Enzymes from Marine Polar Regions and Their Biotechnological Applications
Abstract
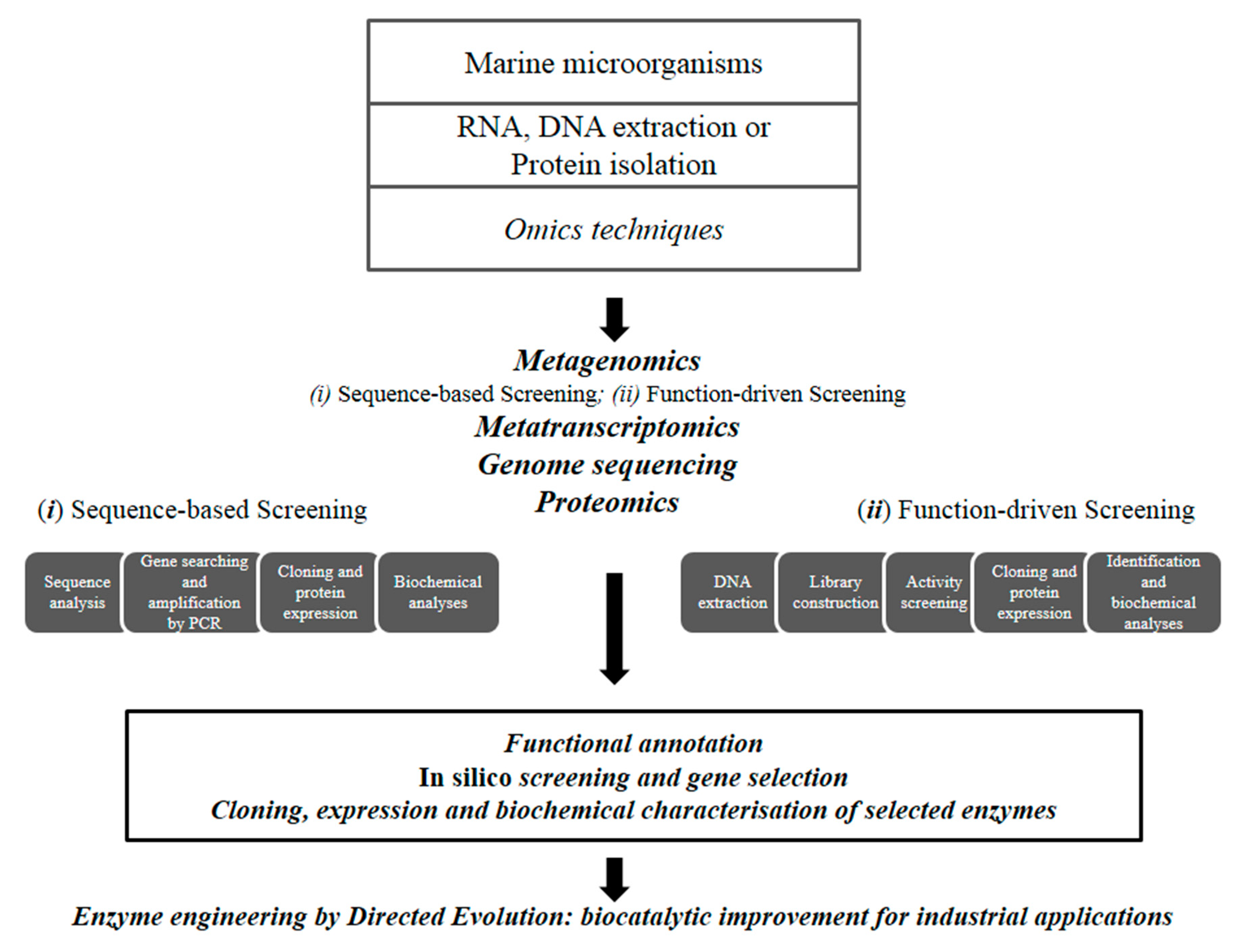
:1. Introduction
2. Methods for Enzyme Discovery and Engineering
Enzyme Engineering
3. Cold-Adapted Enzymes and Their Biotechnological Applications
3.1. Applications of Cold-Adapted Enzymes
- (1)
- They are cost-effective, e.g., lower amounts are required, due to higher catalytic efficiency at low temperature;
- (2)
- They can catalyze reactions at temperatures where competitive, undesirable chemical reactions are slowed down. This property is particularly relevant in the food industry, where deterioration and loss of thermolabile nutrients can occur at room temperature;
- (3)
- They catalyze the desired reactions at temperatures where bacterial contamination is reduced. There is a number of advantages in working at lower temperature (around 10–15 °C) than those currently used for large-scale industrial production;
- (4)
- Most cold-adapted enzymes can be inactivated by moderate heat due to their thermolability, avoiding chemical-based inactivation. A striking application of this property has been described for the design of live vaccines. Mesophilic pathogens were engineered for production of thermolabile homologs of essential enzymes, making them temperature-sensitive (TS). The engineered strains are inactivated at mammalian body temperatures, thus losing pathogenicity, but retaining their entire antigenic repertoire. Duplantis and colleagues [61] were able to entirely shift the lifestyle of Francisella (F. novicida), responsible for tularaemia disease in mice, by substituting its genes encoding essential enzymes with those identified in an Arctic bacterium. The authors applied the same approach to Salmonella enterica and the Gram-positive Mycobacterium [62]. The TS S. enterica strains were shown to be safe in research or diagnostic laboratories but were still capable of stimulating a protective immune response [63]. The TS strain of M. tuberculosis could be a safe research or diagnostic strain that is incapable of causing serious disease in humans while being identical to wild-type M. tuberculosis except for the TS phenotype [62].
3.2. Structural Features of Cold-Adapted Enzymes
3.3. Examples of Biotechnological Applications of Polar Enzymes
3.3.1. Glycoside Hydrolases
3.3.2. Proteases
3.3.3. Lipases and Esterases
3.3.4. Phosphatases
3.3.5. Other Hydrolases
3.3.6. Other Enzymes
4. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, M.; Coscolin, C.; Santiago, G.; Chow, J.; Stogios, P.J.; Bargiela, R.; Gertler, C.; Navarro-Fernandez, J.; Bollinger, A.; Thies, S.; et al. Determinants and Prediction of Esterase Substrate Promiscuity Patterns. ACS Chem. Biol. 2018, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- BCC. Global Markets for Enzymes in Industrial Applications; BCC Publishing: Wellesley, MA, USA, 2018. [Google Scholar]
- SmithersGroup. The Future of Marine Biotechnology for Industrial Applications to 2025; SmithersGroup: Akron, OH, USA, 2015. [Google Scholar]
- Di Donato, P.; Buono, A.; Poli, A.; Finore, I.; Abbamondi, G.R.; Nicolaus, B.; Lama, L. Exploring Marine Environments for the Identification of Extremophiles and Their Enzymes for Sustainable and Green Bioprocesses. Sustainability 2019, 11, 149. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Verde, C.; Giordano, D.; Bellas, C.M.; di Prisco, G.; Anesio, A.M. Chapter Four—Polar Marine Microorganisms and Climate Change. Adv. Microb. Physiol. 2016, 69, 187–215. [Google Scholar]
- Jakobsson, M. Hypsometry and volume of the Arctic Ocean and its constituent seas. Geochem. Geophys. Geosyst. 2002, 3, 1–18. [Google Scholar] [CrossRef]
- Scher, H.D.; Whittaker, J.M.; Williams, S.E.; Latimer, J.C.; Kordesch, W.E.; Delaney, M.L. Onset of Antarctic Circumpolar Current 30 million years ago as Tasmanian Gateway aligned with westerlies. Nature 2015, 523, 580–583. [Google Scholar] [CrossRef]
- Maldonado, A.; Bohoyo, F.; Galindo-Zaldívar, J.; Hernández-Molina, F.J.; Lobo, F.J.; Lodolo, E.; Martos, Y.M.; Pérez, L.F.; Schreider, A.A.; Somoza, L. A model of oceanic development by ridge jumping: Opening of the Scotia Sea. Glob. Planet. Chang. 2014, 123, 152–173. [Google Scholar] [CrossRef] [Green Version]
- Russell, N.J. Molecular adaptations in psychrophilic bacteria: Potential for biotechnological applications. Adv. Biochem. Eng. Biotechnol. 1998, 61, 1–21. [Google Scholar]
- Cavicchioli, R. On the concept of a psychrophile. ISME J. 2015, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Lu, X.L.; Liu, X.Y.; Gao, Y.; Hu, B.; Jiao, B.H.; Zheng, H. Bioactive natural products from the antarctic and arctic organisms. Mini Rev. Med. Chem. 2013, 13, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.L.; Zhao, F.C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef]
- Nunez-Montero, K.; Barrientos, L. Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics 2018, 7, 90. [Google Scholar] [CrossRef]
- Murray, A.E.; Grzymski, J.J. Diversity and genomics of Antarctic marine micro-organisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 2259–2271. [Google Scholar] [CrossRef]
- Margesin, R.; Miteva, V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 2011, 162, 346–361. [Google Scholar] [CrossRef]
- Casanueva, A.; Tuffin, M.; Cary, C.; Cowan, D.A. Molecular adaptations to psychrophily: The impact of ‘omic’ technologies. Trends Microbiol. 2010, 18, 374–381. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Siddiqui, K.S.; Andrews, D.; Sowers, K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 2002, 13, 253–261. [Google Scholar] [CrossRef]
- de Pascale, D.; De Santi, C.; Fu, J.; Landfald, B. The microbial diversity of Polar environments is a fertile ground for bioprospecting. Mar. Genom. 2012, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Kumar, V.; Ramteke, P.W. Chapter 47—Psychrophilic Enzymes: Potential Biocatalysts for Food Processing. Enzym. Food Biotechnol. 2019, 817–825. [Google Scholar] [CrossRef]
- Dhamankar, H.; Prather, K.L. Microbial chemical factories: Recent advances in pathway engineering for synthesis of value added chemicals. Curr. Opin. Struct. Biol. 2011, 21, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.; Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 2011, 13, 21–35. [Google Scholar] [CrossRef]
- Cavicchioli, R. Microbial ecology of Antarctic aquatic systems. Nat. Rev. Microbiol. 2015, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Miyazaki, K. Functional metagenomics for enzyme discovery: Challenges to efficient screening. Curr. Opin. Biotechnol. 2009, 20, 616–622. [Google Scholar] [CrossRef]
- Ferrer, M.; Beloqui, A.; Timmis, K.N.; Golyshin, P.N. Metagenomics for mining new genetic resources of microbial communities. J. Mol. Microbiol. Biotechnol. 2009, 16, 109–123. [Google Scholar] [CrossRef]
- Pena-Garcia, C.; Martinez-Martinez, M.; Reyes-Duarte, D.; Ferrer, M. High Throughput Screening of Esterases, Lipases and Phospholipases in Mutant and Metagenomic Libraries: A Review. Comb. Chem. High Throughput Screen. 2016, 19, 605–615. [Google Scholar] [CrossRef]
- Ferrer, M.; Mendez-Garcia, C.; Bargiela, R.; Chow, J.; Alonso, S.; Garcia-Moyano, A.; Bjerga, G.E.K.; Steen, I.H.; Schwabe, T.; Blom, C.; et al. Decoding the ocean’s microbiological secrets for marine enzyme biodiscovery. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Zallot, R.; Oberg, N.O.; Gerlt, J.A. ‘Democratized’ genomic enzymology web tools for functional assignment. Curr. Opin. Chem. Biol. 2018, 47, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Behrens, G.A.; Hummel, A.; Padhi, S.K.; Schätzle, S.; Bornscheuer, U.T. Discovery and Protein Engineering of Biocatalysts for Organic Synthesis. Adv. Synth. Catal. 2011, 353, 1615–4150. [Google Scholar] [CrossRef]
- Lauritano, C.; Ianora, A. Overview of Recent EU-Funded Projects. In Grand Challenges in Marine Biotechnology; Springer: Berlin, Germany, 2018; pp. 425–449. [Google Scholar]
- Available online: http://www.inmare-h2020.eu/ (accessed on 15 July 2019).
- Popovic, A.; Hai, T.; Tchigvintsev, A.; Hajighasemi, M.; Nocek, B.; Khusnutdinova, A.N.; Brown, G.; Glinos, J.; Flick, R.; Skarina, T.; et al. Activity screening of environmental metagenomic libraries reveals novel carboxylesterase families. Sci. Rep. 2017, 7, 44103. [Google Scholar] [CrossRef] [PubMed]
- Bastard, K.; Smith, A.A.; Vergne-Vaxelaire, C.; Perret, A.; Zaparucha, A.; De Melo-Minardi, R.; Mariage, A.; Boutard, M.; Debard, A.; Lechaplais, C.; et al. Revealing the hidden functional diversity of an enzyme family. Nat. Chem. Biol. 2014, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Chistoserdova, L. Is metagenomics resolving identification of functions in microbial communities? Microb. Biotechnol. 2014, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Martinez-Martinez, M.; Bargiela, R.; Streit, W.R.; Golyshina, O.V.; Golyshin, P.N. Estimating the success of enzyme bioprospecting through metagenomics: Current status and future trends. Microb. Biotechnol. 2016, 9, 22–34. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, P.A. Transcriptomics in the RNA-seq era. Curr. Opin. Chem. Biol. 2013, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sturmberger, L.; Wallace, P.W.; Glieder, A.; Birner-Gruenberger, R. Synergism of proteomics and mRNA sequencing for enzyme discovery. J. Biotechnol. 2016, 235, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Wright, A.T.; Kozarich, J.W. Activity-based protein profiling: From enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. [Google Scholar] [CrossRef]
- Schittmayer, M.; Birner-Gruenberger, R. Lipolytic proteomics. Mass Spectrom. Rev. 2012, 31, 570–582. [Google Scholar] [CrossRef]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going Green and Cold: Biosurfactants from Low-Temperature Environments to Biotechnology Applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.Q.; Jiang, X.R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Longwell, C.K.; Labanieh, L.; Cochran, J.R. High-throughput screening technologies for enzyme engineering. Curr. Opin. Biotechnol. 2017, 48, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, J. Highly engineered biocatalysts for efficient small molecule pharmaceutical synthesis. Curr. Opin. Biotechnol. 2016, 42, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.L.; Rusli, R.A.; Ollis, D.L. Directed Evolution of Enzymes for Industrial Biocatalysis. ChemBioChem 2016, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tyzack, J.D.; Furnham, N.; Sillitoe, I.; Orengo, C.M.; Thornton, J.M. Understanding enzyme function evolution from a computational perspective. Curr. Opin. Struct. Biol. 2017, 47, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rocha, C.G.; Gamble, C.G.; Lonsdale, R.; Li, A.; Nett, N.; Hoebenreich, S.; Lingnau, J.B.; Wirtz, C.; Fares, C.; Hinrichs, H.; et al. P450-Catalyzed Regio- and Diastereoselective Steroid Hydroxylation: Efficient Directed Evolution Enabled by Mutability Landscaping. ACS Catal. 2018, 8, 3395–3410. [Google Scholar] [CrossRef] [Green Version]
- Agostini, F.; Völler, J.-S.; Koksch, B.; Acevedo-Rocha, C.G.; Kubyshkin, V.; Budisa, N. Biocatalysis with Unnatural Amino Acids: Enzymology Meets Xenobiology. Angew. Chem. Int. Ed. 2017, 56, 9680–9703. [Google Scholar] [CrossRef]
- Bernath, K.; Hai, M.; Mastrobattista, E.; Griffiths, A.D.; Magdassi, S.; Tawfik, D.S. In vitro compartmentalization by double emulsions: Sorting and gene enrichment by fluorescence activated cell sorting. Anal. Biochem. 2004, 325, 151–157. [Google Scholar] [CrossRef]
- Becker, S.; Hobenreich, H.; Vogel, A.; Knorr, J.; Wilhelm, S.; Rosenau, F.; Jaeger, K.E.; Reetz, M.T.; Kolmar, H. Single-cell high-throughput screening to identify enantioselective hydrolytic enzymes. Angew. Chem. Int. Ed. Engl. 2008, 47, 5085–5088. [Google Scholar] [CrossRef]
- Fernandez-Alvaro, E.; Snajdrova, R.; Jochens, H.; Davids, T.; Bottcher, D.; Bornscheuer, U.T. A combination of in vivo selection and cell sorting for the identification of enantioselective biocatalysts. Angew. Chem. Int. Ed. Engl. 2011, 50, 8584–8587. [Google Scholar] [CrossRef]
- Fox, R.J.; Davis, S.C.; Mundorff, E.C.; Newman, L.M.; Gavrilovic, V.; Ma, S.K.; Chung, L.M.; Ching, C.; Tam, S.; Muley, S.; et al. Improving catalytic function by ProSAR-driven enzyme evolution. Nat. Biotechnol. 2007, 25, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Coker, J.A. Extremophiles and biotechnology: Current uses and prospects. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Gerlt, J.A.; Allen, K.N.; Almo, S.C.; Armstrong, R.N.; Babbitt, P.C.; Cronan, J.E.; Dunaway-Mariano, D.; Imker, H.J.; Jacobson, M.P.; Minor, W.; et al. The Enzyme Function Initiative. Biochemistry 2011, 50, 9950–9962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, P.N.; Howard, R.M.; Kumar, R.; Thompson, M.P.; Truppo, M.D.; Turner, N.J. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2018, 2, 409–421. [Google Scholar] [CrossRef]
- Liszka, M.J.; Clark, M.E.; Schneider, E.; Clark, D.S. Nature versus nurture: Developing enzymes that function under extreme conditions. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Duplantis, B.N.; Osusky, M.; Schmerk, C.L.; Ross, D.R.; Bosio, C.M.; Nano, F.E. Essential genes from Arctic bacteria used to construct stable, temperature-sensitive bacterial vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 13456–13460. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.T.; Nano, F.E. Stable, temperature-sensitive recombinant strain of Mycobacterium smegmatis generated through the substitution of a psychrophilic ligA gene. FEMS Microbiol. Lett. 2015, 362, fnv152. [Google Scholar] [CrossRef] [PubMed]
- Duplantis, B.N.; Puckett, S.M.; Rosey, E.L.; Ameiss, K.A.; Hartman, A.D.; Pearce, S.C.; Nano, F.E. Temperature-Sensitive Salmonella enterica Serovar Enteritidis PT13a Expressing Essential Proteins of Psychrophilic Bacteria. Appl. Environ. Microbiol. 2015, 81, 6757–6766. [Google Scholar] [CrossRef]
- Santiago, M.; Ramirez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, Molecular Mechanisms, and Industrial Applications of Cold-Active Enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Miteva, V.; Lantz, S.; Brenchley, J. Characterization of a cryptic plasmid from a Greenland ice core Arthrobacter isolate and construction of a shuttle vector that replicates in psychrophilic high G+C Gram-positive recipients. Extremophiles 2008, 12, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Chernikova, T.N.; Yakimov, M.M.; Golyshin, P.N.; Timmis, K.N. Chaperonins govern growth of Escherichia coli at low temperatures. Nat. Biotechnol. 2003, 21, 1266–1267. [Google Scholar] [CrossRef] [PubMed]
- Georlette, D.; Blaise, V.; Collins, T.; D’Amico, S.; Gratia, E.; Hoyoux, A.; Marx, J.C.; Sonan, G.; Feller, G.; Gerday, C. Some like it cold: Biocatalysis at low temperatures. FEMS Microbiol. Rev. 2004, 28, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Laye, V.J.; Karan, R.; Kim, J.M.; Pecher, W.T.; DasSarma, P.; DasSarma, S. Key amino acid residues conferring enhanced enzyme activity at cold temperatures in an Antarctic polyextremophilic beta-galactosidase. Proc. Natl. Acad. Sci. USA 2017, 114, 12530–12535. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Coppola, D.; Russo, R.; Tinajero-Trejo, M.; di Prisco, G.; Lauro, F.; Ascenzi, P.; Verde, C. The globins of cold-adapted Pseudoalteromonas haloplanktis TAC125: From the structure to the physiological functions. Adv. Microb. Physiol. 2013, 63, 329–389. [Google Scholar] [CrossRef]
- Kube, M.; Chernikova, T.N.; Al-Ramahi, Y.; Beloqui, A.; Lopez-Cortez, N.; Guazzaroni, M.E.; Heipieper, H.J.; Klages, S.; Kotsyurbenko, O.R.; Langer, I.; et al. Genome sequence and functional genomic analysis of the oil-degrading bacterium Oleispira antarctica. Nat. Commun. 2013, 4, 2156. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, S.; Claverie, P.; Collins, T.; Georlette, D.; Gratia, E.; Hoyoux, A.; Meuwis, M.A.; Feller, G.; Gerday, C. Molecular basis of cold adaptation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 917–925. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Leiros, H.K.; Pey, A.L.; Innselset, M.; Moe, E.; Leiros, I.; Steen, I.H.; Martinez, A. Structure of phenylalanine hydroxylase from Colwellia psychrerythraea 34H, a monomeric cold active enzyme with local flexibility around the active site and high overall stability. J. Biol. Chem. 2007, 282, 21973–21986. [Google Scholar] [CrossRef] [PubMed]
- Sonan, G.K.; Receveur-Brechot, V.; Duez, C.; Aghajari, N.; Czjzek, M.; Haser, R.; Gerday, C. The linker region plays a key role in the adaptation to cold of the cellulase from an Antarctic bacterium. Biochem. J. 2007, 407, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Mouri, Y.; Takada, Y. Contribution of Three Different Regions of Isocitrate Dehydrogenases from Psychrophilic and Psychrotolerant Bacteria to Their Thermal Properties. Curr. Microbiol. 2018, 75, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.W.; Liao, M.L.; Meng, X.L.; Somero, G.N. Structural flexibility and protein adaptation to temperature: Molecular dynamics analysis of malate dehydrogenases of marine molluscs. Proc. Natl. Acad. Sci. USA 2018, 115, 1274–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischedda, A.; Ramasamy, K.P.; Mangiagalli, M.; Chiappori, F.; Milanesi, L.; Miceli, C.; Pucciarelli, S.; Lotti, M. Antarctic marine ciliates under stress: Superoxide dismutases from the psychrophilic Euplotes focardii are cold-active yet heat tolerant enzymes. Sci. Rep. 2018, 8, 14721. [Google Scholar] [CrossRef] [PubMed]
- Tosco, A.; Birolo, L.; Madonna, S.; Lolli, G.; Sannia, G.; Marino, G. GroEL from the psychrophilic bacterium Pseudoalteromonas haloplanktis TAC 125: Molecular characterization and gene cloning. Extremophiles 2003, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Satyanarayana, T. Biotechnology of cold-active proteases. Biology 2013, 2, 755–783. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.C.; Collins, T.; D’Amico, S.; Feller, G.; Gerday, C. Cold-adapted enzymes from marine Antarctic microorganisms. Mar. Biotechnol. 2007, 9, 293–304. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Charlton, T.; Ertan, H.; Mohd Omar, S.; Siddiqui, K.S.; Williams, T.J. Biotechnological uses of enzymes from psychrophiles. Microb. Biotechnol. 2011, 4, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.W.F.; Dos Santos, J.A.; Vianna, M.V.; Vieira, J.M.F.; Mallagutti, V.H.; Inforsato, F.J.; Wentzel, L.C.P.; Lario, L.D.; Rodrigues, A.; Pagnocca, F.C.; et al. Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit. Rev. Biotechnol. 2018, 38, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Cieslinski, H.; Kur, J.; Bialkowska, A.; Baran, I.; Makowski, K.; Turkiewicz, M. Cloning, expression, and purification of a recombinant cold-adapted beta-galactosidase from antarctic bacterium Pseudoalteromonas sp. 22b. Protein Expr. Purif. 2005, 39, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, M.; Kur, J.; Bialkowska, A.; Cieslinski, H.; Kalinowska, H.; Bielecki, S. Antarctic marine bacterium Pseudoalteromonas sp. 22b as a source of cold-adapted beta-galactosidase. Biomol. Eng. 2003, 20, 317–324. [Google Scholar] [CrossRef]
- Hoyoux, A.; Jennes, I.; Dubois, P.; Genicot, S.; Dubail, F.; Francois, J.M.; Baise, E.; Feller, G.; Gerday, C. Cold-adapted beta-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl. Environ. Microbiol. 2001, 67, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Gerday, C.; Hoyoux, A.; Marie Francois, J.M.; Dubois, P.; Baise, E.; Jennes, I.; Genicot, S. Cold-Active Beta Galactosidase, the Process for its Preparation and the Use Thereof. U.S. Patent WO2001004276A1, 9 July 1999. [Google Scholar]
- Song, C.; Chi, Z.; Li, J.; Wang, X. beta-Galactosidase production by the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica and lactose hydrolysis. Bioprocess. Biosyst. Eng. 2010, 33, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Alikkunju, A.P.; Sainjan, N.; Silvester, R.; Joseph, A.; Rahiman, M.; Antony, A.C.; Kumaran, R.C.; Hatha, M. Screening and Characterization of Cold-Active beta-Galactosidase Producing Psychrotrophic Enterobacter ludwigii from the Sediments of Arctic Fjord. Appl. Biochem. Biotechnol. 2016, 180, 477–490. [Google Scholar] [CrossRef]
- Schmidt, M.; Stougaard, P. Identification, cloning and expression of a cold-active beta-galactosidase from a novel Arctic bacterium, Alkalilactibacillus ikkense. Environ. Technol. 2010, 31, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kan, G.; Ren, X.; Yu, G.; Shi, C.; Xie, Q.; Wen, H.; Betenbaugh, M. Molecular Cloning and Characterization of a Novel alpha-Amylase from Antarctic Sea Ice Bacterium Pseudoalteromonas sp. M175 and Its Primary Application in Detergent. BioMed Res. Int. 2018, 2018, 3258383. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.; Azhar, M.A.; Shamsir, M.S.; Rabu, A.; Murad, A.M.; Mahadi, N.M.; Illias, R.M. Sequence and structural investigation of a novel psychrophilic alpha-amylase from Glaciozyma antarctica PI12 for cold-adaptation analysis. J. Mol. Model. 2013, 19, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Vardhan Reddy, P.V.; Shiva Nageswara Rao, S.S.; Pratibha, M.S.; Sailaja, B.; Kavya, B.; Manorama, R.R.; Singh, S.M.; Radha Srinivas, T.N.; Shivaji, S. Bacterial diversity and bioprospecting for cold-active enzymes from culturable bacteria associated with sediment from a melt water stream of Midtre Lov´enbreen glacier, an Arctic glacier. Res. Microbiol. 2009, 160, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Chessa, J.P.; Feller, G.; Gerday, C. Purification and characterization of the heat-labile alpha-amylase secreted by the psychrophilic bacterium TAC 240B. Can. J. Microbiol. 1999, 45, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; D’Amico, D.; Gerday, C. Thermodynamic stability of a cold-active alpha-amylase from the Antarctic bacterium Alteromonas haloplanctis. Biochemistry 1999, 38, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.; Roohi, J.M.A.; Ramteke, P.W. An Overview of Cold-active Microbial α-amylase: Adaptation Strategies and Biotechnological Potentials. Biotechnol. Biotechnol. Equip. 2011, 10, 246–258. [Google Scholar]
- Del-Cid, A.; Ubilla, P.; Ravanal, M.C.; Medina, E.; Vaca, I.; Levican, G.; Eyzaguirre, J.; Chavez, R. Cold-active xylanase produced by fungi associated with Antarctic marine sponges. Appl. Biochem. Biotechnol. 2014, 172, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Humphry, D.R.; George, A.; Black, G.W.; Cummings, S.P. Flavobacterium frigidarium sp. nov., an aerobic, psychrophilic, xylanolytic and laminarinolytic bacterium from Antarctica. Int. J. Syst. Evol. Microbiol. 2001, 51, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Wintrode, P.L.; Grayling, R.A.; Rubingh, D.N.; Arnold, F.H. Directed evolution study of temperature adaptation in a psychrophilic enzyme. J. Mol. Biol. 2000, 297, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narinx, E.; Davail, S.; Feller, G.; Gerday, C. Nucleotide and derived amino acid sequence of the subtilisin from the antarctic psychrotroph Bacillus TA39. Biochim. Biophys. Acta 1992, 1131, 111–113. [Google Scholar] [CrossRef]
- Davail, S.; Feller, G.; Narinx, E.; Gerday, C. Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the antarctic psychrophile Bacillus TA41. J. Biol. Chem. 1994, 269, 17448–17453. [Google Scholar]
- Wang, Q.F.; Miao, J.L.; Hou, Y.H.; Ding, Y.; Wang, G.D.; Li, G.Y. Purification and characterization of an extracellular cold-active serine protease from the psychrophilic bacterium Colwellia sp. NJ341. Biotechnol. Lett. 2005, 27, 1195–1198. [Google Scholar] [CrossRef]
- Kulakova, L.; Galkin, A.; Kurihara, T.; Yoshimura, T.; Esaki, N. Cold-active serine alkaline protease from the psychrotrophic bacterium Shewanella strain ac10: Gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 1999, 65, 611–617. [Google Scholar]
- Lario, L.D.; Chaud, L.; Almeida, M.D.G.; Converti, A.; Duraes Sette, L.; Pessoa, A., Jr. Production, purification, and characterization of an extracellular acid protease from the marine Antarctic yeast Rhodotorula mucilaginosa L7. Fungal Biol. 2015, 119, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, J.P.; Rodriguez, V.; Saavedra, M.; Munoz, M.; Salazar, O.; Asenjo, J.A.; Andrews, B.A. Cloning, expression and decoding of the cold adaptation of a new widely represented thermolabile subtilisin-like protease. J. Appl. Microbiol. 2013, 114, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-F.; Hou, Y.-H.; Xu, Z.; Miao, J.-L.; Li, G.-Y. Purification and properties of an extracellular cold-active protease from the psychrophilic bacterium Pseudoalteromonas sp. NJ276. Biochem. Eng. J. 2008, 38, 362–368. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Pazgier, M.; Kalinowska, H.; Bielecki, S. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 2003, 7, 435–442. [Google Scholar] [CrossRef] [PubMed]
- de Pascale, D.; Giuliani, M.; De Santi, C.; Bergamasco, N.; Amoresano, A.; Carpentieri, A.; Parrilli, E.; Tutino, M.L. PhAP protease from Pseudoalteromonas haloplanktis TAC125: Gene cloning, recombinant production in E. coli and enzyme characterization. Polar Sci. 2010, 4, 285–294. [Google Scholar] [CrossRef]
- Huston, A.L.; Methe, B.; Deming, J.W. Purification, characterization, and sequencing of an extracellular cold-active aminopeptidase produced by marine psychrophile Colwellia psychrerythraea strain 34H. Appl. Environ. Microbiol. 2004, 70, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Huston, A.L.; Haeggstrom, J.Z.; Feller, G. Cold adaptation of enzymes: Structural, kinetic and microcalorimetric characterizations of an aminopeptidase from the Arctic psychrophile Colwellia psychrerythraea and of human leukotriene A(4) hydrolase. Biochim. Biophys. Acta 2008, 1784, 1865–1872. [Google Scholar] [CrossRef]
- Pereira, J.Q.; Ambrosini, A.; Passaglia, L.M.P.; Brandelli, A. A new cold-adapted serine peptidase from Antarctic Lysobacter sp. A03: Insights about enzyme activity at low temperatures. Int. J. Biol. Macromol. 2017, 103, 854–862. [Google Scholar] [CrossRef]
- Larsen, A.N.; Moe, E.; Helland, R.; Gjellesvik, D.R.; Willassen, N.P. Characterization of a recombinantly expressed proteinase K-like enzyme from a psychrotrophic Serratia sp. FEBS J. 2006, 273, 47–60. [Google Scholar] [CrossRef]
- Xie, B.B.; Bian, F.; Chen, X.L.; He, H.L.; Guo, J.; Gao, X.; Zeng, Y.X.; Chen, B.; Zhou, B.C.; Zhang, Y.Z. Cold adaptation of zinc metalloproteases in the thermolysin family from deep sea and arctic sea ice bacteria revealed by catalytic and structural properties and molecular dynamics: New insights into relationship between conformational flexibility and hydrogen bonding. J. Biol. Chem. 2009, 284, 9257–9269. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Gromek, E.; Kalinowska, H.; Zielińska, M. Biosynthesis and properties of an extracellular metalloprotease from the Antarctic marine bacterium Sphingomonas paucimobilis. J. Biotechnol. 1999, 70, 53–60. [Google Scholar] [CrossRef]
- Denner, E.B.; Mark, B.; Busse, H.J.; Turkiewicz, M.; Lubitz, W. Psychrobacter proteolyticus sp. nov., a psychrotrophic, halotolerant bacterium isolated from the Antarctic krill Euphausia superba Dana, excreting a cold-adapted metalloprotease. Syst. Appl. Microbiol. 2001, 24, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://arcticzymes.com/technology/proteinase/ (accessed on 15 July 2019).
- Wi, A.R.; Jeon, S.J.; Kim, S.; Park, H.J.; Kim, D.; Han, S.J.; Yim, J.H.; Kim, H.W. Characterization and a point mutational approach of a psychrophilic lipase from an arctic bacterium, Bacillus pumilus. Biotechnol. Lett. 2014, 36, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- de Pascale, D.; Cusano, A.M.; Autore, F.; Parrilli, E.; di Prisco, G.; Marino, G.; Tutino, M.L. The cold-active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family. Extremophiles 2008, 12, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Lee, J.H.; Kwon, M.H.; Song, H.E.; An, J.Y.; Eom, S.H.; Lee, S.G.; Kim, H.J. Purification, characterization and preliminary X-ray diffraction analysis of a cold-active lipase (CpsLip) from the psychrophilic bacterium Colwellia psychrerythraea 34H. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Irgens, R.L.; Gosink, J.J.; Staley, J.T. Polaromonas vacuolata gen. nov., sp. nov., a psychrophilic, marine, gas vacuolate bacterium from Antarctica. Int. J. Syst. Bacteriol. 1996, 46, 822–826. [Google Scholar] [CrossRef]
- Parra, L.P.; Reyes, F.; Acevedo, J.P.; Salazar, O.; Andrews, B.A.; Asenjo, J.A. Cloning and fusion expression of a cold-active lipase from marine Antarctic origin. Enzym. Microb. Technol. 2008, 42, 371–377. [Google Scholar] [CrossRef]
- Xuezheng, L.; Shuoshuo, C.; Guoying, X.; Shuai, W.; Ning, D.; Jihong, S. Cloning and heterologous expression of two cold-active lipases from the Antarctic bacterium Psychrobacter sp. G. Polar Res. 2010, 29, 421–429. [Google Scholar] [CrossRef]
- Parra, L.P.; Espina, G.; Devia, J.; Salazar, O.; Andrews, B.; Asenjo, J.A. Identification of lipase encoding genes from Antarctic seawater bacteria using degenerate primers: Expression of a cold-active lipase with high specific activity. Enzym. Microb. Technol. 2015, 68, 56–61. [Google Scholar] [CrossRef]
- Feller, G.; Thiry, M.; Arpigny, J.L.; Gerday, C. Cloning and expression in Escherichia coli of three lipase-encoding genes from the psychrotrophic antarctic strain Moraxella TA144. Gene 1991, 102, 111–115. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Zeng, R. Cloning, expression, and characterization of a cold-adapted lipase gene from an antarctic deep-sea psychrotrophic bacterium, Psychrobacter sp 7195. J. Microbiol. Biotechnol. 2007, 17, 604–610. [Google Scholar] [PubMed]
- Yang, X.; Lin, X.; Fan, T.; Bian, J.; Huang, X. Cloning and expression of lipP, a gene encoding a cold-adapted lipase from Moritella sp.2-5-10-1. Curr. Microbiol. 2008, 56, 194–198. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Michaud, L.; de Pascale, D.; De Domenico, M.; di Prisco, G.; Fani, R.; Bruni, V. Lipolytic activity of Antarctic cold-adapted marine bacteria (Terra Nova Bay, Ross Sea). J. Appl. Microbiol. 2006, 101, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, X.T.; Liu, J.W. Purification and characterization of a novel cold-adapted phytase from Rhodotorula mucilaginosa strain JMUY14 isolated from Antarctic. J. Basic Microbiol. 2015, 55, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Al Khudary, R.; Venkatachalam, R.; Katzer, M.; Elleuche, S.; Antranikian, G. A cold-adapted esterase of a novel marine isolate, Pseudoalteromonas arctica: Gene cloning, enzyme purification and characterization. Extremophiles 2010, 14, 273–285. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Leiros, H.K.; Di Scala, A.; de Pascale, D.; Altermark, B.; Willassen, N.P. Biochemical characterization and structural analysis of a new cold-active and salt-tolerant esterase from the marine bacterium Thalassospira sp. Extremophiles 2016, 20, 323–336. [Google Scholar] [CrossRef]
- Lemak, S.; Tchigvintsev, A.; Petit, P.; Flick, R.; Singer, A.U.; Brown, G.; Evdokimova, E.; Egorova, O.; Gonzalez, C.F.; Chernikova, T.N.; et al. Structure and activity of the cold-active and anion-activated carboxyl esterase OLEI01171 from the oil-degrading marine bacterium Oleispira antarctica. Biochem. J. 2012, 445, 193–203. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, S.; Aurilia, V.; Marabotti, A.; Gonnelli, M.; Strambini, G. Structure and dynamics of cold-adapted enzymes as investigated by phosphorescence spectroscopy and molecular dynamics studies. 2. The case of an esterase from Pseudoalteromonas haloplanktis. J. Phys. Chem. B 2009, 113, 13171–13178. [Google Scholar] [CrossRef]
- Aurilia, V.; Parracino, A.; Saviano, M.; Rossi, M.; D’Auria, S. The psychrophilic bacterium Pseudoalteromonas halosplanktis TAC125 possesses a gene coding for a cold-adapted feruloyl esterase activity that shares homology with esterase enzymes from gamma-proteobacteria and yeast. Gene 2007, 397, 51–57. [Google Scholar] [CrossRef]
- Cieslinski, H.; Bialkowska, A.M.; Dlugolecka, A.; Daroch, M.; Tkaczuk, K.L.; Kalinowska, H.; Kur, J.; Turkiewicz, M. A cold-adapted esterase from psychrotrophic Pseudoalteromas sp. strain 643A. Arch. Microbiol. 2007, 188, 27–36. [Google Scholar] [CrossRef]
- De Santi, C.; Altermark, B.; Pierechod, M.M.; Ambrosino, L.; de Pascale, D.; Willassen, N.P. Characterization of a cold-active and salt tolerant esterase identified by functional screening of Arctic metagenomic libraries. BMC Biochem. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, J.T.; Kang, S.G.; Lee, J.H.; Kim, S.J. Characterization and its potential application of two esterases derived from the arctic sediment metagenome. Mar. Biotechnol. 2009, 11, 307–316. [Google Scholar] [CrossRef]
- Kang, J.H.; Woo, J.H.; Kang, S.G.; Hwang, Y.O.; Kim, S.J. A cold-adapted epoxide hydrolase from a strict marine bacterium, Sphingophyxis alaskensis. J. Microbiol. Biotechnol. 2008, 18, 1445–1452. [Google Scholar] [PubMed]
- Alterio, V.; Aurilia, V.; Romanelli, A.; Parracino, A.; Saviano, M.; D’Auria, S.; De Simone, G. Crystal structure of an S-formylglutathione hydrolase from Pseudoalteromonas haloplanktis TAC125. Biopolymers 2010, 93, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Yoo, W.; Park, S.-H.; Le, L.T.H.L.; Jeong, C.-S.; Ryu, B.H.; Shin, S.C.; Kim, H.-W.; Park, H.; Kim, K.K.; et al. Structural and functional characterization of a novel cold-active S-formylglutathione hydrolase (SfSFGH) homolog from Shewanella frigidimarina, a psychrophilic bacterium. Microb. Cell Fact. 2019, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Ramya, L.N.; Pulicherla, K.K. Molecular insights into cold active polygalacturonase enzyme for its potential application in food processing. J. Food Sci. Technol. 2015, 52, 5484–5496. [Google Scholar] [CrossRef] [PubMed]
- Elleuche, S.; Qoura, F.M.; Lorenz, U.; Rehn, T.; Brück, T.; Antranikian, G. Cloning, expression and characterization of the recombinant cold-active type-I pullulanase from Shewanella arctica. J. Mol. Catal. B Enzym. 2015, 116, 70–77. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Pazgier, M.; Donachie, S.; Kalinowska, H. Invertase and a-glucosidase production by the endemic Antarctic marine yeast Leucosporidium antarcticum. Pol. Polar Res. 2005, 26, 125–136. [Google Scholar]
- Violot, S.; Aghajari, N.; Czjzek, M.; Feller, G.; Sonan, G.K.; Gouet, P.; Gerday, C.; Haser, R.; Receveur-Brechot, V. Structure of a full length psychrophilic cellulase from Pseudoalteromonas haloplanktis revealed by X-ray diffraction and small angle X-ray scattering. J. Mol. Biol. 2005, 348, 1211–1224. [Google Scholar] [CrossRef]
- Lonhienne, T.; Zoidakis, J.; Vorgias, C.E.; Feller, G.; Gerday, C.; Bouriotis, V. Modular structure, local flexibility and cold-activity of a novel chitobiase from a psychrophilic Antarctic bacterium. J. Mol. Biol. 2001, 310, 291–297. [Google Scholar] [CrossRef]
- Rina, M.; Pozidis, C.; Mavromatis, K.; Tzanodaskalaki, M.; Kokkinidis, M.; Bouriotis, V. Alkaline phosphatase from the Antarctic strain TAB5. Properties and psychrophilic adaptations. Eur. J. Biochem. 2000, 267, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Koutsioulis, D.; Wang, E.; Tzanodaskalaki, M.; Nikiforaki, D.; Deli, A.; Feller, G.; Heikinheimo, P.; Bouriotis, V. Directed evolution on the cold adapted properties of TAB5 alkaline phosphatase. Protein Eng. Des. Sel. 2008, 21, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.neb.com/products/m0289-antarctic-phosphatase#Product%20Information (accessed on 15 July 2019).
- Tsuruta, H.; Mikami, B.; Higashi, T.; Aizono, Y. Crystal structure of cold-active alkaline phosphatase from the psychrophile Shewanella sp. Biosci. Biotechnol. Biochem. 2010, 74, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.takarabio.com/products/cloning/modifying-enzymes/nucleases/ cryonase-cold-active-nuclease (accessed on 15 July 2019).
- Available online: https://arcticzymes.com/technology/hl-exol/ (accessed on 15 July 2019).
- Wang, Y.; Hou, Y.; Nie, P.; Wang, Y.; Ren, X.; Wei, Q.; Wang, Q. A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206. Molecules 2019, 24, 2229. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://international.neb.com/products/m0372-antarctic-thermolabile-udg#Product%20 Information (accessed on 15 July 2019).
- Tsigos, I.; Velonia, K.; Smonou, I.; Bouriotis, V. Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. TAE123. Eur. J. Biochem. 1998, 254, 356–362. [Google Scholar] [CrossRef]
- Galkin, A.; Kulakova, L.; Ashida, H.; Sawa, Y.; Esaki, N. Cold-adapted alanine dehydrogenases from two antarctic bacterial strains: Gene cloning, protein characterization, and comparison with mesophilic and thermophilic counterparts. Appl. Environ. Microbiol. 1999, 65, 4014–4020. [Google Scholar]
- Wang, Y.; Hou, Y.; Wang, Y.; Zheng, L.; Xu, X.; Pan, K.; Li, R.; Wang, Q. A Novel Cold-Adapted Leucine Dehydrogenase from Antarctic Sea-Ice Bacterium Pseudoalteromonas sp. ANT178. Mar. Drugs 2018, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Yamamoto, N.; Shimoke, K.; Uesato, S.; Ikeuchi, T.; Fujioka, T. Purification, characterization, and overexpression of psychrophilic and thermolabile malate dehydrogenase of a novel antarctic psychrotolerant, Flavobacterium frigidimaris KUC-1. Biosci. Biotechnol. Biochem. 2005, 69, 2146–2154. [Google Scholar] [CrossRef]
- Fedoy, A.E.; Yang, N.; Martinez, A.; Leiros, H.K.; Steen, I.H. Structural and functional properties of isocitrate dehydrogenase from the psychrophilic bacterium Desulfotalea psychrophila reveal a cold-active enzyme with an unusual high thermal stability. J. Mol. Biol. 2007, 372, 130–149. [Google Scholar] [CrossRef]
- Yoneda, K.; Sakuraba, H.; Muraoka, I.; Oikawa, T.; Ohshima, T. Crystal structure of UDP-galactose 4-epimerase-like L-threonine dehydrogenase belonging to the intermediate short-chain dehydrogenase-reductase superfamily. FEBS J. 2010, 277, 5124–5132. [Google Scholar] [CrossRef]
- Merlino, A.; Russo Krauss, I.; Castellano, I.; De Vendittis, E.; Rossi, B.; Conte, M.; Vergara, A.; Sica, F. Structure and flexibility in cold-adapted iron superoxide dismutases: The case of the enzyme isolated from Pseudoalteromonas haloplanktis. J. Struct. Biol. 2010, 172, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jiang, Y.H.; Miao, J.L.; Wang, Q.F.; Zhang, B.T.; Li, G.Y. Purification and characterization of a cold-active iron superoxide dismutase from a Psychrophilic Bacterium, Marinomonas sp. NJ522. Biotechnol. Lett. 2006, 28, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Wang, Y.F.; Hou, Y.H.; Shi, Y.L.; Han, H.; Miao, M.; Wu, Y.Y.; Liu, Y.P.; Yue, X.N.; Li, Y.J. Cloning, expression and biochemical characterization of recombinant superoxide dismutase from Antarctic psychrophilic bacterium Pseudoalteromonas sp. ANT506. J. Basic Microbiol. 2016, 56, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Im, H.; Lee, K. Expression and Purification of Recombinant Superoxide Dismutase (PaSOD) from Psychromonas arctica in Escherichia coli. Bull. Korean Chem. Soc. 2011, 32, 2405–2409. [Google Scholar] [CrossRef]
- Kan, G.; Wen, H.; Wang, X.; Zhou, T.; Shi, C. Cloning and characterization of iron-superoxide dismutase in Antarctic yeast strain Rhodotorula mucilaginosa AN5. J. Basic Microbiol. 2017, 57, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, M.; Liu, W.; Zhang, B. Purification and characterization of a psychrophilic catalase from Antarctic Bacillus. Can. J. Microbiol. 2008, 54, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Barnwell, C.V.; Grunden, A.M. Characterization of recombinant glutathione reductase from the psychrophilic Antarctic bacterium Colwellia psychrerythraea. Extremophiles 2015, 19, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, H.; Cui, B.; Hou, Y.; Wang, Y.; Wang, Q. A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506: Cloning and heterologous expression of the gene and characterization of recombinant enzyme. Bioengineered 2017, 8, 742–749. [Google Scholar] [CrossRef]
- Cotugno, R.; Rosaria Ruocco, M.; Marco, S.; Falasca, P.; Evangelista, G.; Raimo, G.; Chambery, A.; Di Maro, A.; Masullo, M.; De Vendittis, E. Differential cold-adaptation among protein components of the thioredoxin system in the psychrophilic eubacterium Pseudoalteromonas haloplanktis TAC 125. Mol. BioSyst. 2009, 5, 519–528. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, Y.; Shi, Y.; Han, X.; Chen, Q.; Hu, Z.; Liu, Y.; Li, Y. Cloning, expression, purification, and characterization of glutaredoxin from Antarctic sea-ice bacterium Pseudoalteromonas sp. AN178. BioMed Res. Int. 2014, 2014, 246871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Wang, Y.; Lu, Z.; Song, C.; Xu, Y.; Wei, N.; Wang, Q. Cloning, expression and enzymatic characteristics of a 2-Cys peroxiredoxin from Antarctic sea-ice bacterium Psychrobacter sp. ANT206. Int. J. Biol. Macromol. 2019, 129, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Birolo, L.; Tutino, M.L.; Fontanella, B.; Gerday, C.; Mainolfi, K.; Pascarella, S.; Sannia, G.; Vinci, F.; Marino, G. Aspartate aminotransferase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. Cloning, expression, properties, and molecular modelling. Eur. J. Biochem. 2000, 267, 2790–2802. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Q.; Hou, Y.; Hong, Y.; Han, X.; Yi, J.; Qu, J.; Lu, Y. Molecular cloning, expression and enzymatic characterization of glutathione S-transferase from Antarctic sea-ice bacteria Pseudoalteromonas sp. ANT506. Microbiol. Res. 2014, 169, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Angelaccio, S.; Florio, R.; Consalvi, V.; Festa, G.; Pascarella, S. Serine hydroxymethyltransferase from the cold adapted microorganism Psychromonas ingrahamii: A low temperature active enzyme with broad substrate specificity. Int. J. Mol. Sci. 2012, 13, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Albino, A.; Marco, S.; Di Maro, A.; Chambery, A.; Masullo, M.; De Vendittis, E. Characterization of a cold-adapted glutathione synthetase from the psychrophile Pseudoalteromonas haloplanktis. Mol. BioSyst. 2012, 8, 2405–2414. [Google Scholar] [CrossRef]
- Georlette, D.; Jonsson, Z.O.; Van Petegem, F.; Chessa, J.; Van Beeumen, J.; Hubscher, U.; Gerday, C. A DNA ligase from the psychrophile Pseudoalteromonas haloplanktis gives insights into the adaptation of proteins to low temperatures. Eur. J. Biochem. 2000, 267, 3502–3512. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Del Prete, S.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, characterization and anion inhibition studies of a gamma-carbonic anhydrase from the Antarctic bacterium Colwellia psychrerythraea. Bioorg. Med. Chem. 2016, 24, 835–840. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, characterization and anion inhibition studies of a new gamma-carbonic anhydrase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Bioorg. Med. Chem. 2015, 23, 4405–4409. [Google Scholar] [CrossRef]
- Angeli, A.; Del Prete, S.; Osman, S.M.; AlOthman, Z.; Donald, W.A.; Capasso, C.; Supuran, C.T. Activation Studies of the gamma-Carbonic Anhydrases from the Antarctic Marine Bacteria Pseudoalteromonas haloplanktis and Colwellia psychrerythraea with Amino Acids and Amines. Mar. Drugs 2019, 17, 238. [Google Scholar] [CrossRef]
- Truong, L.V.; Tuyen, H.; Helmke, E.; Binh, L.T.; Schweder, T. Cloning of two pectate lyase genes from the marine Antarctic bacterium Pseudoalteromonas haloplanktis strain ANT/505 and characterization of the enzymes. Extremophiles 2001, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Kim, S.J.; Lee, C.W.; Kim, H.W.; Park, H.H.; Kim, H.M.; Park, H.; Park, H.; Lee, J.H. Crystal structure of UbiX, an aromatic acid decarboxylase from the psychrophilic bacterium Colwellia psychrerythraea that undergoes FMN-induced conformational changes. Sci. Rep. 2015, 5, 8196. [Google Scholar] [CrossRef]
- Do, H.; Lee, C.W.; Han, S.J.; Lee, S.G.; Kim, H.J.; Park, H.; Lee, J.H. Purification, crystallization and preliminary X-ray crystallographic studies of FMN-bound and FMN-free forms of aromatic acid decarboxylase (CpsUbiX) from the psychrophilic bacterium Colwellia psychrerythraea 34H. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Yun, J.S.; Lee, C.W.; Choi, Y.J.; Kim, H.Y.; Kim, Y.J.; Park, H.; Chang, J.H.; Lee, J.H. Crystal Structure and Comparative Sequence Analysis of GmhA from Colwellia psychrerythraea Strain 34H Provides Insight into Functional Similarity with DiaA. Mol. Cells 2015, 38, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- See Too, W.C.; Few, L.L. Cloning of triose phosphate isomerase gene from an antarctic psychrophilic Pseudomonas sp. by degenerate and splinkerette PCR. World J. Microbiol. Biotechnol. 2010, 26, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Rentier-Delrue, F.; Mande, S.C.; Moyens, S.; Terpstra, P.; Mainfroid, V.; Goraj, K.; Lion, M.; Hol, W.G.; Martial, J.A. Cloning and overexpression of the triosephosphate isomerase genes from psychrophilic and thermophilic bacteria. Structural comparison of the predicted protein sequences. J. Mol. Biol. 1993, 229, 85–93. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Marx, J.C.; Gerday, C.; Feller, G. Activity-stability relationships in extremophilic enzymes. J. Biol. Chem. 2003, 278, 7891–7896. [Google Scholar] [CrossRef] [PubMed]
- Tutino, M.L.; Duilio, A.; Moretti, M.A.; Sannia, G.; Marino, G. A rolling-circle plasmid from Psychrobacter sp. TA144: Evidence for a novel rep subfamily. Biochem. Biophys. Res. Commun. 2000, 274, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kazuoka, T.; Takigawa, S.; Arakawa, N.; Hizukuri, Y.; Muraoka, I.; Oikawa, T.; Soda, K. Novel psychrophilic and thermolabile L-threonine dehydrogenase from psychrophilic Cytophaga sp. strain KUC-1. J. Bacteriol. 2003, 185, 4483–4489. [Google Scholar] [CrossRef]
- Tutino, M.L.; Birolo, L.; Fontanella, B.; Mainolfi, K.; Vinci, F.; Sannia, G.; Marino, G. Aspartate aminotransferase from Moraxella TAC125: An unusual psychrophilic enzyme. In Cold-Adapted Organisms; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef]
- Speciale, G.; Thompson, A.J.; Davies, G.J.; Williams, S.J. Dissecting conformational contributions to glycosidase catalysis and inhibition. Curr. Opin. Struct. Biol. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, G.Z.; Ferreira, D.; Vermelho, A.B. Marine extremophiles: A source of hydrolases for biotechnological applications. Mar. Drugs 2015, 13, 1925–1965. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.P.; Wierzbicki, L.E. Beta-galactosidase technology: A solution to the lactose problem. CRC Crit. Rev. Food Technol. 1975, 5, 325–356. [Google Scholar] [CrossRef]
- Karan, R.; Capes, M.D.; DasSarma, P.; DasSarma, S. Cloning, overexpression, purification, and characterization of a polyextremophilic beta-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol. 2013, 13, 3. [Google Scholar] [CrossRef]
- Xu, K.; Tang, X.; Gai, Y.; Mehmood, M.; Xiao, X.; Wang, F. Molecular characterization of cold-inducible beta-galactosidase from Arthrobacter sp. ON14 isolated from Antarctica. J. Microbiol. Biotechnol. 2011, 21, 236–242. [Google Scholar] [PubMed]
- Bialkowska, A.M.; Cieslinski, H.; Nowakowska, K.M.; Kur, J.; Turkiewicz, M. A new beta-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: Gene cloning, purification and characterization. Arch. Microbiol. 2009, 191, 825–835. [Google Scholar] [CrossRef]
- Makowski, K.; Bialkowska, A.; Szczesna-Antczak, M.; Kalinowska, H.; Kur, J.; Cieslinski, H.; Turkiewicz, M. Immobilized preparation of cold-adapted and halotolerant Antarctic beta-galactosidase as a highly stable catalyst in lactose hydrolysis. FEMS Microbiol. Ecol. 2007, 59, 535–542. [Google Scholar] [CrossRef]
- Van de Voorde, I. Evaluation of the cold-active Pseudoalteromonas haloplanktis β-galactosidase enzyme for lactose hydrolysis in whey permeate as primary step of d-tagatose production. Process. Biochem. 2014, 49, 2134–2140. [Google Scholar] [CrossRef]
- Asghar, S.; Lee, C.R.; Park, J.S.; Chi, W.J.; Kang, D.K.; Hong, S.K. Identification and biochemical characterization of a novel cold-adapted 1,3-alpha-3,6-anhydro-L-galactosidase, Ahg786, from Gayadomonas joobiniege G7. Appl. Microbiol. Biotechnol. 2018, 102, 8855–8866. [Google Scholar] [CrossRef]
- Roohi, R.; Kuddus, M.; Saima, S. Cold-active detergent-stable extracellular α-amylase from Bacillus cereus GA6: Biochemical characteristics and its perspectives in laundry detergent formulation. J. Biochem. Technol. 2013, 4, 636–644. [Google Scholar]
- Qin, Y.; Huang, Z.; Liu, Z. A novel cold-active and salt-tolerant alpha-amylase from marine bacterium Zunongwangia profunda: Molecular cloning, heterologous expression and biochemical characterization. Extremophiles 2014, 18, 271–281. [Google Scholar] [CrossRef]
- Dornez, E.; Verjans, P.; Arnaut, F.; Delcour, J.A.; Courtin, C.M. Use of psychrophilic xylanases provides insight into the xylanase functionality in bread making. J. Agric. Food Chem. 2011, 59, 9553–9562. [Google Scholar] [CrossRef]
- Collins, T.; Meuwis, M.A.; Stals, I.; Claeyssens, M.; Feller, G.; Gerday, C. A novel family 8 xylanase, functional and physicochemical characterization. J. Biol. Chem. 2002, 277, 35133–35139. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef]
- Li, Q.; Yi, L.; Marek, P.; Iverson, B.L. Commercial proteases: Present and future. FEBS Lett. 2013, 587, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Vojcic, L.; Pitzler, C.; Korfer, G.; Jakob, F.; Ronny, M.; Maurer, K.H.; Schwaneberg, U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef]
- Elleuche, S.; Schafers, C.; Blank, S.; Schroder, C.; Antranikian, G. Exploration of extremophiles for high temperature biotechnological processes. Curr. Opin. Microbiol. 2015, 25, 113–119. [Google Scholar] [CrossRef]
- Kuddus, M.; Ramteke, P.W. Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit. Rev. Microbiol. 2012, 38, 330–338. [Google Scholar] [CrossRef]
- Tindbaek, N.; Svendsen, A.; Oestergaard, P.R.; Draborg, H. Engineering a substrate-specific cold-adapted subtilisin. Protein Eng. Des. Sel. 2004, 17, 149–156. [Google Scholar] [CrossRef]
- Bialkowska, A.M.; Krysiak, J.; Florczak, T.; Szulczewska, K.M.; Wanarska, M.; Turkiewicz, M. The psychrotrophic yeast Sporobolomyces roseus LOCK 1119 as a source of a highly active aspartic protease for the in vitro production of antioxidant peptides. Biotechnol. Appl. Biochem. 2018, 65, 726–738. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, S.M.; Choi, J.I. Purification, Characterization, and Cloning of a Cold-Adapted Protease from Antarctic Janthinobacterium lividum. J. Microbiol. Biotechnol. 2018, 28, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fornbacke, M.; Clarsund, M. Cold-adapted proteases as an emerging class of therapeutics. Infect. Dis. Ther. 2013, 2, 15–26. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Joseph, B.; Ramteke, P.W.; Thomas, G. Cold active microbial lipases: Some hot issues and recent developments. Biotechnol. Adv. 2008, 26, 457–470. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Brieva, R.; Gotor, V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B Enzym. 2006, 40, 111–120. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Busto, E.; Gotor, V. Candida antarctica Lipase B: An Ideal Biocatalyst for the Preparation of Nitrogenated Organic Compounds. Adv. Synth. Catal. 2006, 348, 797–812. [Google Scholar] [CrossRef]
- Maharana, A.K.; Singh, S.M. A cold and organic solvent tolerant lipase produced by Antarctic strain Rhodotorula sp. Y-23. J. Basic Microbiol. 2018, 58, 331–342. [Google Scholar] [CrossRef]
- Ramle, Z.; Rahim, R.A. Psychrophilic Lipase from Arctic Bacterium. Trop. Life Sci. Res. 2016, 27, 151–157. [Google Scholar] [CrossRef]
- Arifin, A.R.; Kim, S.J.; Yim, J.H.; Suwanto, A.; Kim, H.K. Isolation and biochemical characterization of Bacillus pumilus lipases from the Antarctic. J Microbiol. Biotechnol. 2013, 23, 661–667. [Google Scholar] [CrossRef]
- Florczak, T.; Daroch, M.; Wilkinson, M.C.; Bialkowska, A.; Bates, A.D.; Turkiewicz, M.; Iwanejko, L.A. Purification, characterisation and expression in Saccharomyces cerevisiae of LipG7 an enantioselective, cold-adapted lipase from the Antarctic filamentous fungus Geomyces sp. P7 with unusual thermostability characteristics. Enzyme Microb. Technol. 2013, 53, 18–24. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; De Boeck, G.; Becker, K. Phytate and phytase in fish nutrition. J. Anim. Physiol. Anim. Nutr. 2012, 96, 335–364. [Google Scholar] [CrossRef]
- Rebello, S.; Jose, L.; Sindhu, R.; Aneesh, E.M. Molecular advancements in the development of thermostable phytases. Appl. Microbiol. Biotechnol. 2017, 101, 2677–2689. [Google Scholar] [CrossRef]
- Park, I.; Cho, J. The phytase from antarctic bacterial isolate, Pseudomonas sp. JPK1 as a potential tool for animal agriculture to reduce manure phosphorus excretion. Afr. J. Agric. Res. 2011, 6, 1398–1406. [Google Scholar]
- Awazu, N.; Shodai, T.; Takakura, H.; Kitagawa, M.; Mukai, H.; Kato, I. Microorganism-Derived Psychrophilic Endonuclease. U.S. Patent 8,034,597, 11 October 2011. [Google Scholar]
- Thallinger, B.; Prasetyo, E.N.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 2013, 8, 97–109. [Google Scholar] [CrossRef]
- See-Too, W.S.; Convey, P.; Pearce, D.A.; Chan, K.G. Characterization of a novel N-acylhomoserine lactonase, AidP, from Antarctic Planococcus sp. Microb. Cell Fact. 2018, 17, 179. [Google Scholar] [CrossRef]
- Medigue, C.; Krin, E.; Pascal, G.; Barbe, V.; Bernsel, A.; Bertin, P.N.; Cheung, F.; Cruveiller, S.; D’Amico, S.; Duilio, A.; et al. Coping with cold: The genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 2005, 15, 1325–1335. [Google Scholar] [CrossRef]
- Methe, B.A.; Nelson, K.E.; Deming, J.W.; Momen, B.; Melamud, E.; Zhang, X.; Moult, J.; Madupu, R.; Nelson, W.C.; Dodson, R.J.; et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 2005, 102, 10913–10918. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Morel, M.A.; Brana, V.; Morales, D.; Martinez-Lopez, W.; Castro-Sowinski, S. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles 2017, 21, 409–418. [Google Scholar] [CrossRef]

| Molecular Adaptation | Effect | Reference |
|---|---|---|
| Decreased number of hydrogen bonds and salt bridges | Increased flexibility | [69,72] |
| Reduced proline and arginine content | Increased molecular entropy | [23,74] |
| Increased surface charged residues | Increased conformational flexibility | [23] |
| Reduced frequency of surface, inter-domain and inter-subunit ionic linkages and ion-network | Increased conformational flexibility and reduced enthalphic contribution to stability | [75] |
| Reduced core hydrophobicity/increased surface hydrophobicity | Reduced hydrophobic effect/ entropic destabilization | [70] |
| Increased accessibility of active site | Increased flexibility for substrate and cofactor binding | [76] |
| Loop extensions | Reduced stability | [77] |
| Marine Polar-Active Enzymes | Reaction | Organism Source | Origin of Sample | Applications/Potential Uses | References |
|---|---|---|---|---|---|
| HYDROLASES: EC 3 (Type of reaction: Hydrolytic cleavage AB + H2O → AOH + BH) | |||||
| β-galactosidase | Hydrolysis of lactose into its constituent monosaccharides | Pseudoalteromonas sp. 22b | Alimentary tract of Antarctic krill Thyssanoessa macrura | Candidates for lactose removal from dairy products at low temperatures | [86,87] |
| β-galactosidase | Pseudoalteromonas haloplanktis TAE 79 | Antarctic seawater | [88] | ||
| β-galactosidase | Pseudoalteromonas haloplanktis LMG P-19143 | Antarctic seawater | [89] | ||
| β-galactosidase | Guehomyces pullulans | Antarctic sea sediment | [90] | ||
| β-galactosidase | Enterobacter ludwigii | Sediment samples of Kongsfgord, Arctic | [91] | ||
| β-galactosidase | Alkalilactibacillus ikkense | Ikka columns in South-West Greenland | [92] | ||
| α-Amylase | Cleavage of α-1,4-glycosidic linkages in starch molecules to generate smaller polymers of glucose units | Pseudoalteromonas sp. M175 | Antarctic sea-ice | Detergent additive for its stain removal efficiency | [93] |
| α-Amylase§ | Glaciozyma antarctica PI12 | Antarctic sea-ice | Additives in processed food, in detergents for cold washing, in waste-water treatment, in bioremediation in cold climates and in molecular biology applications | [94] | |
| α-Amylase | Bacterial strains | Sediment samples from Midtre Lovènbreen Arctic glacier | [95] | ||
| α-Amylase | Alteromonas sp. TAC 240B | Antarctic seawater | [96] | ||
| α-Amylase | Pseudoalteromonas haloplanktis * | Antarctic seawater | [97,98] | ||
| Xylanase | Hydrolysis of the main chain of xylan to oligosaccharides, which in turn are degraded to xylose | Cladosporium sp. | Antarctic marine sponges | Additives in textile and food industries, and bioremediation | [99] |
| Xylanase | Flavobacterium frigidarium sp. | Antarctic shallow-water marine sediment | [100] | ||
| Serine protease (Subtilisin) | Cleavage of peptide bonds | Bacillus TA39 | Antarctic seawater | Additives in low-temperature food processing, food and textile industries, leather processing, detergent industry | [101,102] |
| Serine protease (Subtilisin) | Bacillus TA41 | Antarctic seawater | [101,103] | ||
| Serine protease | Colwellia sp. NJ341 | Antarctic sea-ice | [104] | ||
| Serine alkaline protease | Shewanella sp. Ac10u | Antarctic seawater | [105] | ||
| Acid protease | Rhodotorula mucilaginosa L7 | Antarctic marine alga | [106] | ||
| Subtilisin-like serine protease | Pseudoalteromonas sp., Marinobacter sp., Psychrobacter sp., Polaribacter sp. | Antarctic seawater and thorax, abdomen and head of krill (Euphausia superba Dana) | [107] | ||
| Protease | Pseudoalteromonas sp. NJ276 | Antarctic sea-ice | [108] | ||
| Subtilisin-like Serine proteinase | Leucosporidium antarcticum 171 | Antarctic sub-glacial waters | [109] | ||
| Aminopeptidase | Pseudoalteromonas haloplanktis TAC125 | Antarctic seawater | [110] | ||
| Aminopeptidase | Colwellia psychrerythraea 34H | Greenland continental shelf sediment samples | [111,112] | ||
| Serine peptidase | Lysobacter sp. A03 | Penguin feathers in Antarctica | [113] | ||
| Serine peptidase | Serratia sp. | Coastal seawater in Northern Norway | [114] | ||
| Metalloprotease | Pseudoalteromonas sp. SM495 | Arctic sea-ice (Canadian Basin) | [115] | ||
| Metalloprotease | Sphingomonas paucimobilis | Stomach of Antarctic krill, Euphausia superba Dana | [116] | ||
| Metalloprotease | Psychrobacter proteolyticus sp. | Stomach of Antarctic krill Euphausia superba Dana | [117] | ||
| Endopeptidase | Microbial source | Arctic marine microbial source | Candidate for molecular biology application: digestion of chromatin (ArcticZymes) | [118] | |
| Lipase | Hydrolysis of long-chain triacylglycerol substances with the formation of an alcohol and a carboxylic acid | Bacillus pumilus ArcL5 | Arctic seawater (Chukchi Sea) | Detergent additives used at low temperatures and biocatalysts for the biotransformation of heat-labile compounds | [119] |
| Lipase | Pseudoalteromonas haloplanktis TAC125 | Antarctic seawater | [120] | ||
| Lipase | Colwellia psychrerythraea 34H | Arctic seawater | [121] | ||
| Lipase | Polaromonas vacuolata | Antarctic seawater | [122] | ||
| Lipase | Psychrobacter sp. | Antarctic seawater | [123,124] | ||
| Lipase | Shewanella frigidimarina | Antarctic seawater | [125] | ||
| Lipase | Bacterial strains | Arctic sediment samples from the snout of Midtre Lovènbreen glacier up to the convergence point with the sea | [95] | ||
| Lipase | Psychrobacter sp. TA144 ** | Antarctic seawater | [126] | ||
| Lipase | Psychrobacter sp. 7195 | Antarctic deep-sea sediment (Prydz Bay) | [127] | ||
| Lipase | Moritella sp. 2-5-10-1 | Antarctic deep-sea water | [128] | ||
| Lipase | Pseudoalteromonas sp., Psychrobacter sp., Vibrio sp. | Antarctic seawater samples (Ross Sea) | [129] | ||
| Phytase | Hydrolysis of phytate to phosphorylated myo-inositol derivatives | Rhodotorula mucilaginosa JMUY14 | Antarctic deep-sea sediment | Candidate for feed applications, especially in aquaculture | [130] |
| Esterase | Hydrolysis of simple esters, usually only triglycerides composed of fatty acids shorter than C 8 | Pseudoalteromonas arctica | Arctic sea-ice from Spitzbergen, Norway | Additives in laundry detergents and biocatalysts for the biotransformation of labile compounds at low temperatures | [131] |
| Esterase | Thalassospira sp. | Arctic sea fan (Paramuricea placomus), Vestfjorden area (Northern Norway) | [132] | ||
| Esterase | Oleispira antarctica | Antarctic coastal waters | [73,133] | ||
| Esterase | Pseudoalteromas haloplanktis TAC125 | Antarctic seawater | [134,135] | ||
| Esterase | Pseudoalteromas sp. 643A | Alimentary tract of Antarctic krill Euphasia superba Dana | [136] | ||
| Esterase | Marine Arctic metagenomics libraries | Arctic seawater and sediment from Barents Sea and Svalbard | Candidate for organic synthesis reactions and cheese ripening processes | [137,138] | |
| Epoxide hydrolase | Hydrolysis of an epoxide to its corresponding vicinal diol with the addition of a water molecule to the oxirane ring | Sphingophyxis alaskensis | Arctic seawater | Candidate for the production of enantiopure epoxides in the pharmaceutical industry | [139] |
| S-formylglutathione hydrolase | Hydrolysis of S-formylglutathione to formic acid and glutathione | Pseudoalteromonas haloplanktis TAC125 | Antarctic seawater | Candidates for chemical synthesis and industrial pharmaceutics | [140] |
| S-formylglutathione hydrolase | Shewanella frigidimarina | Antarctic marine environment | [141] | ||
| Polygalacturonase (pectin depolymerase) | Cleavage of glycosidic bonds between galacturonic acid residues | Pseudoalteromonas haloplanktis | Antarctic seawater | Additive in food industries, such as clarification of juice, in the process of vinification, yield and color enhancement and in the mashing of fruits | [142] |
| Pullulanase | Hydrolysis of α-1,6-glycosidic bonds in pullulan to produce maltotriose | Shewanella arctica | Seawater samples in Spitsbergen, Norway | Additive in food and biofuel industries | [143] |
| Invertase | Hydrolysis of the terminal non-reducing β-fructofuranoside residue in sucrose, raffinose and related β-D-fructofuranosides | Leucosporidium antarcticum | Antarctic seawater | Not defined (ND) | [144] |
| α-glucosidase | Hydrolysis of the non-reducing terminal α-glucopyranoside residues from various α-glucosides and related compounds | Leucosporidium antarcticum | Antarctic seawater | Additive in detergent and food industries | [144] |
| Cellulase | Hydrolysis of the β-1,4-D-glycosidic linkages in cellulose | Pseudoalteromonas haloplanktis | Antarctic seawater | Additive in detergent industry | [145] |
| Chitobiase | Hydrolysis of chitobiose to N-acetylglucosamine | Arthrobacter sp. TAD20 | Antarctic sea sediments | ND | [146] |
| Alkaline phosphatase | Hydrolysis and transphosphorylation of a wide variety of phosphate monoesters | TAB5 strain | Antarctica^ | Candidate for molecular biology application: dephosphorylation of DNA (New England Biolabs) | [147,148,149] |
| Alkaline phosphatase | Shewanella sp. | Intestine of Antarctic shellfish | Candidate for molecular biology application | [150] | |
| Pyrophosphatase | Catalysis of the conversion of one ion of pyrophosphate to two phosphate ions | Oleispira antarctica | Antarctic deep sea | ND | [73] |
| Glycerophosphodiesterase | Catalysis of the hydrolysis of a glycerophosphodiester | Oleispira antarctica | Antarctic deep sea | ND | [73] |
| Endonuclease (Cryonase) | Cleavage of the phosphodiester bond the middle of a polynucleotide chain | Shewanella sp. Ac10 | Antarctic seawater | Candidate for molecular biology application: digestion of all types of DNA and RNA at cold temperatures (Takara-Clontech) | [151] |
| Exonuclease | Cleavage of the phosphodiester bond at either the 3′ or the 5′ end | Arctic marine bacterium | Arctic marine microbial source | Candidate for molecular biology application: 3′-5′ exonuclease specific for single stranded DNA (ArcticZymes) | [152] |
| Ribonuclease | Hydrolysis of the phosphodiester bonds among the nucleic acid residues of RNA | Psychrobacter sp. ANT206 | Antarctic sea-ice | Candidate for molecular biology applications | [153] |
| Uracil-DNA glycosylase | Hydrolysis of the N-glycosidic bond from deoxyuridine to release uracil | Antarctic marine bacterium | Antarctic marine microbial source | Candidate for molecular biology application: release of free uracil from uracil-containing single-stranded or double-stranded DNA (New England Biolabs) | [154] |
| OXIDOREDUCTASES: EC 1 (Type of reaction: Transfer of hydrogen or oxygen or electrons between molecules AH + B → A + BH; A + O → AO; A-+B→A+B-) | |||||
| Phenylalanine hydroxylase | Catalysis of the hydroxylation of L-Phe to form tyrosine | Colwellia psychrerythraea 34H | Arctic marine sediments | ND | [76] |
| Alcohol dehydrogenase | Catalysis of the interconversion of alcohols to their corresponding carbonyl compounds | Moraxella sp. TAE123 | Antarctic seawater | Candidate for asymmetric synthesis | [155] |
| Alanine dehydrogenase | Catalysis of reversible deamination of L-alanine to pyruvate | Shewanella sp. Ac10u, Carnobacterium sp. St2 | Antarctic seawater | Candidate for enantioselective production of optically active amino acids | [156] |
| Leucine dehydrogenase | Catalysis of reversible L-leucine and other branched chain L-amino acids deamination reaction to the corresponding α-keto acid | Pseudoalteromonas sp. ANT178 | Antarctic sea-ice | Candidate for medical and pharmaceutical industry applications | [157] |
| Malate dehydrogenase | Catalysis of reversible oxidation of malate to oxalacetate | Flavobacterium frigidimaris KUC-1 | Antarctic seawater | Candidate for detection and production of malate under cold conditions | [158] |
| Isocitrate dehydrogenase | Catalysis of decarboxylation of isocitrate to α-ketoglutarate and CO2 | Desulfotalea psychrophila | Arctic marine sediments | ND | [159] |
| L-threonine dehydrogenase | Catalysis of dehydrogenation at the β-carbon (C3) position of L-threonine | Flavobacterium frigidimaris KUC-1 *** | Antarctic seawater | ND | [160] |
| Superoxide dismutase | Catalysis of the dismutation of superoxide anion radicals into molecular oxygen and hydrogen peroxide | Pseudoalteromonas haloplanktis | Antarctic seawater | Candidates for applications in agriculture, cosmetics, food, healthcare products and medicines | [161] |
| Superoxide dismutase | Marinomonas sp. NJ522 | Antarctic sea-ice | [162] | ||
| Superoxide dismutase | Pseudoalteromonas sp. ANT506 | Antarctic sea-ice | [163] | ||
| Superoxide dismutase | Psychromonas arctica | Arctic sea-ice and sea-water samples | [164] | ||
| Superoxide dismutase | Rhodotorula mucilaginosa AN5 | Antarctic sea-ice | [165] | ||
| Catalase | Catalysis of degradation of hydrogen peroxide into water and molecular oxygen | Bacillus sp. N2a | Antarctic seawater | Candidate for textile and cosmetic industries | [166,167] |
| Glutathione reductase | Catalysis of the reduction of oxidized glutathione to produce reduced glutathione | Colwellia psychrerythraea | Antarctic seawater | Candidate as an antioxidant enzyme in heterologous systems | [168] |
| Glutathione peroxidase | Catalysis of the reduction of hydrogen peroxide and other organic peroxides | Pseudoalteromonas sp. ANT506 | Antarctic sea-ice | ND | [169] |
| Thioredoxin reductase | Catalysis of the reduction of thioredoxin | Pseudoalteromonas haloplanktis TAC125 | Antarctic seawater | ND | [170] |
| Glutaredoxin | Catalysis of the reduction of protein disulfides in glutathione-dependent reactions | Pseudoalteromonas sp. AN178 | Antarctic sea-ice | ND | [171] |
| Peroxiredoxin | Catalysis of the reduction of hydrogen peroxide, peroxynitrite and a wide range of organic hydroperoxides | Psychrobacter sp. ANT206 | Antarctic sea-ice | Candidate for food and pharmaceutical industries | [172] |
| Dihydroorotate oxidase | Catalysis of the stereospecific oxidation of (S)-dihydroorotate to orotate | Oleispira antarctica | Antarctic deep sea | ND | [73] |
| TRANSFERASES: EC 2 (Type of reaction: Transfer of groups of atoms AB + C → A + BC) | |||||
| Aspartate aminotransferase | Catalysis of transamination reaction of L-aspartate and α-ketoglutarate into the corresponding oxaloacetate and L-glutamate | Pseudoalteromonas haloplanktis TAC125 **** | Antarctic seawater | ND | [173] |
| Glutathione S-transferase | Catalysis of conjugation of reduced glutathione with various electrophilic compounds and ROS | Pseudoalteromonas sp. ANT506 | Antarctic sea-ice | ND | [174] |
| Hydroxymethyl-transferase | Catalysis of reversible conversion of L-serine and tetrahydropteroylglutamate to glycine and 5,10-methylenetetrahydropteroylglutamate. Cleavage of many 3-hydroxyamino acids and decarboxylation of aminomalonate | Psychromonas ingrahamii | Arctic polar sea-ice | Candidate as a pharmaceutical, agrochemicals and food additive | [175] |
| LIGASES: EC 6 (Type of reaction: Covalent joining of two molecules coupled with the hydrolysis of an energy rich bond in ATP or similar triphosphates A + B+ ATP → AB + ADP + Pi) | |||||
| Glutathione synthetase | Catalysis of formation of glutathione from L-γ-glutamylcysteine and glycine | Pseudoalteromonas haloplanktis | Antarctic seawater | ND | [176] |
| DNA ligase | Catalysis of the formation of a phosphodiester bond between adjacent 5′-phosphoryl and 3′-hydroxyl groups in double stranded DNA | P. haloplanktis TAE 72 | Antarctic seawater | Candidate for applications in molecular biology | [177] |
| LYASES: EC 4 (Type of reaction: Cleavage of C-C, C-O, C-S, C-N or other bonds by other means than by hydrolysis or oxidation RCOCOOH → RCOH + CO2) | |||||
| γ-carbonic anhydrase | Catalysis of CO2 hydration to bicarbonate and protons | Colwellia psychrerythraea | Antarctic cold ice sediments | Candidates for biomedical applications | [178] |
| γ-carbonic anhydrase | Pseudoalteromonas haloplanktis | Antarctic seawater | [179,180] | ||
| Pectate lyase | Cleavage of the α-1,4 glycosidic bonds of polygalacturonic acid into simple sugars | Pseudoalteromonas haloplanktis ANT/505 | Antarctic sea-ice | Candidate for detergent industry | [167,181] |
| Acid decarboxylase | Catalysis of decarboxylation of 3-octaprenyl-4-hydroxybenzoate to produce 2-polyprenylphenol | Colwellia psychrerythraea 34H | Arctic marine sediments | ND | [182,183] |
| ISOMERASES: EC 5 (Type of reaction: Transfer of group from one position to another within one molecule AB → BA) | |||||
| Sedoheptulose 7- phosphate isomerase | Catalysis of the conversion of sedoheptulose 7-phosphate to D-glycero-D-mannoheptose 7-phosphate | Colwellia psychrerythraea 34H | Arctic marine sediments | Candidate for biocatalysis under low water conditions | [184] |
| Triose phosphate isomerase§ | Catalysis of the isomerization of dihydroxyacetone phosphate to D-glyceraldehyde 3-phosphate | Pseudomonas sp. π9 | Antarctic sea-ice | [185] | |
| Triose phosphate isomerase | Moraxella sp. TA137 | Intestine of Antarctic fish | [186] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, S.; Coppola, D.; di Prisco, G.; Giordano, D.; Verde, C. Enzymes from Marine Polar Regions and Their Biotechnological Applications. Mar. Drugs 2019, 17, 544. https://doi.org/10.3390/md17100544
Bruno S, Coppola D, di Prisco G, Giordano D, Verde C. Enzymes from Marine Polar Regions and Their Biotechnological Applications. Marine Drugs. 2019; 17(10):544. https://doi.org/10.3390/md17100544
Chicago/Turabian StyleBruno, Stefano, Daniela Coppola, Guido di Prisco, Daniela Giordano, and Cinzia Verde. 2019. "Enzymes from Marine Polar Regions and Their Biotechnological Applications" Marine Drugs 17, no. 10: 544. https://doi.org/10.3390/md17100544
APA StyleBruno, S., Coppola, D., di Prisco, G., Giordano, D., & Verde, C. (2019). Enzymes from Marine Polar Regions and Their Biotechnological Applications. Marine Drugs, 17(10), 544. https://doi.org/10.3390/md17100544








