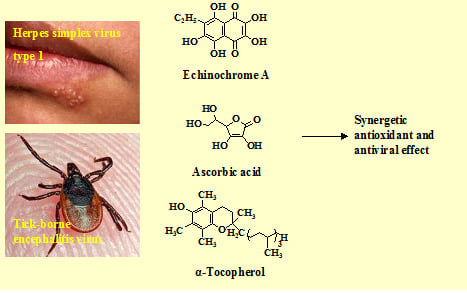
Antiviral and Antioxidant Properties of Echinochrome A
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity of Ech Formulations Alone or Combined with Other Antioxidants
2.2. Antioxidant Activity of the Formulations Against LPS-Induced ROS Formation in Vero Cells
2.3. Cytotoxicity and Antiviral Activity of Formulations.
2.4. Time-of-Formulation-Addition Assay
3. Discussion
4. Materials and Methods
4.1. Viruses and Cell Cultures
4.2. Studied Formulations
- Ascorbic acid (Asc) 99.8%, pharmaceutical, AppliChem, Germany.
- α-Tocopherol (Toc) ≥96%, pharmaceutical, Carl Roth, Germany.
- The composition of antioxidants Ech + Asc + Toc at the weight ratio of 5:5:1.
- The composition containing Asc and Toc at the weight ratio of 5:1.
- Ribavirin®, pharmaceutical, Vertex, Russia.
- Acyclovir®, pharmaceutical, Belmedpreparation, Republic of Belarus.
4.3. Determination of Antioxidant Activity of the Formulations
4.4. Antioxidant Activity of the Formulations Against LPS-Induced ROS Formation in Vero Cells
4.5. Cytotoxicity Assay of the Formulations
4.6. Antiviral Activity ASSAY of Formulations
4.7. Time-of-Formulation-Addition assay
- -
- Pretreatment of cells with formulations. Monolayer of cells was pretreated with formulations in triplicate and incubated at 37 °C for one hour. Thereafter, the cells were washed and infected with virus at 37 °C for one hour. The cells were washed to remove unabsorbed virus and incubated with maintenance medium until CPE was observed.
- -
- Pretreatment of virus with formulations. The virus was mixed with formulations at a ratio 1:1 (v/v), incubated for one hour at 37 °C, then applied to monolayer of cells in triplicate. After one hour adsorption at 37 °C, cells were washed, and maintenance medium was added, followed by incubation until CPE was observed.
- -
- Treatment of infected cells. Monolayer of cells was infected with the virus at 37 °C for one hour, then washed, treated with tested formulations in triplicate, and incubated until CPE was appeared.
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Gullberg, R.C.; Steel, J.J.; Moon, S. Oxidative stress influences positive strand RNA virus genome. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef]
- Kumar, S.; Misra, U.K.; Kalita, J.; Khanna, V.K.; Khan, M.Y. Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem. Int. 2009, 55, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Zaharicheva, T.A.; Koval’skij, Ju.G.; Lebed’ko, O.A.; Mzhel’skaja, T.V. Oxidative stress in patients with tick-borne encephalitis in the Far East of the Russian Federation. Dal’nevost. Zhurn Infekc. Patol. 2012, 20, 41–45. (In Russian) [Google Scholar]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovacs, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. Neurovirol. 2007, 13, 416–425. [Google Scholar] [CrossRef]
- Sebastiano, M.; Chastel, O.; de Thoisy, B.; Eens, M.; Costantini, D. Oxidative stress favours herpes virus infection in vertebrates: A meta-analysis. Curr. Zool. 2016, 62, 325–332. [Google Scholar] [CrossRef]
- Valyi-Nagy, T.; Dermody, T.S. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol. Histopathol. 2005, 20, 957–967. [Google Scholar] [CrossRef]
- Schwarz, K.B. Oxidative stress during viral infection: A review. Free Radic. Biol. Med. 1996, 21, 641–649. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta 2014, 1842, 1282–1294. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Mukherjee, A.; Bodhankar, S.L. Antioxidant for treatment of diabetic nephropathy: A systematic review and meta-analysis. Chem. Biol. Interact. 2017, 278, 212–221. [Google Scholar] [CrossRef]
- Krylova, N.V.; Popov, A.M.; Leonova, G.N. Antioxidants as Potential Antiviral Agents for Flavivirus Infections. Antibiot Khimioter 2016, 61, 25–31. [Google Scholar] [PubMed]
- Malvy, D.J.M.; Richard, M.J.; Arnaud, J.; Favier, A.; Amedee-Manesme, O. Relationship of plasma malondialdehyde, vitamin E and antioxidant micronutrients to human immunodeficiency virus-1 seropositivity. Clin. Chim. Acta 1994, 224, 89–94. [Google Scholar] [CrossRef]
- Allard, J.P.; Aghdassi, E.; Chau, J.; Tam, C.; Kovacs, C.M.; Salit, I.E.; Walmsley, S.L. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. Aids 1998, 12, 1653–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, D.; Hsu, W.L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxidative Med. Cell. Longev. 2018, 2018, 5919237. [Google Scholar] [CrossRef] [PubMed]
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 66. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Maximov, O.B.; Mischenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A.A. Composition Comprising di-and Trisodium Salts of Echinochrome for Treating Ocular Conditions. European Patent 1121929, 3 November 2004. [Google Scholar]
- Elyakov, G.B.; Maximov, O.B.; Mischenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A.A. Drug preparation “Histochrome” for treating acute myocardial infarction and ischemic heart diseases. European Patent 1121930, 14 November 2007. [Google Scholar]
- Olcott, H.S.; Einset, E. A weighing method for measuring the induction period of marine and other oils. J. Am. Oil Chem. Soc. 1958, 35, 161–162. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Slavova-Kazakova, A.K.; Angelova, S.E.; Kumar, P.; Malhotra, S.; Singh, B.K.; Saso, L.; Prasad, A.K.; Parmar, V.S. Protective effects of new antioxidant compositions of 4-methylcoumarins and related compounds with DL-α-tocopherol and L-ascorbic acid. J. Sci. Food Agric. 2018, 98, 3784–3794. [Google Scholar] [CrossRef]
- Stonik, V.A.; Gusev, E.I.; Martynov, M.Yu.; Guseva, M.R.; Shchukin, I.A.; Agafonova, I.G.; Mishchenko, N.P.; Fedoreev, A.S. New medications for treatment of hemorrhagic stroke. High-resolution MRI in evaluation of histochrome in experimental hemorrhagic stroke. Dokl. Boil. Sci. 2005, 405, 421–423. [Google Scholar] [CrossRef]
- Uchide, N.; Toyoda, H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef] [PubMed]
- Torky, Z.A.; Hossain, M.M. Pharmacological evaluation of the hibiscus herbal extract against herpes simples virus-type 1 as an antiviral drug in vitro. Int. J. Virol. 2017, 13, 68–79. [Google Scholar] [CrossRef]
- Garrett, R.; Romanos, M.T.V.; Borges, R.M.; Santos, M.G.; Rocha, L.; Silva, A.J.R.D. Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Rev. Bras. Farmacogn. 2012, 22, 306–313. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar] [PubMed]
- Li, D.; Baert, L.; Xia, M.; Zhong, W.; Jiang, X.; Uyttendaele, M. Effects of a variety of food extracts and juices on the specific binding ability of norovirus GII.4 P particles. J. Food Protect. 2012, 75, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Leonova, G.N.; Maystrovskaya, O.S.; Kondratov, I.G.; Takashima, I.; Belikov, S. The nature of replication of tick-borne encephalitis virus strains isolated from residents of the Russian Far East with inapparent and clinical forms of infection. Virus Res. 2014, 189, 34–42. [Google Scholar] [CrossRef]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. Antioxidant activity of polyphenols from the Far East yew-sprouted plant. Pharm. Chem. J. 2007, 41, 29–34. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Kalinovsky, A.I.; Menchinskaya, E.S.; Pislyagin, E.A.; Dmitrenok, P.S. The Influence on LPS-Induced ROS Formation in Macrophages of Capelloside A, a New Steroid Glycoside from the Starfish Ogmaster Capella. Nat. Prod. Commun. 2015, 10, 1937–1940. [Google Scholar]
- Matsuda, M.; Shigeta, S.; Okutani, K. Antiviral activities of marine Pseudomonas polysaccharides and their oversulfated derivatives. Mar. Biotechnol. 1999, 1, 68–73. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef] [PubMed]


| Antioxidants and their Compositions | Δτ, h | The Efficiency of the Composition Compared to Ech |
|---|---|---|
| Ech | 100 ± 5 | - |
| Asc | 24 ± 3 | - |
| Toc | 125 ± 7 | - |
| Ech + Asc (1:1) | 69 ± 4 | No effect |
| Ech + Toc (1:1) | 201 ± 8 * | Synergism 8% |
| Asc + Toc (2:1) | 195 ± 7 * | Synergism 7% |
| Ech + Asc + Toc (5:5:1) | 223 ± 10 ** | Synergism 40% |
| Control-linetol | 24 ± 2 |
| Formulation | TBEV | HSV-1 | ||||
|---|---|---|---|---|---|---|
| CC50 (µg/mL) | IC50 (µg/mL) | SI | CC50 (µg/mL) | IC50 (µg/mL) | SI | |
| Ech + Asc + Toc (5:5:1) | 57.9 ± 2.3 * | 12.6 ± 1.5 ** | 4.8 ± 0.5 ** | 66.7 ± 3.2 * | 11.2 ± 1.2 ** | 6.0 ± 0.6 ** |
| Ech | 54.4 ± 1.8 * | 21.8 ± 2.6 * | 2.5 ± 0.2 * | 60.5 ± 3.1 * | 18.8 ± 2.1 * | 3.2 ± 0.3 * |
| Asc + Toc (5:1) | 521.7 ± 5.3 | 1304 ± 145 | 0.4 ± 0.1 | 530.9 ± 9.4 | 885 ± 97 | 0.6 ± 0.1 |
| Ribavirin | 2010 ± 180 | 30.5 ± 4.6 | 66.0 ± 5.4 | |||
| Acyclovir | 1470 ± 160 | 10.8 ± 1.2 | 133.6 ± 12.0 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. https://doi.org/10.3390/md16120509
Fedoreyev SA, Krylova NV, Mishchenko NP, Vasileva EA, Pislyagin EA, Iunikhina OV, Lavrov VF, Svitich OA, Ebralidze LK, Leonova GN. Antiviral and Antioxidant Properties of Echinochrome A. Marine Drugs. 2018; 16(12):509. https://doi.org/10.3390/md16120509
Chicago/Turabian StyleFedoreyev, Sergey A., Natalia V. Krylova, Natalia P. Mishchenko, Elena A. Vasileva, Evgeny A. Pislyagin, Olga V. Iunikhina, Vyacheslav F. Lavrov, Oksana A. Svitich, Linna K. Ebralidze, and Galina N. Leonova. 2018. "Antiviral and Antioxidant Properties of Echinochrome A" Marine Drugs 16, no. 12: 509. https://doi.org/10.3390/md16120509
APA StyleFedoreyev, S. A., Krylova, N. V., Mishchenko, N. P., Vasileva, E. A., Pislyagin, E. A., Iunikhina, O. V., Lavrov, V. F., Svitich, O. A., Ebralidze, L. K., & Leonova, G. N. (2018). Antiviral and Antioxidant Properties of Echinochrome A. Marine Drugs, 16(12), 509. https://doi.org/10.3390/md16120509