Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1
Abstract
:1. Introduction
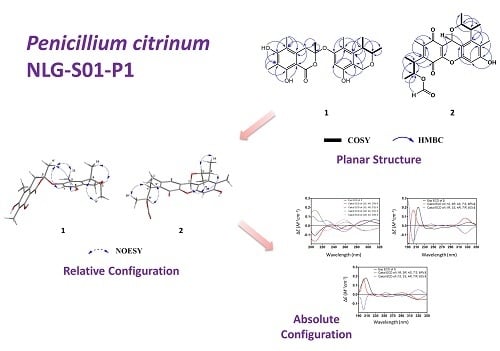
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. ECD Calculation
3.5. Antibacterial Assay
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, B.R.; Capon, R.J.; Lacey, E.; Tennant, S.; Gill, J.H. Citrinin revisited: From monomers to dimers and beyond. Org. Biomol. Chem. 2006, 4, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Kitabatake, N.; Trivedi, A.B.; Doi, E. Thermal Decomposition and Detoxification of Citrinin under Various Moisture Conditions. J. Agric. Food Chem. 1991, 39, 2240–2244. [Google Scholar] [CrossRef]
- Trivedi, A.B.; Doi, E.; Kitabatake, N. Toxic Compounds Formed on Prolonged Heating of Citrinin under Watery Conditions. J. Food Sci. 1993, 58, 229–232. [Google Scholar] [CrossRef]
- Hirota, M.; Mehta, A.; Yoneyama, K.; Kitabatake, N. A Major Decomposition Product, Citrinin H2, from Citrinin on Heating with Moisture. Biosci. Biotechnol. Biochem. 2002, 66, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentrivedi, A.; Hirota, M.; Doi, E.; Kitabatake, N. Formation of a New Toxic Compound, Citrinin H1, from Citrinin on Mild Heating in Water. J. Chem. Soc. Perkin Trans. 1 1993, 2167–2171. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.W.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; de Oliveira Santos, J.V.; de Alencar, M.V.; Júnior, A.L.; Paz, M.F.; de Brito, M.D.; e Sousa, J.M.; et al. A Comprehensive Review on Biological Properties of Citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Wakana, D.; Hosoe, T.; Itabashi, T.; Okada, K.; Takaki, G.M.D.; Yaguchi, T.; Fukushima, K.; Kawai, K. New Citrinin Derivatives Isolated from Penicillium citrinum. J. Nat. Med. 2006, 60, 279–284. [Google Scholar] [CrossRef]
- Ngan, N.T.; Quang, T.H.; Kim, K.W.; Kim, H.J.; Sohn, J.H.; Kang, D.G.; Lee, H.S.; Kim, Y.C.; Oh, H. Anti-inflammatory Effects of Secondary Metabolites Isolated from the Marine-derived Fungal Strain Penicillium sp. Sf-5629. Arch. Pharm. Res. 2017, 40, 328–337. [Google Scholar] [CrossRef]
- Xin, Z.H.; Tian, L.; Zhu, T.J.; Wang, W.L.; Du, L.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isocoumarin Derivatives from the Sea Squirt-derived Fungus Penicillium stoloniferum QY2-10 and the Halotolerant Fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007, 30, 816–819. [Google Scholar] [CrossRef]
- Shibano, M.; Naito, H.; Taniguchi, M.; Wang, N.H.; Baba, K. Two Isocoumarins from Pleurospermum angelicoides. Chem. Pharm. Bull. 2006, 54, 717–718. [Google Scholar] [CrossRef]
- Holler, U.; Konig, G.M.; Wright, A.D. Three New Metabolites from Marine-derived Fungi of the Genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.L.; Zhao, Y.X.; Deng, Y.Y.; Dai, H.F. A New Cytotoxic Isocoumarin from Endophytic Fungus Penicillium sp. 091402 of the Mangrove Plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Lin, X.P.; Liu, J.; Kaliyaperumal, K.; Ai, W.; Ju, Z.R.; Yang, B.; Wang, J.; Yang, X.W.; Liu, Y. Ascomycotin A, a New Citromycetin Analogue Produced by Ascomycota sp. Ind19f07 Isolated from Deep Sea Sediment. Nat. Prod. Res. 2015, 29, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum psu-f51. Tetrahedron 2012, 68, 8245–8250. [Google Scholar] [CrossRef]
- Krohn, K.; Florke, U.; Rao, M.S.; Steingrover, K.; Aust, H.J.; Draeger, S.; Schulz, B. Metabolites from fungi 15. New Isocoumarins from an Endophytic Fungus Isolated from the Canadian Thistle Cirsium arvense. Nat. Prod. Lett. 2001, 15, 353–361. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Chhillar, A.K.; Gahlaut, A. Evaluation of Antibacterial Potential of Plant Extracts Using Resazurin Based Microtiter Dilution Assay. Int. J. Pharm. Pharm. Sci. 2013, 5, 372–376. [Google Scholar]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Low, A.L.; Mohamad, S.A.; Khong, H.Y.; Sufian, A.S.; Manshoor, N.; Takayama, H. Malaysianol B, an Oligostilbenoid Derivative from Dryobalanops lanceolata. Fitoterapia 2012, 83, 1569–1575. [Google Scholar] [CrossRef]
- Coban, A.Y. Rapid Determination of Methicillin Resistance among Staphylococcus aureus Clinical Isolates by Colorimetric Methods. J. Clin. Microbiol. 2012, 50, 2191–2193. [Google Scholar] [CrossRef]
- Han, S.B.; Shin, Y.J.; Hyon, J.Y.; Wee, W.R. Cytotoxicity of Voriconazole on Cultured Human Corneal Endothelial Cells. Antimicrob. Agents Chemother. 2011, 55, 4519–4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Li, D.; Peng, J.; Zhu, T.; Gu, Q.; Li, D. Penicitols A-C and Penixanacid A from the Mangrove-derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 2015, 78, 306–310. [Google Scholar] [CrossRef] [PubMed]





| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | |
| 1 | 58.9, CH2 | 4.54, d (15.4) | 61.3, CH | 5.29, s |
| 1 | 58.9, CH2 | 4.61, d (15.4) | ||
| 3 | 73.8, CH | 3.85, qd (6.54, 2.3) | 79.6, CH | 4.03, qd (6.5, 4.6) |
| 4 | 35.1, CH | 2.64, qd (6.87, 2.3) | 37.7, CH | 2.97, qd (7.4, 4.6) |
| 4a | 137.9, C | 140.6, C | ||
| 5 | 112.7, C | 120.1, C | ||
| 6 | 154.2, C | 156.1, C | ||
| 7 | 99.7, CH | 6.32, s | 99.6, CH | 6.61, s |
| 8 | 150.8, C | 144.9, C | ||
| 8a | 111.8, C | 111.1, C | ||
| 9 | 17.4, CH3 | 1.19, d (6.5) | 14.8, CH3 | 1.32, d (6.5) |
| 10 | 19.9, CH3 | 1.21, d (6.9) | 22.0, CH3 | 1.26, d (7.4) |
| 11 | 9.5, CH3 | 2.06, s | 10.0, CH3 | 2.17, s |
| 1′ | 170.2, C | 179.8, C | ||
| 2′ | 143.0, C | |||
| 3′ | 102.3, C | 142.3, C | ||
| 4′ | 36, CH2 | 3.09, d (18.0) | 184.8, C | |
| 4′ | 36, CH2 | 3.20, d (18.0) | ||
| 4′a | 134.5, C | |||
| 5′ | 113.4, C | 115.2, C | ||
| 6′ | 159.8, C | 149.0, C | ||
| 7′ | 100.5, C | 40.3, CH | 3.22, qd (7.3, 2.4) | |
| 8′ | 160, C | 72.3, CH | 5.48, qd (6.1,2.4) | |
| 8′a | 108.7, C | |||
| 9′ | 27.4, CH3 | 1.71, s | 20.5, CH3 | 1.34, d (7.3) |
| 10′ | 7.3, CH3 | 2.13, s | 18.3, CH3 | 1.39, d (6.1) |
| 11′ | 10.3, CH3 | 2.13, s | 11.4, CH3 | 2.14, s |
| 12′ | 160.5, CH | 7.97, s | ||
| OH-6 | 8.82, s | |||
| OH-8′ | 11.82, s | |||
| OH-8 | 7.98, s | |||
| OH-6′ | 7.82, s | |||
| Compounds | MIC (μg/mL) | IC50 (μM) | |||||
|---|---|---|---|---|---|---|---|
| MRSA 1 | MRSA 2 | VV | VC | VR | A549 | HeLa | |
| 1 | 7.8 ± 0.8 | 7.6 ± 0.5 | NA | 30.1 ± 1.2 | NA | 23.2 ± 1.2 | 4.1 ± 0.8 |
| 2 | 7.3 ± 0.8 | 7.8 ± 0.9 | NA | 32.7± 1.9 | NA | 45.2 ± 0.9 | 42.3 ± 0.6 |
| 3 | 15.3 ± 0.6 | 15.5 ± 0.8 | NA | 32.3 ± 0.3 | NA | 46.3 ± 0.7 | 35.6 ± 0.9 |
| 4 | 16.3 ± 0.5 | 32.2 ± 0.3 | NA | NA | NA | 23.1 ± 0.9 | 17.7 ± 0.3 |
| 5 | 15.2 ± 0.4 | 16.1 ± 0.3 | 16.6 ± 0.4 | 15.3 ± 0.4 | 32.3 ± 0.3 | 40.0 ± 0.3 | 42.2 ± 0.5 |
| 6 | NA | NA | NA | NA | NA | NA | NA |
| 7 | NA | NA | 32.4 ± 0.5 | NA | 33.3 ± 0.2 | NA | NA |
| 8 | NA | NA | NA | NA | NA | 25.1 ± 1.0 | 27.7 ± 0.2 |
| 9 | NA | NA | NA | NA | 33.1 ± 0.7 | 33.1 ± 0.9 | 16.7 ± 0.1 |
| 10 | NA | NA | 15.7 ± 0.6 | 15.6 ± 0.5 | 32.3 ± 0.3 | 42.0 ± 0.3 | 22.2 ± 0.4 |
| 11 | 33.6 ± 0.2 | 32.1 ± 0.1 | NA | NA | NA | 37.7 ± 0.3 | NA |
| 12 | 32.1 ± 0.4 | 32.4 ± 0.1 | 32.1 ± 0.5 | NA | NA | NA | NA |
| 13 | NA | NA | NA | NA | 32.7 ± 0.2 | NA | NA |
| erythromycin | NT | NT | 2.0 ± 0.0 | 7.7 ± 0.2 | 3.9 ± 0.1 | NT | NT |
| chloramphenicol | 7.6 ± 0.2 | 7.5 ± 0.1 | NT | NT | NT | NT | NT |
| doxorubicin | NT | NT | NT | NT | NT | 0.1 ± 0.0 | 0.5 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liao, Y.; Zhang, B.; Gao, M.; Ke, W.; Li, F.; Shao, Z. Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1. Mar. Drugs 2019, 17, 46. https://doi.org/10.3390/md17010046
Wang W, Liao Y, Zhang B, Gao M, Ke W, Li F, Shao Z. Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1. Marine Drugs. 2019; 17(1):46. https://doi.org/10.3390/md17010046
Chicago/Turabian StyleWang, Weiyi, Yanyan Liao, Beibei Zhang, Maolin Gao, Wenqian Ke, Fang Li, and Zongze Shao. 2019. "Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1" Marine Drugs 17, no. 1: 46. https://doi.org/10.3390/md17010046
APA StyleWang, W., Liao, Y., Zhang, B., Gao, M., Ke, W., Li, F., & Shao, Z. (2019). Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1. Marine Drugs, 17(1), 46. https://doi.org/10.3390/md17010046