Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig
Abstract
:1. Introduction
2. Results
2.1. Animal Performance and Faecal Scores
2.2. Volatile Fatty Acid Analysis
2.3. Large Intestinal Microbiota
2.3.1. Bacterial Richness and Diversity Analysis
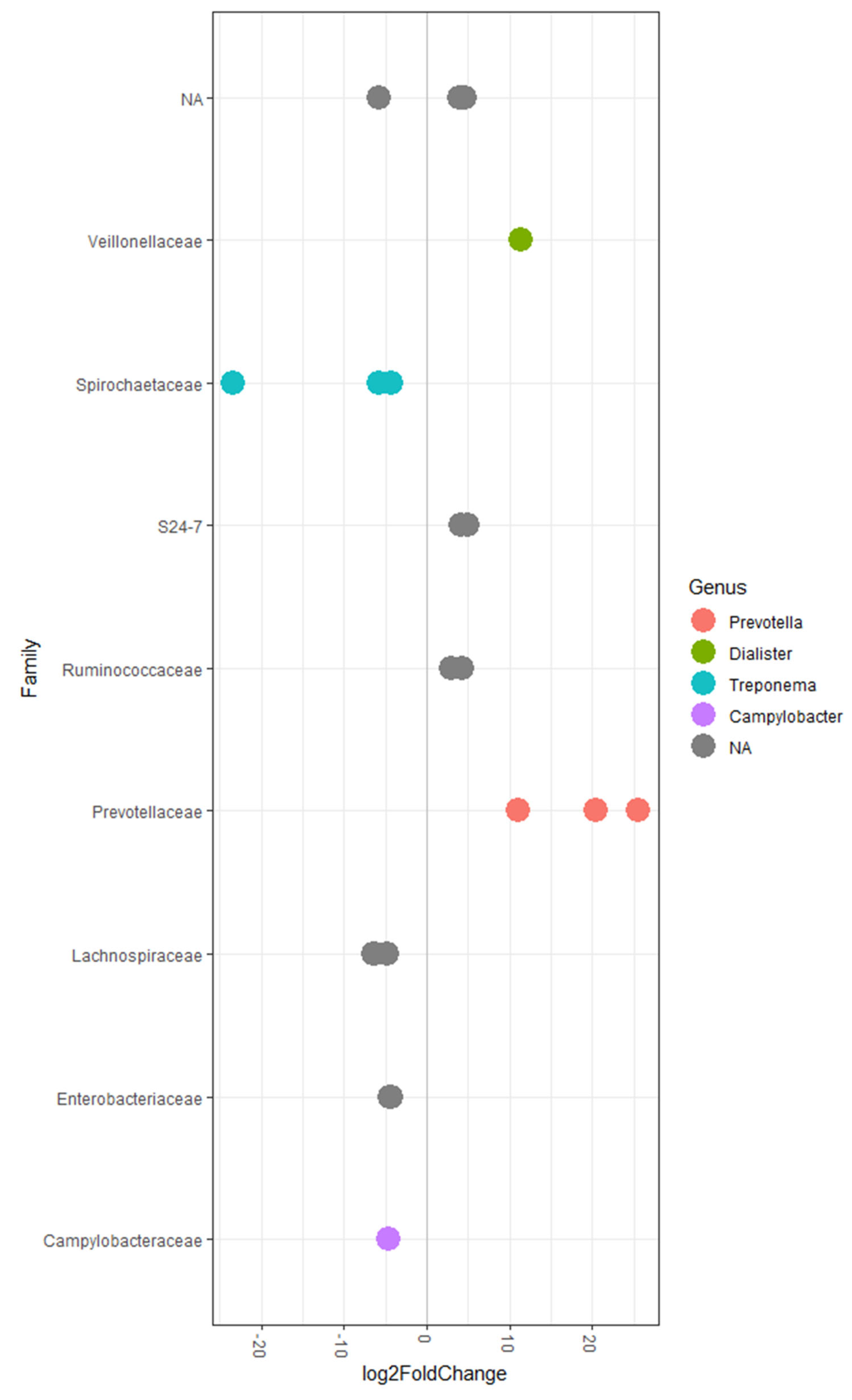
2.3.2. Differential Abundance Analysis of Bacterial Taxa in Caecal Digesta and Their Correlations with Performance Traits
2.3.3. Differential Abundance Analysis of Bacterial Taxa in Colonic Digesta and Their Correlations with Performance Traits
3. Discussion
4. Materials and Methods
4.1. Experimental Design, Animal Management, and Diets
4.2. Feed Analysis
4.3. Volatile Fatty Acid Analysis
4.4. Microbiological Analyses
4.4.1. Microbial DNA Extraction
4.4.2. Illumina Sequencing
4.5. Bioinformatic and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sweeney, T.; O’Doherty, J.V. Marine macroalgal extracts to maintain gut homeostasis in the weaning piglet. Domest. Anim. Endocrinol. 2016, 56, S84–S89. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Doherty, J.V.; Bouwhuis, M.A.; Sweeney, T. Novel marine polysaccharides and maternal nutrition to stimulate gut health and performance in post-weaned pigs. Anim. Prod. Sci. 2017, 57, 2376–2385. [Google Scholar] [CrossRef]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Selective detection, enumeration and identification of potentially probiotic Lactobacillus and Bifidobacterium species in mixed bacterial populations. Int. J. Food Microbiol. 1997, 35, 1–27. [Google Scholar] [CrossRef]
- Conway, T.; Cohen, P.S. Commensal and Pathogenic Escherichia coli Metabolism in the Gut. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Gad, S.A.; El-Baky, R.M.A.; Ahmed, A.B.F.; Gad, G.F.M. In Vitro evaluation of probiotic potential of five lactic acid bacteria and their antimicrobial activity against some enteric and food-borne pathogens. Afr. J. Microbiol. Res. 2016, 10, 400–409. [Google Scholar] [CrossRef]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’Doherty, J.V. Effect of purified beta-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Heim, G.; Walsh, A.M.; Sweeney, T.; Doyle, D.N.; O’Shea, C.J.; Ryan, M.T.; O’Doherty, J.V. Effect of seaweed-derived laminarin and fucoidan and zinc oxide on gut morphology, nutrient transporters, nutrient digestibility, growth performance and selected microbial populations in weaned pigs. Br. J. Nutr. 2014, 111, 1577–1585. [Google Scholar] [CrossRef]
- Reilly, P.; O’Doherty, J.V.; Pierce, K.M.; Callan, J.J.; O’Sullivan, J.T.; Sweeney, T. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2008, 2, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.P.; Dal Bello, F.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. The effects of liquid versus spray-dried Laminaria digitata extract on selected bacterial groups in the piglet gastrointestinal tract (GIT) microbiota. Anaerobe 2013, 21, 1–8. [Google Scholar] [CrossRef]
- Heim, G.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2014, 112, 1955–1965. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary Polysaccharide from Enteromorpha Clathrata Modulates Gut Microbiota and Promotes the Growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167. [Google Scholar] [CrossRef] [Green Version]
- Rattigan, R.; Sweeney, T.; Maher, S.; Thornton, K.; Rajauria, G.; O’Doherty, J.V. Laminarin-rich extract improves growth performance, small intestinal morphology, gene expression of nutrient transporters and the large intestinal microbial composition of piglets during the critical post-weaning period. Br. J. Nutr. 2019, 123, 255–263. [Google Scholar] [CrossRef]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajarillo, E.A.B.; Chae, J.-P.; Balolong, M.P.; Kim, H.B.; Kang, D.-K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Nie, Y.; Chen, J.; Zhang, Y.; Wang, Z.; Fan, Q.; Yan, X. Gradual Changes of Gut Microbiota in Weaned Miniature Piglets. Front. Microbiol. 2016, 7, 1727. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, L.L.; Naughton, P.J.; Hedemann, M.S.; Jensen, B.B. Effects of physical properties of feed on microbial ecology and survival of Salmonella enterica serovar Typhimurium in the pig gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 3485–3492. [Google Scholar] [CrossRef] [Green Version]
- Hojberg, O.; Canibe, N.; Knudsen, B.; Jensen, B.B. Potential rates of fermentation in digesta from the gastrointestinal tract of pigs: Effect of feeding fermented liquid feed. Appl. Environ. Microbiol. 2003, 69, 408–418. [Google Scholar] [CrossRef] [Green Version]
- O’Doherty, J.V.; Dillon, S.; Figat, S.; Callan, J.J.; Sweeney, T. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed. Sci. Technol. 2010, 157, 173–180. [Google Scholar] [CrossRef]
- Roubos-van den Hil, P.J.; Litjens, R.; Oudshoorn, A.-K.; Resink, J.W.; Smits, C.H.M. New perspectives to the enterotoxigenic E. coli F4 porcine infection model: Susceptibility genotypes in relation to performance, diarrhoea and bacterial shedding. Vet. Microbiol. 2017, 202, 58–63. [Google Scholar] [CrossRef]
- Dodd, D.; Mackie, R.I.; Cann, I.K. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol. Microbiol. 2011, 79, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J.; Bayer, E.A. Plant cell wall breakdown by anaerobic microorganisms from the Mammalian digestive tract. Ann. N. Y. Acad. Sci. 2008, 1125, 280–288. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Spatial Heterogeneity and Co-occurrence of Mucosal and Luminal Microbiome across Swine Intestinal Tract. Front. Microbiol. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the Extraction of Polysaccharides and Antioxidants from Macroalgae Using Sequential Hydrothermal-Assisted Extraction Followed by Ultrasound and Thermal Technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvant, D.; Perez, J.M.; Tran, G. Table of Composition and Nutritional Value of Feed Materials. Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses, Fish; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre and non starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Clarke, L.C.; Sweeney, T.; Curley, E.; Duffy, S.K.; Rajauria, G.; O’Doherty, J.V. The variation in chemical composition of barley feed with or without enzyme supplementation influences nutrient digestibility and subsequently affects performance in piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 799–809. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Hamady, M.; Lozupone, C.; Knight, R. Fast UniFrac: Facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010, 4, 17–27. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009; p. 216. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
| Control | Laminarin | SEM | p value | |
|---|---|---|---|---|
| Caecum | ||||
| Total VFA1 | 127.55 | 137.94 | 6.49 | 0.274 |
| Acetic acid | 92.57 | 98.85 | 4.79 | 0.368 |
| Propionic acid | 19.85 | 21.75 | 1.55 | 0.401 |
| Butyric acid | 12.76 | 15.15 | 1.29 | 0.211 |
| Isobutyric acid | 0.63 | 0.45 | 0.11 | 0.313 |
| Isovaleric acid | 0.45 | 0.44 | 0.06 | 0.879 |
| Valeric acid | 1.27 | 1.28 | 0.18 | 0.980 |
| Ace:Prop ratio | 4.69 | 4.76 | 0.29 | 0.870 |
| Colon | ||||
| Total VFA | 130.62 | 157.83 | 7.37 | 0.027 |
| Acetic acid | 95.36 | 107.12 | 6.03 | 0.034 |
| Propionic acid | 19.04 | 22.26 | 1.91 | 0.094 |
| Butyric acid | 11.77 | 17.96 | 1.78 | 0.050 |
| Isobutyric acid | 1.03 | 1.23 | 0.22 | 0.536 |
| Isovaleric acid | 0.89 | 1.2 | 0.136 | 0.121 |
| Valeric acid | 1.27 | 1.28 | 0.18 | 0.101 |
| Ace:Prop ratio | 5.11 | 5.01 | 0.18 | 0.822 |
| Control | Laminarin | SEM | p value | |
|---|---|---|---|---|
| Caecum | ||||
| Observed | 646.11 | 648.38 | 17.15 | 0.951 |
| Chao1 | 697.07 | 707.97 | 16.70 | 0.763 |
| ACE | 686.77 | 695.85 | 15.82 | 0.791 |
| Shannon | 3.92 | 3.93 | 0.11 | 0.971 |
| Simpson | 0.94 | 0.92 | 0.01 | 0.459 |
| InvSimpson | 19.12 | 19.89 | 2.60 | 0.892 |
| Fisher | 86.93 | 87.04 | 2.66 | 0.985 |
| Colon | ||||
| Observed | 689.50 | 705.00 | 16.16 | 0.657 |
| Chao1 | 739.06 | 748.56 | 13.40 | 0.743 |
| ACE | 725.70 | 735.86 | 14.22 | 0.741 |
| Shannon | 4.43 | 4.47 | 0.11 | 0.886 |
| Simpson | 0.96 | 0.95 | 0.01 | 0.615 |
| InvSimpson | 39.29 | 32.07 | 4.93 | 0.496 |
| Fisher | 94.48 | 96.72 | 2.49 | 0.679 |
| Taxa | Trait | Correlation | AdjPvalue |
|---|---|---|---|
| Prevotellaceae | Acetic | 0.68 | 0.01 |
| Porphyromonadaceae | ADFI | -0.76 | 0.00 |
| Helicobacteraceae | ADFI | -0.75 | 0.00 |
| BS11 | ADFI | -0.75 | 0.00 |
| Bacteroidaceae | ADFI | -0.74 | 0.00 |
| Fusobacteriaceae | ADFI | -0.73 | 0.01 |
| Prevotellaceae | ADFI | 0.53 | 0.03 |
| Helicobacteraceae | ADG | -0.63 | 0.01 |
| BS11 | ADG | -0.63 | 0.02 |
| Porphyromonadaceae | ADG | -0.61 | 0.02 |
| Enterobacteriaceae | ADG | -0.60 | 0.04 |
| Bacteroidaceae | ADG | -0.58 | 0.04 |
| Fusobacteriaceae | ADG | -0.57 | 0.04 |
| Prevotellaceae | ADG | 0.59 | 0.02 |
| BS11 | Buty | -0.72 | 0.00 |
| Porphyromonadaceae | Buty | -0.68 | 0.01 |
| Helicobacteraceae | Buty | -0.68 | 0.01 |
| Bacteroidaceae | Buty | -0.66 | 0.01 |
| Fusobacteriaceae | Buty | -0.65 | 0.01 |
| Enterobacteriaceae | Buty | -0.63 | 0.04 |
| Prevotellaceae | Buty | 0.56 | 0.02 |
| Prevotellaceae | IsoVal | -0.56 | 0.02 |
| Enterobacteriaceae | IsoVal | 0.56 | 0.05 |
| p.2534.18B5 | IsoVal | 0.65 | 0.03 |
| Mogibacteriaceae. | IsoVal | 0.75 | 0.00 |
| Prevotellaceae | Prop | 0.64 | 0.01 |
| Prevotellaceae | Total VFA | 0.76 | 0.00 |
| Taxa | Trait | Correlation | AdjPvalue |
|---|---|---|---|
| Prevotellaceae | Acetic | 0.49 | 0.05 |
| Porphyromonadaceae | ADFI | -0.77 | 0.00 |
| Bacteroidaceae | ADFI | -0.75 | 0.00 |
| Fusobacteriaceae | ADFI | -0.73 | 0.01 |
| Helicobacteraceae | ADFI | -0.71 | 0.01 |
| Prevotellaceae | ADFI | 0.53 | 0.04 |
| Prevotellaceae | ADG | 0.59 | 0.02 |
| Prevotellaceae | Buty | 0.63 | 0.01 |
| Porphyromonadaceae | Buty | -0.61 | 0.03 |
| Prevotellaceae | Prop | 0.69 | 0.01 |
| Prevotellaceae | Total VFA | 0.63 | 0.01 |
| Prevotellaceae | Valer | 0.75 | 0.00 |
| Ingredient (g/kg) | |
|---|---|
| Wheat | 340.0 |
| Full fat soya | 170.0 |
| Flaked wheat | 130.0 |
| Soya bean meal | 105.0 |
| Flaked maize | 70.0 |
| Whey powder | 50.0 |
| Soya oil | 65.0 |
| Vitamins and minerals | 2.5 |
| Sodium bicarbonate | 2.0 |
| Mono calcium phosphate | 4.0 |
| Calcium carbonate (Limestone) | 6.0 |
| Salt | 2.0 |
| Lysine HCL | 4.0 |
| DL-methionine | 1.5 |
| L-threonine | 1.5 |
| Chemical analysis | |
| DM | 866.1 |
| Crude protein (N × 6.25) | 190 |
| Digestible energy (MJ/kg) † | 14.95 |
| Ash | 48.4 |
| Neutral detergent fibre | 114.00 |
| Lysine † | 13.5 |
| Methionine and cysteine † | 7.4 |
| Threonine † | 7.9 |
| Tryptophan † | 2.6 |
| Calcium † | 7.2 |
| Phosphorous † | 6.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigors, S.; O’Doherty, J.V.; Rattigan, R.; McDonnell, M.J.; Rajauria, G.; Sweeney, T. Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Mar. Drugs 2020, 18, 157. https://doi.org/10.3390/md18030157
Vigors S, O’Doherty JV, Rattigan R, McDonnell MJ, Rajauria G, Sweeney T. Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Marine Drugs. 2020; 18(3):157. https://doi.org/10.3390/md18030157
Chicago/Turabian StyleVigors, Stafford, John V O’Doherty, Ruth Rattigan, Mary J McDonnell, Gaurav Rajauria, and Torres Sweeney. 2020. "Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig" Marine Drugs 18, no. 3: 157. https://doi.org/10.3390/md18030157
APA StyleVigors, S., O’Doherty, J. V., Rattigan, R., McDonnell, M. J., Rajauria, G., & Sweeney, T. (2020). Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Marine Drugs, 18(3), 157. https://doi.org/10.3390/md18030157






