Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis
Abstract
:1. Introduction
2. Results
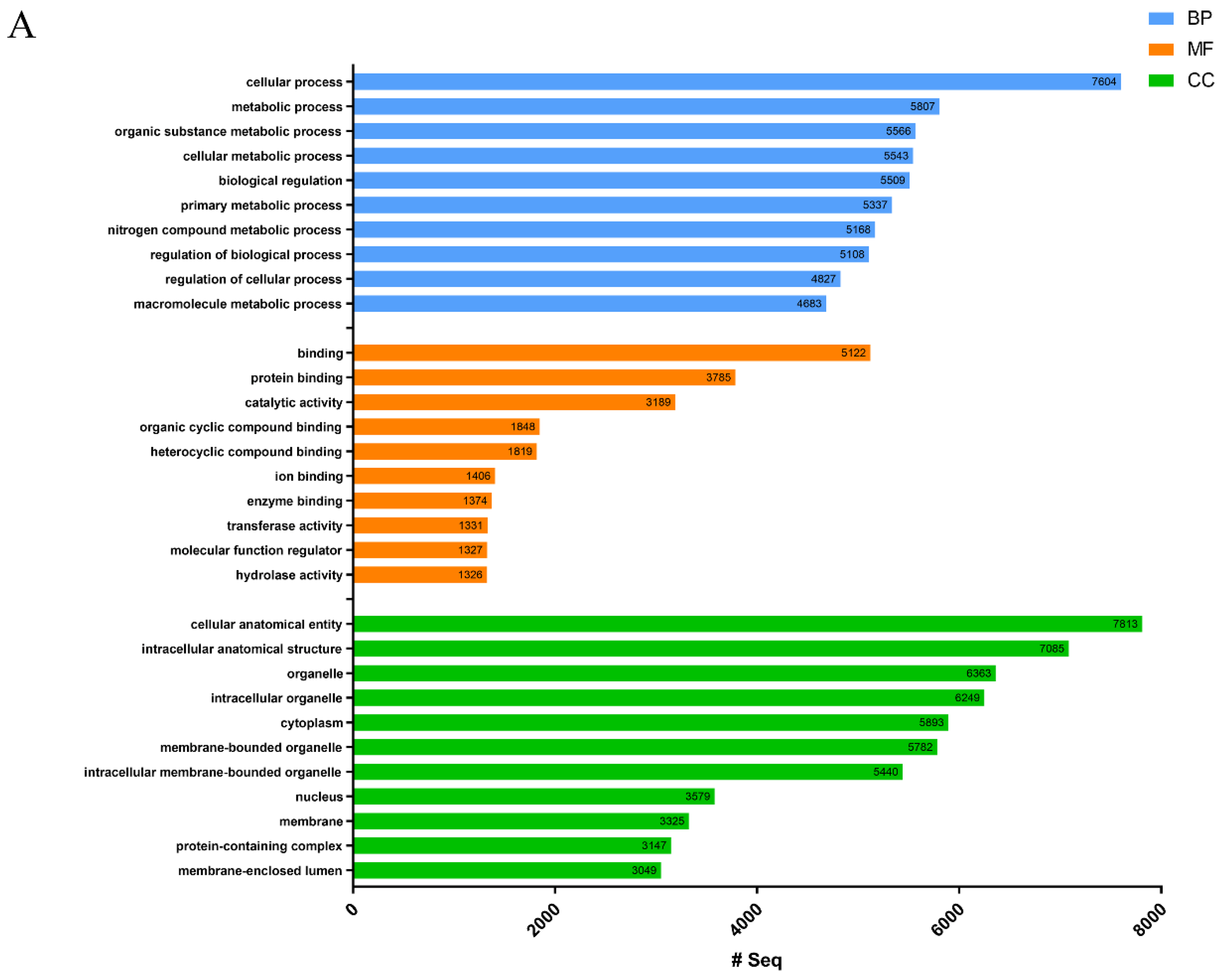
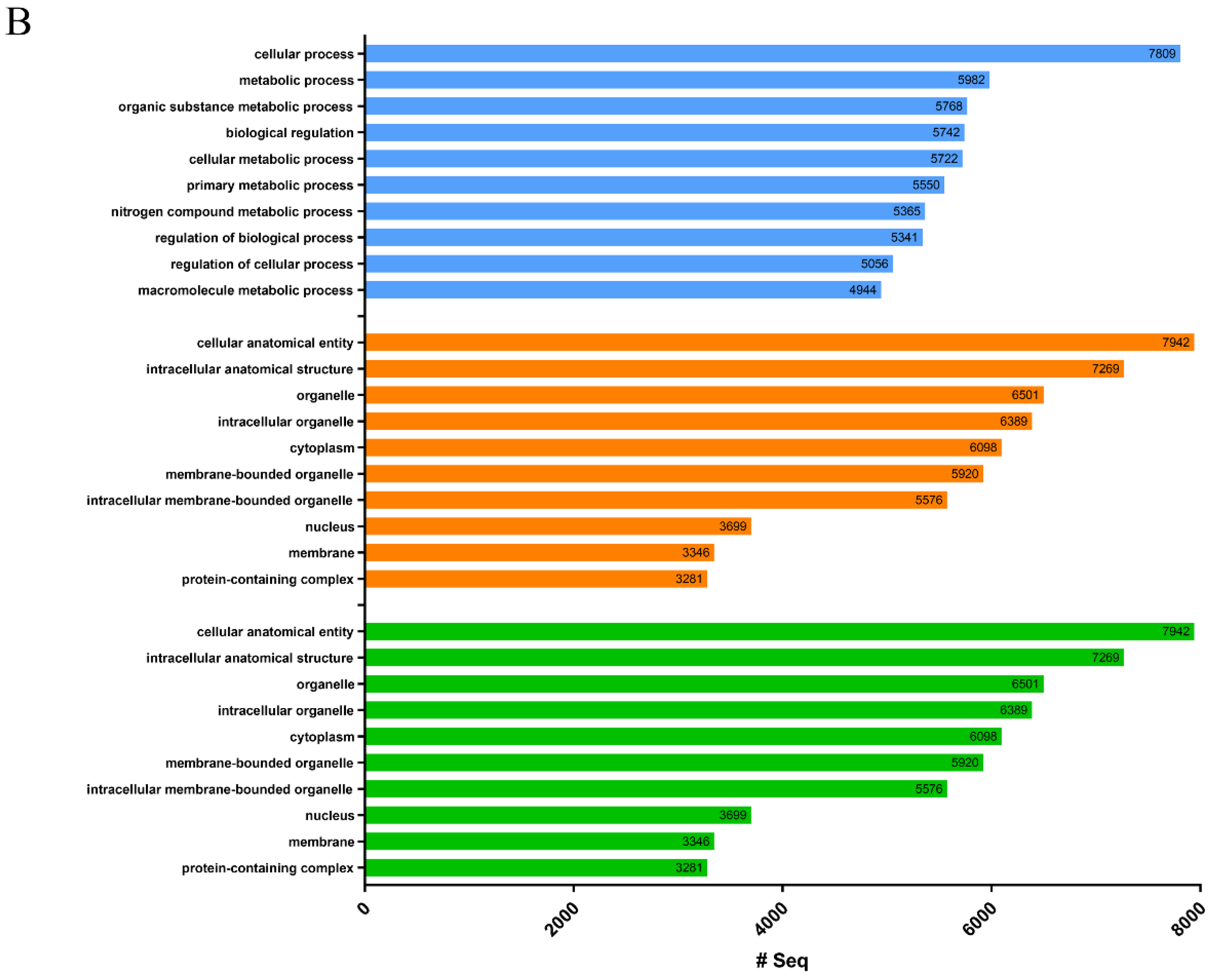
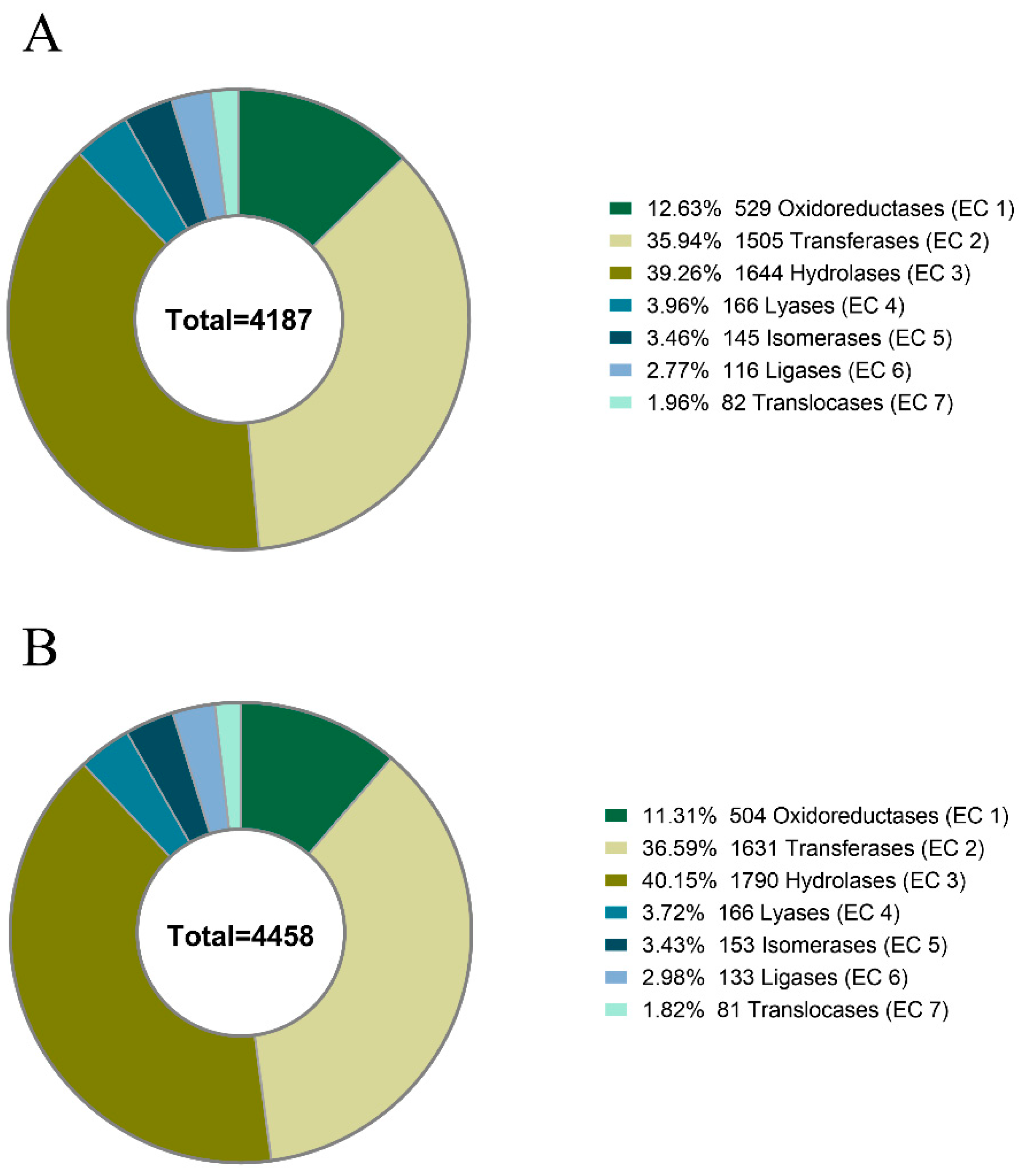
2.1. Transcriptome and Protein Database Construction
2.2. Identification of R. esculentum and S. malayensis Nematocyst Proteins by nano-LC-ESI MS/MS
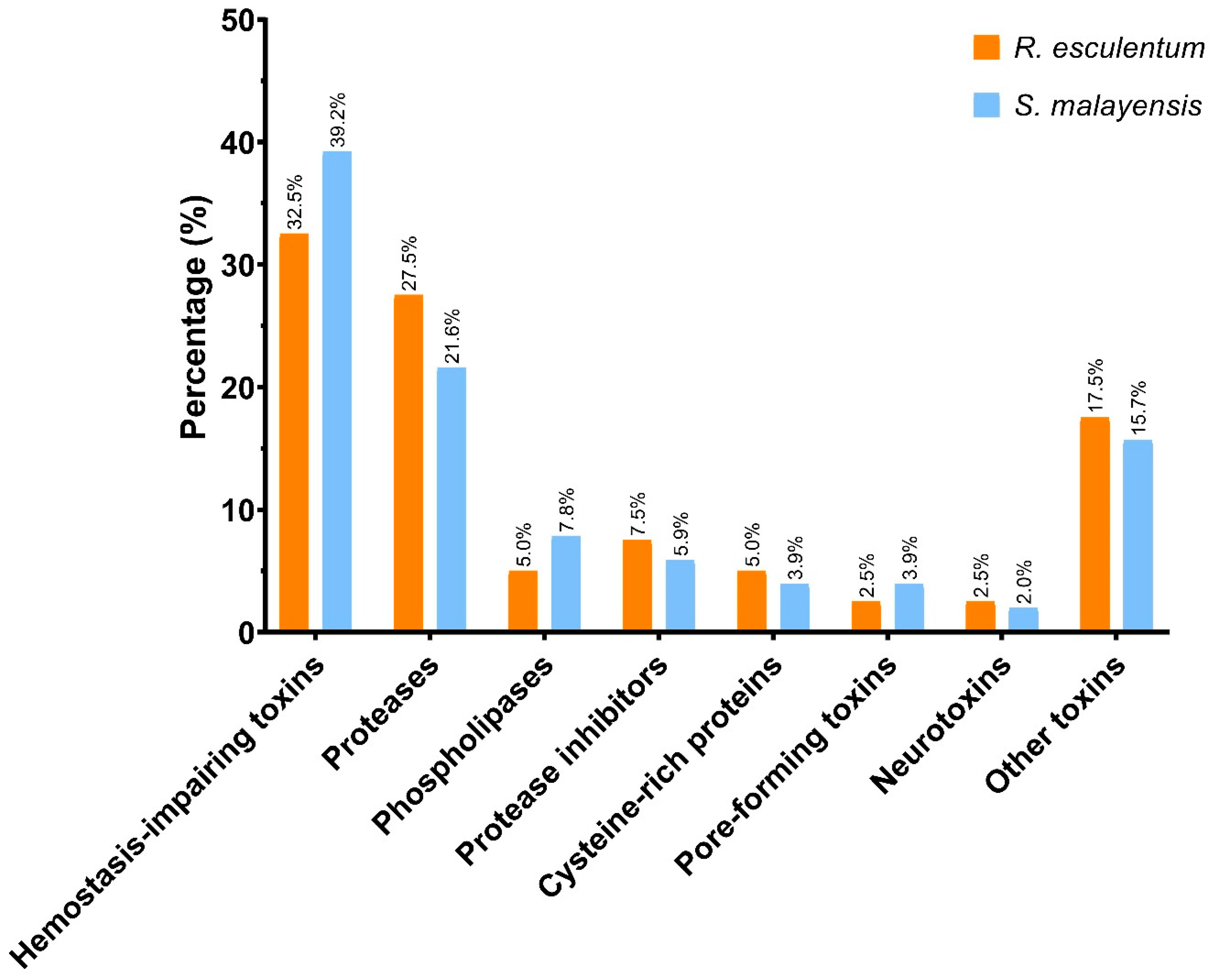
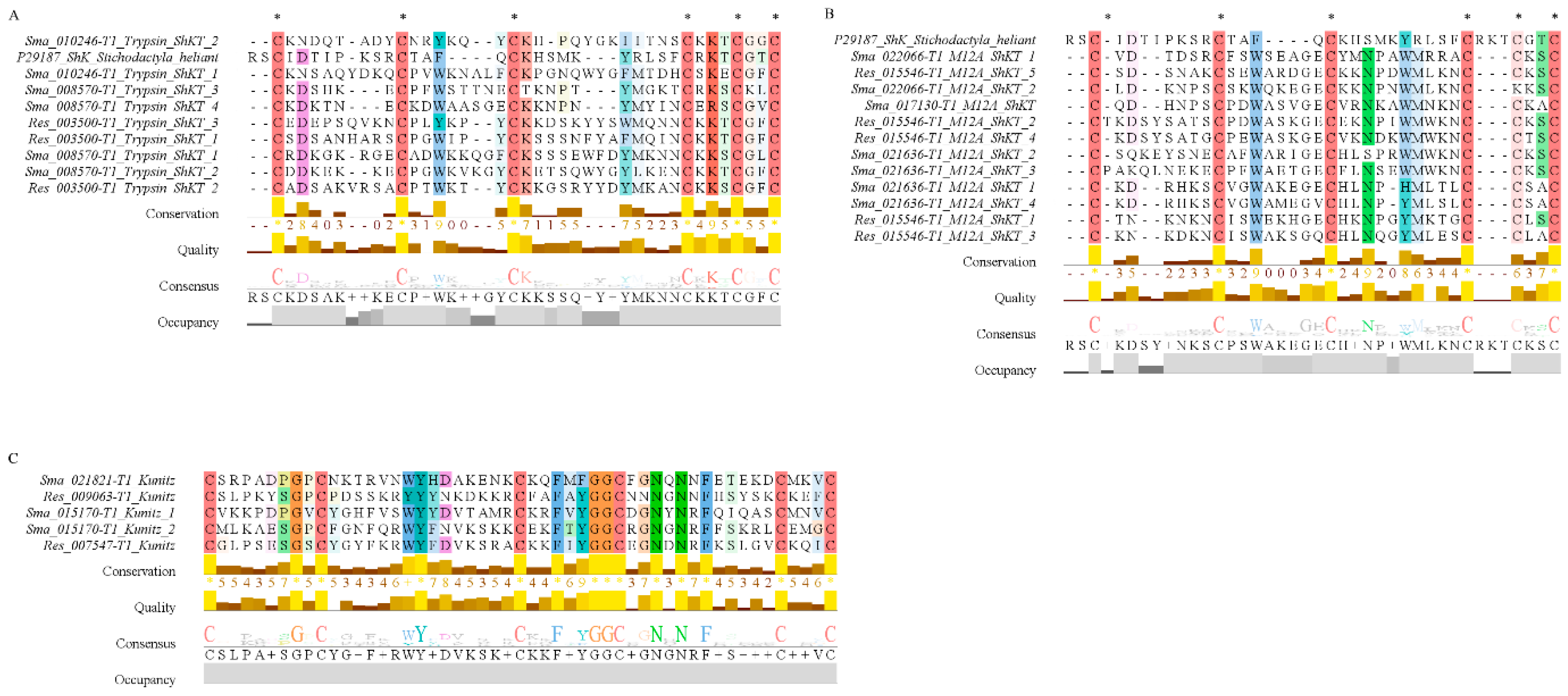
2.3. Functional Analysis of the Putative Toxins
2.4. InterProScan Analysis
3. Discussion
4. Materials and Methods
4.1. Jellyfish Collection
4.2. Sample Preparation for Proteomic Analysis
4.3. Spectral Searches and Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagadorn, J.W.; Dott, R.H.; Damrow, D. Stranded on a Late Cambrian shoreline: Medusae from central Wisconsin. Geology 2002, 30, 147–150. [Google Scholar] [CrossRef]
- Kayal, E.; Roure, B.; Philippe, H.; Collins, A.G.; Lavrov, D.V. Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evol. Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shostak, S. Cnidaria (Coelenterates). In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- WoRMS—World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=browser&accepted=1&id[]=2#focus (accessed on 20 October 2020).
- Beckmann, A.; Özbek, S. The Nematocyst: A molecular map of the Cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R. Venomology of Marine Cnidarians. In Biology and Ecology of Venomous Marine Cnidarians; Springer Singapore: Singapore, 2020; pp. 287–320. ISBN 9789811516030.0. [Google Scholar]
- Remigante, A.; Costa, R.; Morabito, R.; LaSpada, G.; Marino, A.; Dossena, S. Impact of scyphozoan venoms on human health and current first aid options for stings. Toxins 2018, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, M.; Uye, S.; Burnett, J.; Mianzan, H. Stings of edible jellyfish (Rhopilema hispidum, Rhopilema esculentum and Nemopilema nomurai) in Japanese waters. Toxicon 2006, 48, 713–716. [Google Scholar] [CrossRef]
- Fenner, P.J. Venomous jellyfish of the world. S. Pac. Underw. Med. Soc. J. 2005, 35, 131–138. [Google Scholar]
- Balamurugan, E.; Reddy, B.V.; Menon, V.P. Antitumor and antioxidant role of Chrysaora quinquecirrha (sea nettle) nematocyst venom peptide against ehrlich ascites carcinoma in Swiss Albino mice. Mol. Cell. Biochem. 2010, 338, 69–76. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.K.; Kim, M.; Pyo, M.J.; Kim, M.; Yang, S.; Won, C.K.; Yoon, W.D.; Han, C.H.; Kang, C.; et al. Anticancer Effect of Nemopilema nomurai Jellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evid. Based Complement. Altern. Med. 2017, 2017, 2752716. [Google Scholar] [CrossRef] [Green Version]
- Ayed, Y.; Sghaier, R.M.; Laouini, D.; Bacha, H. Evaluation of anti-proliferative and anti-inflammatory activities of Pelagia noctiluca venom in Lipopolysaccharide/Interferon-γ stimulated RAW264.7 macrophages. Biomed. Pharmacother. 2016, 84, 1986–1991. [Google Scholar] [CrossRef]
- Ayed, Y.; Dellai, A.; Mansour, H.B.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Taxonomy: “Metazoa [33208]” (Keyword: Toxin OR Annotation: (Type: “Tissue Specificity” Venom)) and Reviewed: Yes. Available online: https://www.uniprot.org/uniprot/?query=taxonomy%3A%22Metazoa+%5B33208%5D%22+AND+%28keyword%3Atoxin++OR+annotation%3A%28type%3A%22tissue+specificity%22+AND+venom%29%29+AND+reviewed%3Ayes (accessed on 28 October 2020).
- Nong, W.; Cao, J.; Li, Y.; Qu, Z.; Sun, J.; Swale, T.; Yip, H.Y.; Qian, P.Y.; Qiu, J.W.; Kwan, H.S.; et al. Jellyfish genomes reveal distinct homeobox gene clusters and conservation of small RNA processing. Nat. Commun. 2020, 11, 3151. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2018, 47, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungo, F.; Bougueleret, L.; Xenarios, I.; Poux, S. The UniProtKB/Swiss-Prot Tox-Prot program: A central hub of integrated venom protein data. Toxicon 2012, 60, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacesa, R.; Barlow, D.J.; Long, P.F. Machine learning can differentiate venom toxins from other proteins having non-toxic physiological functions. PeerJ Comput. Sci. 2016, 2016, e90. [Google Scholar] [CrossRef]
- Negi, S.S.; Schein, C.H.; Ladics, G.S.; Mirsky, H.; Chang, P.; Rascle, J.B.; Kough, J.; Sterck, L.; Papineni, S.; Jez, J.M.; et al. Functional classification of protein toxins as a basis for bioinformatic screening. Sci. Rep. 2017, 7, 13940. [Google Scholar] [CrossRef]
- Hargreaves, A.D.; Swain, M.T.; Hegarty, M.J.; Logan, D.W.; Mulley, J.F. Restriction and recruitment-gene duplication and the origin and evolution of snake venom toxins. Genome Biol. Evol. 2014, 6, 2088–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ompraba, G.; Chapeaurouge, A.; Doley, R.; Devi, K.R.; Padmanaban, P.; Venkatraman, C.; Velmurugan, D.; Lin, Q.; Kini, R.M. Identification of a novel family of snake venom proteins veficolins from cerberus rynchops using a venom gland transcriptomics and proteomics approach. J. Proteome Res. 2010, 9, 1882–1893. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Pan, Y.; Tian, M.; Li, Y.; He, C.; Dong, Y.; Sun, Y.; Zhou, Z. Chromosome-level reference genome of the jellyfish Rhopilema esculentum. GigaScience 2020, 9, giaa036. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish venomics and venom gland transcriptomics analysis of Stomolophus meleagris to reveal the toxins associated with sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef]
- Choudhary, I.; Hwang, D.H.; Lee, H.; Yoon, W.D.; Chae, J.; Han, C.H.; Yum, S.; Kang, C.; Kim, E. Proteomic analysis of novel components of nemopilema nomurai jellyfish venom: Deciphering the mode of action. Toxins 2019, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Jiang, G.; Wang, T.; Zhang, J.; Liu, W.; Xu, Z.; Zhang, J.; Xiao, L. An integrated transcriptomic and proteomic analysis reveals toxin arsenal of a novel Antarctic jellyfish Cyanea sp. J. Proteom. 2019, 208, 103483. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.; Wang, B.; Wang, Q.; Liu, G.; Wang, T.; He, Q.; Zhang, L. Unique Diversity of Sting-Related Toxins Based on Transcriptomic and Proteomic Analysis of the Jellyfish Cyanea capillata and Nemopilema nomurai (Cnidaria: Scyphozoa). J. Proteome Res. 2019, 18, 436–448. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake venomics of the lesser antillean pit vipers bothrops caribbaeus and Bothrops lanceolatus: Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef]
- Öhler, M.; Georgieva, D.; Seifert, J.; VonBergen, M.; Arni, R.K.; Genov, N.; Betzel, C. The venomics of bothrops alternatus is a pool of acidic proteins with predominant hemorrhagic and coagulopathic activities. J. Proteome Res. 2010, 9, 2422–2437. [Google Scholar] [CrossRef]
- Valenzuela, J.G.; Garfield, M.; Rowton, E.D.; Pham, V.M. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J. Exp. Biol. 2004, 207, 3717–3729. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, G.S.; Junqueira-de-Azevedo, I.L.M.; Lopes-Ferreira, M.; Lorenzini, D.M.; Ho, P.L.; Moura-da-Silva, A.M. Transcriptome analysis of expressed sequence tags from the venom glands of the fish Thalassophryne nattereri. Biochimie 2006, 88, 693–699. [Google Scholar] [CrossRef]
- Ponce, D.; Brinkman, D.L.; Potriquet, J.; Mulvenna, J. Tentacle transcriptome and venom proteome of the pacific sea nettle, Chrysaora fuscescens (Cnidaria: Scyphozoa). Toxins 2016, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.; Miyake, Y.; Kobayashi, K.; Murata, Y.; Iizasa, S.; Iizasa, E.; Yamasaki, S.; Hirakawa, N.; Hara, H.; Yoshida, H.; et al. Essential roles of C-type lectin Mincle in induction of neuropathic pain in mice. Sci. Rep. 2019, 9, 872. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, D.L.; Jia, X.; Potriquet, J.; Kumar, D.; Dash, D.; Kvaskoff, D.; Mulvenna, J. Transcriptome and venom proteome of the box jellyfish Chironex fleckeri. BMC Genom. 2011. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yu, H.; Yue, Y.; Liu, S.; Xing, R.; Chen, X.; Li, P. Combined proteomics and transcriptomics identifies sting-related toxins of jellyfish Cyanea nozakii. J. Proteom. 2016, 148, 57–64. [Google Scholar] [CrossRef]
- Moran, Y.; Praher, D.; Schlesinger, A.; Ayalon, A.; Tal, Y.; Technau, U. Analysis of Soluble Protein Contents from the Nematocysts of a Model Sea Anemone Sheds Light on Venom Evolution. Mar. Biotechnol. 2013, 15, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jung, E.; Kang, C.; Yoon, W.D.; Kim, J.S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef]
- Almeida, F.M.; Pimenta, A.M.C.; DeFigueiredo, S.G.; Santoro, M.M.; Martin-Eauclaire, M.F.; Diniz, C.R.; DeLima, M.E. Enzymes with gelatinolytic activity can be found in Tityus bahiensis and Tityus serrulatus venoms. Toxicon 2002, 40, 1041–1045. [Google Scholar] [CrossRef]
- Serrano, S.M.T.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef]
- Li, A.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Inhibitory Effect of Metalloproteinase Inhibitors on Skin Cell Inflammation Induced by Jellyfish Nemopilema nomurai Nematocyst Venom. Toxins 2019, 11, 156. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Weber, J.A.; Lee, N.; Park, S.G.; Cho, Y.S.; Bhak, Y.; Lee, N.; Jeon, Y.; Jeon, S.; Luria, V.; et al. The genome of the giant Nomura’s jellyfish sheds light on the early evolution of active predation. BMC Biol. 2019, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Ye, M.; Xu, J.; Guo, C.; Zheng, H.; Hu, J.; Chen, J.; Wang, Y.; Xu, S.; Yan, X. Lipid Profile in Different Parts of Edible Jellyfish Rhopilema esculentum. J. Agric. Food Chem. 2015, 63, 8283–8291. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Liu, D.; Wang, Q.; Ruan, Z.; He, Q.; Zhang, L. Global transcriptome analysis of the tentacle of the Jellyfish Cyanea capillata using deep sequencing and expressed sequence tags: Insight into the toxin-and degenerative disease-related transcripts. PLoS ONE 2015, 10, e0142680. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, C.; Li, R.; Xing, R.; Liu, S.; Li, P. Factors influencing hemolytic activity of venom from the jellyfish Rhopilema esculentum Kishinouye. Food Chem. Toxicol. 2007, 45, 1173–1178. [Google Scholar] [CrossRef]
- Hseu, M.-J.; Yen, C.-H.; Tzeng, M.-C. Crocalbin: A new calcium-binding protein that is also a binding protein for crotoxin, a neurotoxic phospholipase A 2. FEBS Lett. 1999, 445, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Dodds, D.; Schlimgen, A.K.; Lu, S.-Y.; Perin, M.S. Novel Reticular Calcium Binding Protein Is Purified on Taipoxin Columns. J. Neurochem. 2002, 64, 2339–2344. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; Mcanulla, C.; Mcwilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Mourão, C.B.F.; Schwartz, E.F. Protease inhibitors from marine venomous animals and their counterparts in terrestrial venomous animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef] [Green Version]
- Gorman, L.M.; Judge, S.J.; Fezai, M.; Jemaà, M.; Harris, J.B.; Caldwell, G.S. The venoms of the lesser (Echiichthys vipera) and greater (Trachinus draco) weever fish—A review. Toxicon X 2020, 6, 100025. [Google Scholar] [CrossRef]
- Sung, J.M.L.; Low, K.S.Y.; Khoo, H.E. Characterization of the mechanism underlying stonustoxin-mediated relaxant response in the rat aorta in vitro. Biochem. Pharmacol. 2002, 63, 1113–1118. [Google Scholar] [CrossRef]
- Yew, W.S.; Khoo, H.E. The role of tryptophan residues in the hemolytic activity of stonustoxin, a lethal factor from stonefish (Synanceja horrida) venom. Biochimie 2000, 82, 251–257. [Google Scholar] [CrossRef]
- Pennington, M.W.; Byrnes, M.E.; Zaydenberg, I.; Khaytin, I.; de Chastonay, J.; Krafte, D.S.; Hill, R.; Mahnir, V.M.; Volberg, W.A.; Gorczyca, W. Chemical synthesis and characterization of ShK toxin: A potent potassium channel inhibitor from a sea anemone. Int. J. Pept. Protein Res. 1995, 46, 354–358. [Google Scholar] [CrossRef]
- Chandy, K.G.; Norton, R.S. Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune disease. Curr. Opin. Chem. Biol. 2017, 38, 97–107. [Google Scholar] [CrossRef]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [Green Version]
- Tarcha, E.J.; Chi, V.; Muñoz-Elías, E.J.; Bailey, D.; Londono, L.M.; Upadhyay, S.K.; Norton, K.; Banks, A.; Tjong, I.; Nguyen, H.; et al. Durable pharmacological responses from the peptide ShK-186, a specific Kv1.3 channel inhibitor that suppresses T cell mediators of autoimmune disease. J. Pharmacol. Exp. Ther. 2012, 342, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK. Mar. Drugs 2015, 13, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Pennington, M.W.; Harunur Rashid, M.; Tajhya, R.B.; Beeton, C.; Kuyucak, S.; Norton, R.S. A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3. FEBS Lett. 2012, 586, 3996–4001. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.C.; Huq, R.; Chhabra, S.; Beeton, C.; Pennington, M.W.; Smith, B.J.; Norton, R.S. N-terminally extended analogues of the K+ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3. FEBS J. 2015, 282, 2247–2259. [Google Scholar] [CrossRef] [Green Version]
- Pennington, M.W.; Beeton, C.; Galea, C.A.; Smith, B.J.; Chi, V.; Monaghan, K.P.; Garcia, A.; Rangaraju, S.; Giuffrida, A.; Plank, D.; et al. Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes. Mol. Pharmacol. 2009, 75, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Liao, Q.; Li, S.; Siu, S.W.I.; Yang, B.; Huang, C.; Chan, J.Y.W.; Morlighem, J.É.R.L.; Wong, C.T.T.; Rádis-Baptista, G.; Lee, S.M.Y. Novel Kunitz-like Peptides Discovered in the Zoanthid Palythoa caribaeorum through Transcriptome Sequencing. J. Proteome Res. 2018, 17, 891–902. [Google Scholar] [CrossRef]
- Ranasinghe, S.L.; Rivera, V.; Boyle, G.M.; McManus, D.P. Kunitz type protease inhibitor from the canine tapeworm as a potential therapeutic for melanoma. Sci. Rep. 2019, 9, 16207. [Google Scholar] [CrossRef] [Green Version]
- Farkas, H.; Varga, L. Ecallantide is a novel treatment for attacks of hereditary angioedema due to C1 inhibitor deficiency. Clin. Cosmet. Investig. Dermatol. 2011, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]


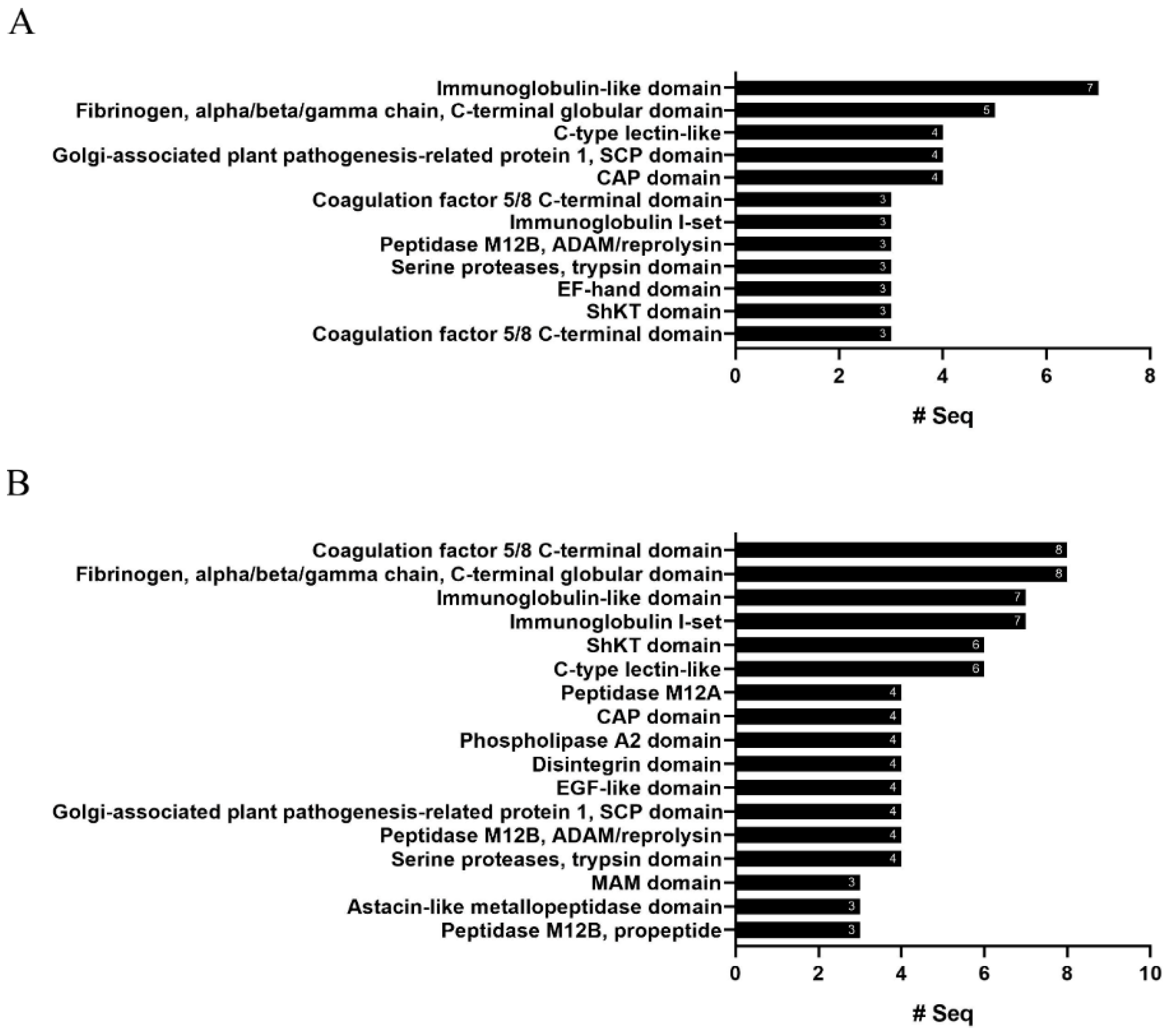
| R. esculentum | S. malayensis | |
|---|---|---|
| Proteins sequences | 18,923 | 26,914 |
| Proteins sequences annotated with GO terms | 8786 | 9138 |
| GO terms | 143,350 | 153,009 |
| Biological process GO terms | 80,786 | 24,533 |
| Molecular function GO terms | 22,970 | 41,992 |
| Cellular component GO terms | 39,612 | 86,484 |
| Enzymes | 4187 | 4485 |
| Putative toxins | 190 | 186 |
| R. esculentum | S. malayensis | |||
|---|---|---|---|---|
| Toxin Family | UniProt Accession | Description | UniProt Accession | Description |
| Hemostasis-impairing toxins | ||||
| A7X3Z7 | C-type lectin lectoxin-Lio2 (CTL) | C6JUN9 | C-type lectin (CTL) | |
| Q593B6 | Coagulation factor V (cleaved into coagulation factor V heavy chain and coagulation factor V light chain) | Q593B6 | Coagulation factor V (cleaved into coagulation factor V heavy chain and coagulation factor V light chain) | |
| Q66S03 | Galactose-specific lectin nattectin (CTL) | Q4QXT9 | Coagulation factor X (EC 3.4.21.6) (cleaved into factor X light chain, factor X heavy chain, and activated factor Xa heavy chain) | |
| D8VNS8 | Ryncolin-2 | Q66S03 | Galactose-specific lectin nattectin (CTL) | |
| D8VNT0 | Ryncolin-4 | D8VNS8 | Ryncolin-2 | |
| P22030 | Snaclec botrocetin subunit beta (platelet coagglutinin) | D8VNT0 | Ryncolin-4 | |
| Q7SZN0 | Venom prothrombin activator pseutarin-C non-catalytic subunit (PCNS) (vPA) (venom coagulation factor Va-like protein) (cleaved into pseutarin-C non-catalytic subunit heavy chain and pseutarin-C non-catalytic subunit light chain) | Q56EB0 | Snaclec bothrojaracin subunit beta (BJC subunit beta) | |
| A6MFK7 | Venom prothrombin activator vestarin-D1 (vPA) (EC 3.4.21.6) (venom coagulation factor Xa-like protease) (cleaved into vestarin-D1 light chain and vestarin-D1 heavy chain) | Q58L90 | Venom prothrombin activator omicarin-C non-catalytic subunit (vPA) (venom coagulation factor Va-like protein) (cleaved into omicarin-C non-catalytic subunit heavy chain and omicarin-C non-catalytic subunit light chain) | |
| Q58L91 | Venom prothrombin activator oscutarin-C non-catalytic subunit (vPA) (venom coagulation factor Va-like protein) (cleaved into oscutarin-C non-catalytic subunit heavy chain and oscutarin-C non-catalytic subunit light chain) | |||
| Q7SZN0 | Venom prothrombin activator pseutarin-C non-catalytic subunit (PCNS) (vPA) (venom coagulation factor Va-like protein) (cleaved into pseutarin-C non-catalytic subunit heavy chain and pseutarin-C non-catalytic subunit light chain) | |||
| A6MFK8 | Venom prothrombin activator vestarin-D2 (vPA) (EC 3.4.21.6) (venom coagulation factor Xa-like protease) (cleaved into vestarin-D2 light chain and vestarin-D2 heavy chain) | |||
| Proteases | ||||
| C9D7R2 | Astacin-like metalloprotease toxin 2 (EC 3.4.24.-) (Loxosceles astacin-like protease 2) (LALP2) | A0FKN6 | Astacin-like metalloprotease toxin 1 (EC 3.4.24.-) (Loxosceles astacin-like protease 1) (LALP) (LALP1) | |
| Q76B45 | Blarina toxin (BLTX) (EC 3.4.21.-) | P0DM62 | Astacin-like metalloprotease toxin 5 (EC 3.4.24.-) (Loxosceles astacin-like protease 5) (LALP5) (Fragment) | |
| K7Z9Q9 | Nematocyst-expressed protein 6 (NEP-6) (EC 3.4.24.-) (astacin-like metalloprotease toxin) | Q76B45 | Blarina toxin (BLTX) (EC 3.4.21.-) | |
| W4VS99 | Neprilysin-1 (EC 3.4.24.-) | K7Z9Q9 | Nematocyst-expressed protein 6 (NEP-6) (EC 3.4.24.-) (astacin-like metalloprotease toxin) | |
| B2D0J4 | Venom dipeptidyl peptidase 4 (allergen C) (venom dipeptidyl peptidase IV) (EC 3.4.14.5) (allergen Api m 5) | W4VS99 | Neprilysin-1 (EC 3.4.24.-) | |
| Q7M4I3 | Venom protease (EC 3.4.21.-) (allergen Bom p 4) | B2D0J4 | Venom dipeptidyl peptidase 4 (allergen C) (venom dipeptidyl peptidase IV) (EC 3.4.14.5) (allergen Api m 5) | |
| C9WMM5 | Venom serine carboxypeptidase (EC 3.4.16.5) (allergen Api m 9) | O73795 | Zinc metalloproteinase/disintegrin (cleaved into snake venom metalloproteinase Mt-b (SVMP) (EC 3.4.24.-) and disintegrin) | |
| J3S830 | Zinc metalloproteinase-disintegrin-like 3a (EC 3.4.24.-) (snake venom metalloproteinase) (SVMP) | P20164 | Zinc metalloproteinase-disintegrin-like HR1b (EC 3.4.24.-) (snake venom metalloproteinase) (SVMP) (trimerelysin I) (trimerelysin-1) (cleaved into disintegrin-like 1b) | |
| F8RKW0 | Zinc metalloproteinase-disintegrin-like MTP8 (EC 3.4.24.-) (snake venom metalloproteinase) (SVMP) | A8QL59 | Zinc metalloproteinase-disintegrin-like NaMP (EC 3.4.24.-) (snake venom metalloproteinase) (SVMP) | |
| Q2LD49 | Zinc metalloproteinase-disintegrin-like TSV-DM (EC 3.4.24.-) (snake venom metalloproteinase) (SVMP) | |||
| Phospholipase | ||||
| P80003 | Acidic phospholipase A2 PA4 (PLA2) (EC 3.1.1.4) (phosphatidylcholine 2-acylhydrolase) (cleaved into acidic phospholipase A2 PA2) | P80003 | Acidic phospholipase A2 PA4 (PLA2) (EC 3.1.1.4) (phosphatidylcholine 2-acylhydrolase) (cleaved into acidic phospholipase A2 PA2) | |
| P16354 | Phospholipase A2 isozymes PA3A/PA3B/PA5 (PLA2) (EC 3.1.1.4) (phosphatidylcholine 2-acylhydrolase) | I7GQA7 | Phospholipase A(2) (EC 3.1.1.4) (phosphatidylcholine 2-acylhydrolase) | |
| P16354 | Phospholipase A2 isozymes PA3A/PA3B/PA5 (PLA2) (EC 3.1.1.4) (phosphatidylcholine 2-acylhydrolase) | |||
| Protease inhibitors | ||||
| B1P1J3 | Cystatin-1 (cystatin JZTX-75) | J3RYX9 | Cystatin-1 | |
| P0C8W3 | Kunitz-type serine protease inhibitor Hg1 (delta-KTx 1.1) | Q6T269 | Kunitz-type serine protease inhibitor bitisilin-3 (two-Kunitz protease inhibitor) (fragment) | |
| W4VSH9 | Kunitz-type U19-barytoxin-Tl1a (U19-BATX-Tl1a) (Kunitz-type serine protease inhibitor Kunitz-1) | |||
| Pore-forming toxins | ||||
| Q91453 | Stonustoxin subunit beta (SNTX subunit beta) (DELTA-synanceitoxin-Sh1b) (DELTA-SYTX-Sh1b) (trachynilysin subunit beta) (TLY subunit beta) | A0ZSK4 | Neoverrucotoxin subunit beta (NeoVTX subunit beta) | |
| Cysteine-rich proteins | ||||
| Q3SB07 | Cysteine-rich venom protein pseudechetoxin-like (CRVP) | A6MFK9 | Cysteine-rich venom protein (CRVP) (cysteine-rich secretory protein) (CRISP) | |
| Q8AVA3 | Cysteine-rich venom protein pseudecin (CRVP Pdc) | P81656 | Venom allergen 5 (antigen 5) (Ag5) (cysteine-rich venom protein) (CRVP) (allergen Pol d 5) | |
| Neurotoxins | ||||
| Q25338 | Delta-latroinsectotoxin-Lt1a (delta-LIT-Lt1a) (delta-latroinsectotoxin) (delta-LIT) | G0LXV8 | Alpha-latrotoxin-Lh1a (Alpha-LTX-Lh1a) (alpha-latrotoxin) (fragment) | |
| Other toxins | ||||
| Q8AY75 | Calglandulin | Q92035 | Acetylcholinesterase (BfAChE) (EC 3.1.1.7) | |
| A7ISW2 | Glutaminyl-peptide cyclotransferase (EC 2.3.2.5) (glutaminyl cyclase) (QC) (glutaminyl-tRNA cyclotransferase) | A7ISW1 | Glutaminyl-peptide cyclotransferase (EC 2.3.2.5) (glutaminyl cyclase) (QC) (glutaminyl-tRNA cyclotransferase) | |
| Q75WF2 | Plancitoxin-1 (EC 3.1.22.1) (plancitoxin I) (plan-I) (cleaved into plancitoxin-1 subunit alpha and plancitoxin-1 subunit beta) | J3S820 | Hyaluronidase (EC 3.2.1.35) (hyaluronoglucosaminidase) (venom-spreading factor) | |
| J3S9D9 | Reticulocalbin-2 (taipoxin-associated calcium-binding protein 49 homolog) | A8QL51 | l-amino-acid oxidase (Bm-LAAO) (LAO) (EC 1.4.3.2) | |
| M5B4R7 | Translationally controlled tumor protein homolog (GTx-TCTP1) | Q75WF2 | Plancitoxin-1 (EC 3.1.22.1) (plancitoxin I) (Plan-I) (cleaved into plancitoxin-1 subunit alpha and plancitoxin-1 subunit beta) | |
| P0DN11 | U-actitoxin-Avd3j (U-AITX-Avd3j) (AsKC7) | J3S9D9 | Reticulocalbin-2 (taipoxin-associated calcium-binding protein 49 homolog) | |
| M5B4R7 | Translationally controlled tumor protein homolog (GTx-TCTP1) | |||
| Q5BLY5 | Venom acid phosphatase Acph-1 (EC 3.1.3.2) (allergen Api m 3) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, T.C.N.; Qu, Z.; Nong, W.; Hui, J.H.L.; Ngai, S.M. Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis. Mar. Drugs 2020, 18, 655. https://doi.org/10.3390/md18120655
Leung TCN, Qu Z, Nong W, Hui JHL, Ngai SM. Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis. Marine Drugs. 2020; 18(12):655. https://doi.org/10.3390/md18120655
Chicago/Turabian StyleLeung, Thomas C. N., Zhe Qu, Wenyan Nong, Jerome H. L. Hui, and Sai Ming Ngai. 2020. "Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis" Marine Drugs 18, no. 12: 655. https://doi.org/10.3390/md18120655
APA StyleLeung, T. C. N., Qu, Z., Nong, W., Hui, J. H. L., & Ngai, S. M. (2020). Proteomic Analysis of the Venom of Jellyfishes Rhopilema esculentum and Sanderia malayensis. Marine Drugs, 18(12), 655. https://doi.org/10.3390/md18120655





