti-Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, K.; Rossant, C.; Ng, S.; Huang, Y.; Butler, M.S.; Buss, A.D. 10-Methoxydihydrofuscin, fuscinarin, and fuscin, novel antagonists of the human CCR5 receptor from Oidiodendron griseum. J. Nat. Prod. 2003, 66, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; D’Souza, L.; Kamat, T.; Rodrigues, C.; Naik, C.G. Batch culture fermentation of Penicillium chrysogenum and a report on the isolation, purification, identification and antibiotic activity of citrinin. Indian J. Mar. Sci. 2009, 38, 38–44. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Proksch, P. Marine-derived fungal metabolites. In Springer Handbook of Marine Biotechnology; Prof, S.-K.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 759–788. [Google Scholar]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Vansteelandt, M.; Blanchet, E.; Egorov, M.; Petit, F.; Toupet, L.; Bondon, A.; Monteau, F.; Le Bizec, B.; Thomas, O.P.; Pouchus, Y.F.; et al. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium Strain. J. Nat. Prod. 2013, 76, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. A 2008, 25, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pyuskyulev, B.; Rindone, B.; Scolastico, C. A new synthesis of fuscin. Tetrahedron 1973, 29, 2849–2850. [Google Scholar] [CrossRef]
- Birch, A.J.; Massy-Westropp, R.A.; Rickards, R.W.; Smith, H. 66. Studies in relation to biosynthesis. Part XIII. Griseofulvin. J. Chem. Soc. 1958, 360–365. [Google Scholar] [CrossRef]
- Chen, L.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre. J. Nat. Prod. 2008, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine targets, protein kinases and pyridoxal kinase. J. Biol. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef] [PubMed]




| MIC (μg/mL) | |||
|---|---|---|---|
| Dihydrosecofuscin | Secofuscin | Control | |
| Enterococcus faecalis ATCC 29212 | 128–256 | >256 | 4 |
| Vancomycin Resistant Enterococcus faecium BM4147 | 128–256 | >256 | NA |
| Staphylococcus aureus ATCC 29213 | 128 * | 256 * | 1–2 |
| Methicillin-resistant Staphylococcus aureus | 128 * | ≥256 | NA |
| Streptococcus equinus NRRL-B-4268 | 64–128 | 256 | NA |
| Cinetobacter baumannii CIP70.34T | 128–256 * | ≥256 | NA |
| Escherichia coli ATCC 25922 | >256 | >256 | 4 |
| Klebsiella pneumoniae ATCC 8045 | 128–256 * | >256 | NA |
| Pseudomonas aeruginosa ATCC 27853 | ≥256 | ≥256 | 4 |
| Protein Kinases | Dihydrosecofuscin |
|---|---|
| Rn_DYRK1A | 63 |
| Mm_CLK1 | 17 |
| Hs_CDK9/CyclinT | 69 |
| Hs_CDK5/p25 | 57 |
| Hs_CDK2/CyclinA | 55 |
| Ssc_GSK3a/b | 57 |
| Ssc_CK1 | 72 |
| Lm_CK1 | 89 |
| Hs_PIM1 | 86 |
| Hs_Haspin | 60 |
| Hs_RIPK3 | 67 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarri, M.; Jégou, C.; Bondon, A.; Pottier, S.; Bach, S.; Baratte, B.; Ruchaud, S.; Barbier, G.; Burgaud, G.; Fleury, Y. Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129. Mar. Drugs 2017, 15, 111. https://doi.org/10.3390/md15040111
Navarri M, Jégou C, Bondon A, Pottier S, Bach S, Baratte B, Ruchaud S, Barbier G, Burgaud G, Fleury Y. Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129. Marine Drugs. 2017; 15(4):111. https://doi.org/10.3390/md15040111
Chicago/Turabian StyleNavarri, Marion, Camille Jégou, Arnaud Bondon, Sandrine Pottier, Stéphane Bach, Blandine Baratte, Sandrine Ruchaud, Georges Barbier, Gaëtan Burgaud, and Yannick Fleury. 2017. "Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129" Marine Drugs 15, no. 4: 111. https://doi.org/10.3390/md15040111
APA StyleNavarri, M., Jégou, C., Bondon, A., Pottier, S., Bach, S., Baratte, B., Ruchaud, S., Barbier, G., Burgaud, G., & Fleury, Y. (2017). Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129. Marine Drugs, 15(4), 111. https://doi.org/10.3390/md15040111