Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum grandiflorum (Verrill, 1879)
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedure
3.2. Animal Material
3.3. Isolation of Bathyptilones and Enbepeanone
3.4. X-Ray Crystallography
3.5. Biological Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soldatou, S.; Baker, B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017, 34, 585–626. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [Green Version]
- Trimurtulu, G.; Faulkner, D.J.; Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.G.; Jameson, G.B. Alkaloids from the Antarctic sponge Kirkpatrickia varialosa. part 2: variolin A and N(3′)-methyl tetrahydrovariolin B. Tetrahedron 1994, 50, 3993–4000. [Google Scholar] [CrossRef]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.G.; Parkin, S.; Hope, H. Alkaloids from the Antarctic sponge Kirkpatrickia varialosa. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide a, a cytotoxic macrolide from the antarctic tunicate Synoicum adareanum. J. Am. Chem. Soc. 2006, 128, 5630–5631. [Google Scholar] [CrossRef] [PubMed]
- von Salm, J.L.; Witowski, C.G.; Fleeman, R.M.; McClintock, J.B.; Amsler, C.D.; Shaw, L.N.; Baker, B.J. Darwinolide, a new diterpene scaffold that inhibits methicillin-resistant Staphylococcus aureus biofilm from the Antarctic sponge Dendrilla membranosa. Org. Lett. 2016, 18, 2596–2599. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.L.; von Salm, J.L.; Clark, S.; Ferlita, S.; Nemani, P.; Azhari, A.; Rice, C.A.; Wilson, N.G.; Kyle, D.E.; Baker, B.J. Keikipukalides, furanocembrane diterpenes from the Antarctic deep sea octocoral Plumerella delicatissima. J. Nat. Prod. 2018, 81, 117–123. [Google Scholar] [CrossRef]
- Shilling, A.J.; von Salm, J.L.; Sanchez, A.R.; Kee, Y.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Anverenes B-E, new polyhalogenated monoterpenes form the Antarctic re alga Plocamium cartilagineum. Mar. Drugs 2019, 17, 230. [Google Scholar] [CrossRef]
- Knestrick, M.A.; Wilson, N.G.; Roth, A.; Adams, J.H.; Baker, B.J. Friomaramide, a highly modified linear hexapeptide from an Antarctic sponge Inflatella coelosphaeroides. J. Nat. Prod. 2019, 82, 2354–2358. [Google Scholar] [CrossRef]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T. A review of chemistry and biological activities of the Indonesian octocorallia. J. Appl. Pharm. Sci. 2017, 7, 219–227. [Google Scholar]
- Su, Y.D.; Su, J.H.; Hwang, T.L.; Wen, Z.H.; Sheu, J.H.; Wu, Y.C.; Sung, P.J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.C.; Shen, Y.C.; Lin, Y.S.; Hwang, T.L.; Kuo, Y.H.; Khalil, A.T. Frajunolides E-K, briarane diterpenes from Junceella fragilis. J. Nat. Prod. 2008, 71, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.F.; Di Su, Y.; Hwang, T.L.; Liao, Z.J.; Tsui, K.H.; Wen, Z.H.; Wu, Y.C.; Sung, P.J.; Schmidt, T.J. Briarenols C-E, new polyoxygenated briaranes from the octocoral Briareum excavatum. Molecules 2017, 22, 475. [Google Scholar] [CrossRef] [PubMed]
- Lei, H. Diterpenoids of gorgonian corals: chemistry and bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.P.; Tang, H.; Sun, P.; Liu, B.S.; Zhuang, C.L.; Yi, Y.H.; Zhang, W. Chemistry and bioactivity of briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; La, M.-P.; Li, L.; Li, X.-B.; Tang, H.; Liu, B.-S.; Krohn, K.; Sun, P.; Yi, Y.-H.; Zhang, W. Bioactive 11,20-epoxy-3,5(16)-diene briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. J. Nat. Prod. 2011, 74, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.B.; Butler, M.S.; Healy, P.C.; Quinn, R.J. Anthoptilides A-E, new briarane diterpenes from the Australian sea pen Anthoptilum cf. kukenthali. 2000, 318–321. [Google Scholar]
- Garcia-Fernàndez, J. The genesis and evolution of homeobox gene clusters. Nat. Rev. Genet. 2005, 6, 881–892. [Google Scholar] [CrossRef]
- Stern, M.; Gierse, A.; Tan, S.; Bicker, G. Human ntera2 cells as a predictive in vitro test system for developmental neurotoxicity. Arch. Toxicol. 2014, 88, 127–136. [Google Scholar] [CrossRef]
- Tharmarajah, L.; Samarakoon, S.R.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.H.; Senathilake, K.S.; Rajagopalan, U.; Galhena, P.B.; Thabrew, I. In vitro anticancer effect of gedunin on human teratocarcinomal (Ntera-2) cancer stem-like cells. Biomed Res. Int. 2017, 2413197. [Google Scholar] [CrossRef]
- France, S.C.; Hoover, L.L. Analysis of variation in mitochondrial DNA sequences (ND3, ND4L, MSH) among Octocorallia (= Alcyonaria)(Cnidaria: Anthozoa). Bull. Biol. Soc. Wash. 2001, 10, 110–118. [Google Scholar]
- France, S.C.; Hoover, L.L. DNA sequences of the mitochondrial COI gene have low levels of divergence among deep-sea octocorals (Cnidaria: Anthozoa). Hydrobiologia 2002, 471, 149–155. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucl. Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.I.; Hopkins, K.; Wheeler, A.J.; Allcock, A.L.; Yesson, C. Novel diversity in mitochondrial genomes of deep-sea Pennatulacea (Cnidaria: Anthozoa: Octocorallia). Mitochondrial DNA Part A 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.C. The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea). PLoS one 2011, 6, e22747. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX3 User Manual; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker. SAINT v8.35a. Data Reduction Software; Bruker AXS Inc.: Madison, WI, USA, 2017. [Google Scholar]
- Sheldrick, G.M. SADABS program for empirical absorption correction. Ph.D. Thesis, University of Gottingen, Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Phase annealing in shelx-90: direct methods for larger structures. Acta Crystallogr. Sect. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with shelxl. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hooft, R.W.W.; Straver, L.H.; Spek, A.L. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J. Appl. Crystallogr. 2008, 41, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]

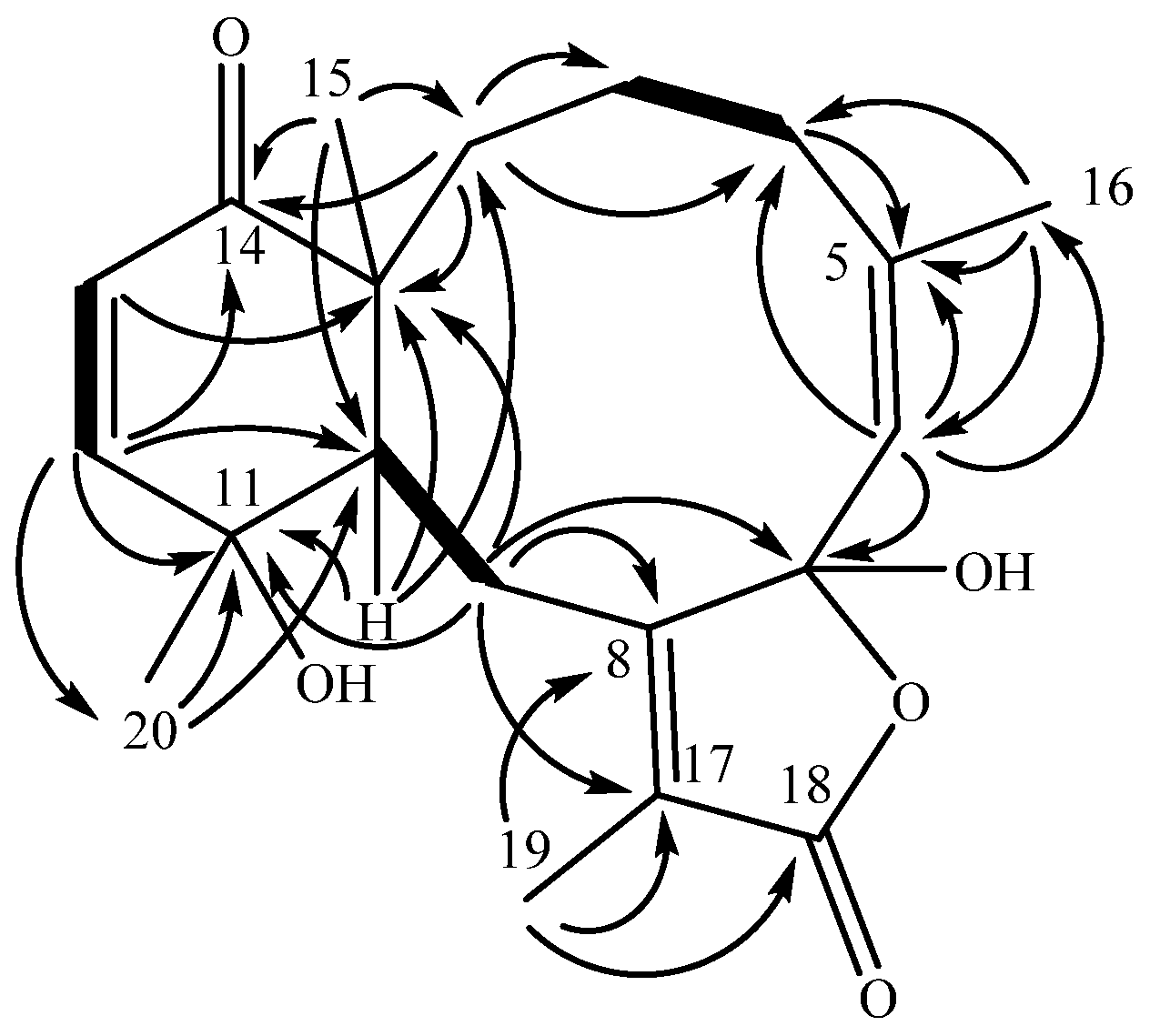
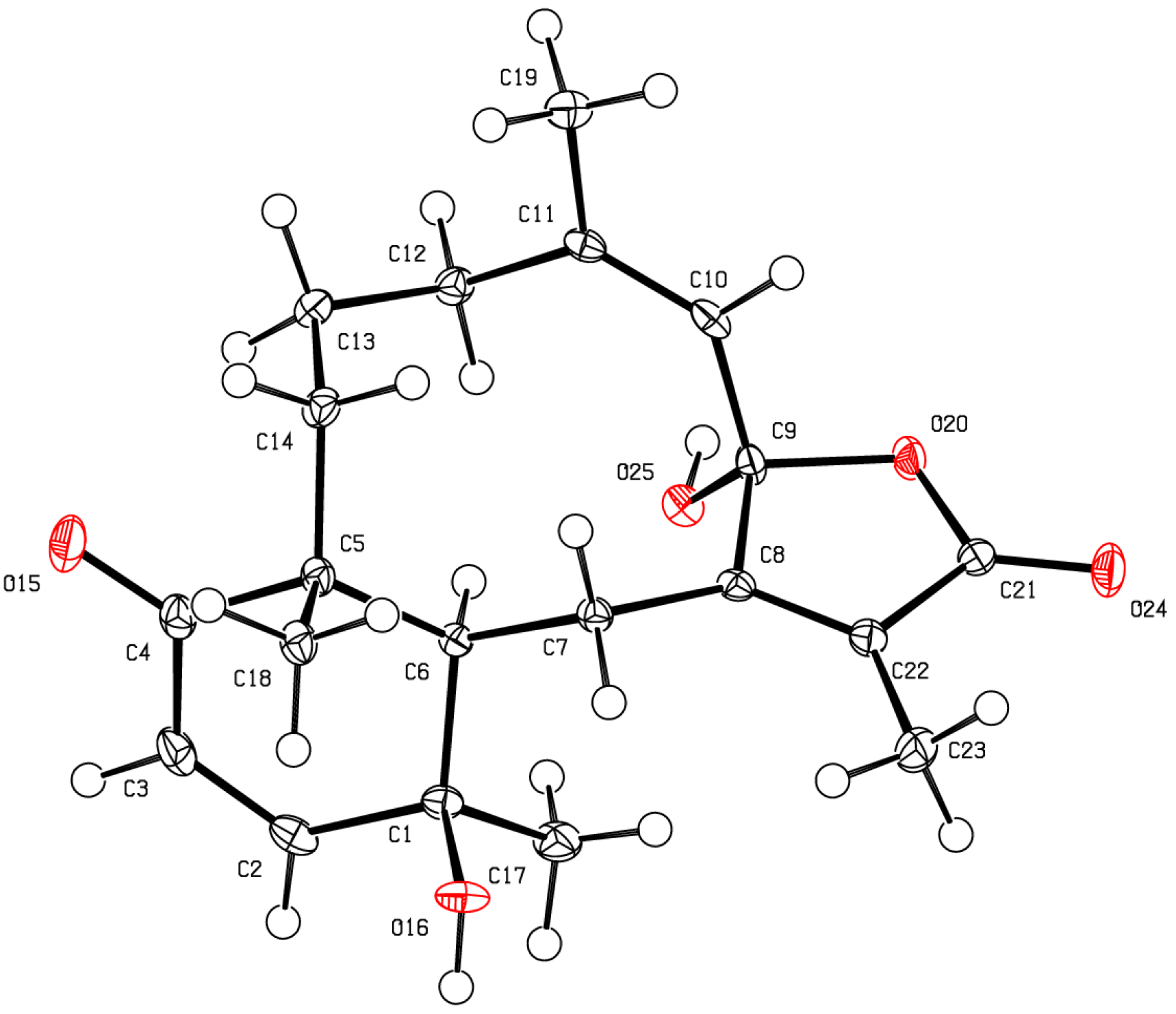


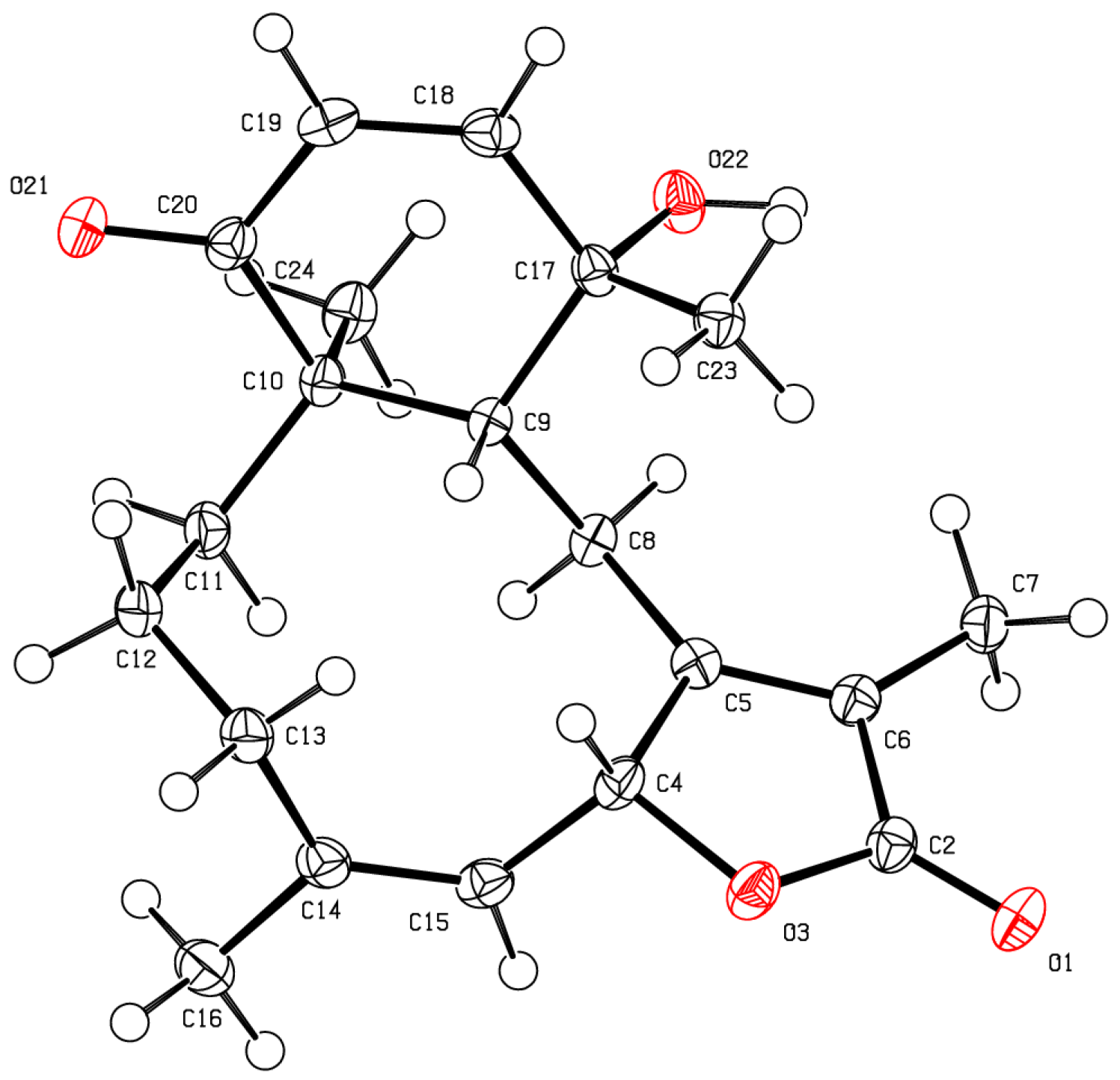

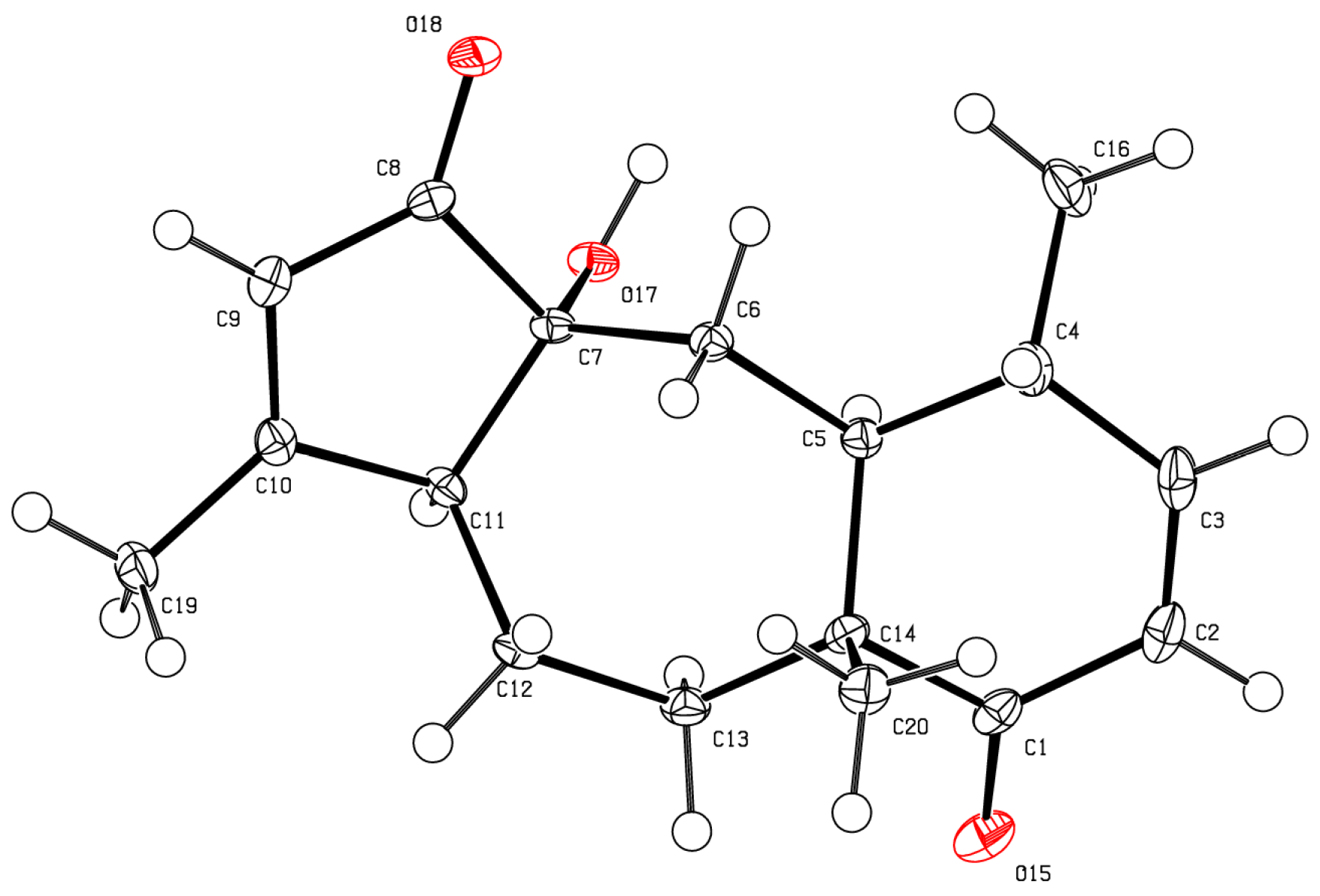


| Position | 13C, type | 1H (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 50.2, C | ||
| 2 | 21.8, CH2 | a 1.54, m b 1.84, m | 14 1, 4 |
| 3 | 31.6, CH2 | a 1.52, m b 1.98, m | 2 1, 2, 4, 10, 15 |
| 4 | 29.5, CH2 | a 1.85, m b 3.52, br t | 5, 6 |
| 5 | 145.3, C | ||
| 6 | 124.1, CH | 5.35, s | 4, 5, 7, 16 |
| 7 | 106.2, C | ||
| 8 | 160.7, C | ||
| 9 | 22.7, CH2 | a 2.47, d (15.8) b 3.25, dd (9.5, 15.3) | 1, 8, 10, 11, 17 7, 8, 10, 11, 17 |
| 10 | 41.4, CH | 3.14, d (9.5) | 1, 8, 9, 11, 14, 15 |
| 11 | 69.3, C | ||
| 12 | 151.7, CH | 6.63, d (10.0) | 10, 11, 14, 20 |
| 13 | 127.4, CH | 5.97, d (10.0) | 1, 11 |
| 14 | 203.7, C | ||
| 15 | 24.2, CH3 | 1.16, s | 1, 2, 3, 10, 14 |
| 16 | 23.5, CH3 | 1.78, s | 4, 5, 6 |
| 17 | 125.8, C | ||
| 18 | 171.7, C | ||
| 19 | 9.1, CH3 | 1.95, s | 8, 17, 18 |
| 20 | 30.4, CH3 | 1.35, s | 10, 11, 12 |
| 7, 11-OH | 3.52, br |
| Position | Bathyptilone B (2) | Bathyptilone C (3) | ||
|---|---|---|---|---|
| C, type a,c | 1H (J in Hz) b | C, type | 1H (J in Hz) | |
| 1 | 52.5, C | 50.5, C | ||
| 2 | 77.9, CH | 5.89, dd (4.8, 12.9) | 31.5, CH2 | a 1.90, m b 1.56, m |
| 3 | 27.6, CH2 | a 3.93, dt (3.2, 14.7) b 1.89, m | 21.8, CH2 | a 1.95, m b 1.52, m |
| 4 | 26.3, CH2 | a 1.99, m b 2.32, m | 30.3, CH2 | a 2.60, td (13.5, 5.3) b 2.03, m |
| 5 | 146.0, C | 141.2, C | ||
| 6 | 122.8, CH | 5.26, s | 122.4, CH | 5.00, br d (8.6) |
| 7 | 106.3, C | 79.2, CH | 5.66, br d (8.2) | |
| 8 | 161.3, C | 159.4, C | ||
| 9 | 28.4, CH2 | a 3.64, m b 2.40, m | 22.4, CH2 | a 3.28, br dd (15.6, 9.7) b 2.34, br d (15.6) |
| 10 | 41.4, CH | 3.34, t (8.5) | 44.8, CH | 2.42, br d (9.3) |
| 11 | 38.0, CH | 2.45, m | 68.9, C | |
| 12 | 154.8, CH | 6.64, br d (10.3) | 151.2, CH | 6.62, br d (9.7) |
| 13 | 127.0, CH | 5.94, d (10.3) | 127.6, CH | 6.00, br d (9.7) |
| 14 | 201.4, C | 203.2, C | ||
| 15 | 16.7, CH3 | 1.11, s | 24.0, CH3 | 1.14, s |
| 16 | 23.2, CH3 | 1.77, s | 22.7, CH3 | 1.74, s |
| 17 | 124.3, C | 122.9, C | ||
| 18 | 171.7, C | 1.91, s | 163.9, C | |
| 19 | 9.1, CH3 | 9.1, CH3 | 1.94, s | |
| 20 | 18.8, CH3 | 1.14, d (6.5) | 30.8, CH3 | 1.27, s |
| 21 | 169.5, C | |||
| 22 | 21.2, CH3 | 2.03, s | ||
| Position | 13C, type | 1H (J in Hz) |
|---|---|---|
| 1 | 52.8, C | |
| 2 | 31.9, CH2 | 1.14, m 1.39, m |
| 3 | 19.5, CH2 | 1.20, m 1.30, m |
| 4 | 46.3, CH | 2.20, m |
| 5 | 177.5, C | |
| 6 | 133.2, CH | 5.85, s |
| 7 | 205.4, C | |
| 8 | 99.5, C | |
| 9 | 28.3, CH2 | 1.36, m 1.46, dd (12.4, 7.0) |
| 10 | 32.2, CH | 1.35, m |
| 11 | 36.1, CH | 2.08, m |
| 12 | 153.6, CH | 6.57, dd (10.9, 6.2) |
| 13 | 128.0, CH | 6.07, d (10.9) |
| 14 | 202.9, C | |
| 15 | 19.3, CH3 | 1.24, s |
| 16 | 25.7, CH3 | 2.09, s |
| 17 | 19.6, CH3 | 1.09, d (6.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.A.L.; Sanchez, A.; Kee, Y.; Wilson, N.G.; Baker, B.J. Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum grandiflorum (Verrill, 1879). Mar. Drugs 2019, 17, 513. https://doi.org/10.3390/md17090513
Thomas SAL, Sanchez A, Kee Y, Wilson NG, Baker BJ. Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum grandiflorum (Verrill, 1879). Marine Drugs. 2019; 17(9):513. https://doi.org/10.3390/md17090513
Chicago/Turabian StyleThomas, Santana A.L., Anthony Sanchez, Younghoon Kee, Nerida G. Wilson, and Bill J. Baker. 2019. "Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum grandiflorum (Verrill, 1879)" Marine Drugs 17, no. 9: 513. https://doi.org/10.3390/md17090513
APA StyleThomas, S. A. L., Sanchez, A., Kee, Y., Wilson, N. G., & Baker, B. J. (2019). Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum grandiflorum (Verrill, 1879). Marine Drugs, 17(9), 513. https://doi.org/10.3390/md17090513






