New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster patagonicus and Their Anticancer Activity
Abstract
:1. Introduction
2. Results and Discussion
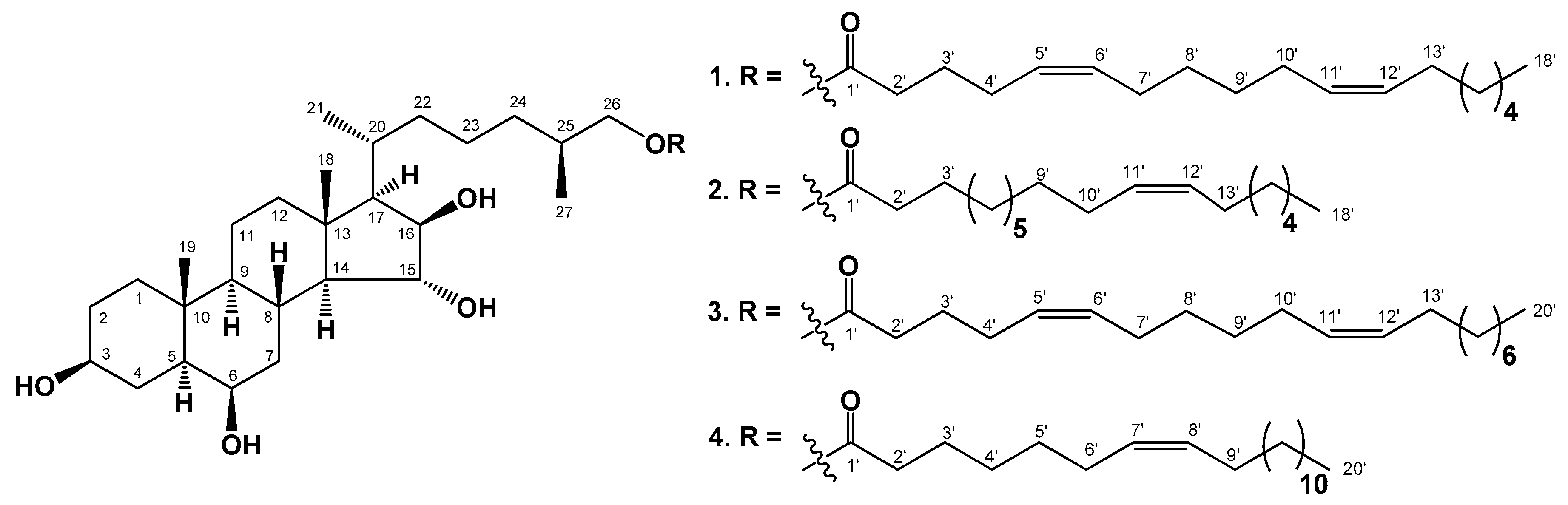
2.1. The Isolation and Structure Elucidation of Compounds 1–4 from C. patagonicus
2.2. In Vitro Anticancer Activity of Compounds 1–4
2.2.1. The Cytotoxic Activity of Compounds 1–4 against Normal and Cancer Cells
2.2.2. The Effect of Compounds 1–4 on the Colony Formation and Growth of Human Cancer Cells
2.2.3. The Effect of Compounds 1–4 on Migration of Human Cancer Cells
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. Methanolysis and Preparation of 4,4-Dimethyloxazoline Derivatives of Fatty Acids
3.6. FAME and DMOX Analysis.
3.7. Bioactivity Assay
3.7.1. Reagents
3.7.2. Cell Cultures
3.7.3. Compounds Preparation
3.7.4. Cell Viability Assay
3.7.5. Soft Agar Assay
3.7.6. Wound Healing Assay
3.7.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomes, A.R.; Freitas, A.C.; Rocha-Santos, T.A.P.; Duarte, A.C. Bioactive compounds derived from echinoderms. RSC Adv. 2014, 4, 29365–29382. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Zollo, F. Steroidal oligoglycosides and polyhydroxysteroids from Echinoderms. Fortschr. Chem. Org. Nat. 1993, 62, 75–308. [Google Scholar]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Iorizzi, M.; De Marino, S.; Zollo, F. Steroidal oligoglycosides from the Asteroidea. Curr. Org. Chem. 2001, 5, 951–973. [Google Scholar] [CrossRef]
- Stonik, V.A.; Ivanchina, N.V.; Kicha, A.A. New polar steroids from starfish. Nat. Prod. Commun. 2008, 3, 1587–1610. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.; Xu, T.H.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y.H. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Advances in Natural Products Discovery; Gomes, A.R., Rocha-Santos, T., Duarte, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; Volume 6, pp. 191–224. [Google Scholar]
- Xia, J.M.; Miao, Z.; Xie, C.L.; Zhang, J.W.; Yang, X.W. Chemical constituents and bioactivities of starfishes: An update. Chem. Biodivers. 2020, 17, e1900638. [Google Scholar] [CrossRef] [Green Version]
- Kicha, A.A.; Ivanchina, N.V.; Malyarenko, T.V.; Kalinovsky, A.I.; Popov, R.S.; Stonik, V.A. Six new polyhydroxylated steroid taurine conjugates, microdiscusols A–F, from the Arctic starfish Asterias microdiscus. Steroids 2019, 150, 108458. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, J.; Huang, R.; Wang, Y.; Xu, T.; Zhou, X.; Liu, Q.; Zeng, F.; Ju, H.; Yang, X.; et al. Polyhydroxy steroids and saponins from China sea starfish Asterina pectinifera and their biological activities. Chem. Pharm. Bull. 2010, 58, 856–858. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Hong, J.; Lee, C.-O.; Im, K.S.; Choi, J.S.; Jung, J.H. Cytotoxic sterols and saponins from the starfish Certonardoa semiregularis. J. Nat. Prod. 2004, 67, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Pizza, C.; Zollo, F. 5α-cholestane-3β,6β,15α,16β,26-pentaol: A polyhydroxylated sterol from the starfish Hacelia attenuata. Tetrahedron Lett. 1982, 23, 1841–1844. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Stonik, V.A. New polyhydroxysteroids and steroid glycosides from the Far East starfish Ceramaster patagonicus. Russ. Chem. Bull. 1997, 46, 186–191. [Google Scholar] [CrossRef]
- Svetashev, V.I. Mild method for preparation of 4,4-dimethyloxazoline derivatives of polyunsaturated fatty acids for GC–MS. Lipids 2011, 46, 463–467. [Google Scholar] [CrossRef]
- Stransky, K.; Jursik, T.; Vitek, A. Standard equivalent chain length values of monoenic and polyenic (methylene interrupted) fatty acids. J. High Resolut. Chromatogr. 1997, 20, 143–158. [Google Scholar] [CrossRef]
- Ling, T.; Lang, W.H.; Maier, J.; Centurion, M.Q.; Rivas, F. Cytostatic and cytotoxic natural products against cancer cell models. Molecules 2019, 24, 2012. [Google Scholar] [CrossRef] [Green Version]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef] [Green Version]
- Tahtamouni, L.; Ahram, M.; Koblinski, J.; Rolfo, C. Molecular regulation of cancer cell migration, invasion, and metastasis. Anal. Cell. Pathol. 2019, 2019, 1356508. [Google Scholar] [CrossRef] [Green Version]
- Kicha, A.A.; Ivanchina, N.V.; Gorshkova, I.A.; Ponomarenko, L.P.; Likhatskaya, G.N.; Stonik, V.A. The distribution of free sterols, polyhydroxysteroids and steroid glycosides in various body components of the starfish Patiria (=Asterina) pectinifera. Comp. Biochem. Physiol. 2001, 128, 43–52. [Google Scholar] [CrossRef]
- Kicha, A.A.; Ivanchina, N.V.; Stonik, V.A. Seasonal variations in the levels of polyhydroxysteroids and related glycosides in the digestive tissues of the starfish Patiria (=Asterina) pectinifera. Comp. Biochem. Physiol. 2003, 136, 897–903. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Grebnev, B.B.; Stonik, V.A.; Dmitrenok, P.S. The distribution of asterosaponins, polyhydroxysteroids and related glycosides in different body components of the Far Eastern starfish Lethasterias fusca. Mar. Drugs 2019, 17, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of all compounds in the manuscripts are available from the authors. |




| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | 1.76 dt (11.4, 3.5) 1.10 m | 39.1 | 15 | 4.40 brd (10.0) | 84.7 |
| 2 | 2.13 m 1.86 m | 32.5 | 16 | 4.66 brd (7.3) | 82.2 |
| 3 | 3.99 m | 71.1 | 17 | 1.55 dd (11.1, 7.3) | 59.3 |
| 4 | 2.45 m 2.04 brd (12.2) | 37.0 | 18 | 1.27 s | 14.9 |
| 5 | 1.35 m | 48.3 | 19 | 1.47 s | 16.2 |
| 6 | 4.15 brd (2.6) | 71.2 | 20 | 2.35 m | 30.0 |
| 7 | 2.95 dt (14.2, 3.5) 1.82 m | 41.2 | 21 | 1.13 d (6.8) | 18.3 |
| 8 | 2.55 qd (11.1, 3.5) | 30.8 | 22 | 1.92 m 1.28 m | 36.4 |
| 9 | 0.96 m | 54.9 | 23 | 1.60 m 1.39 m | 23.9 |
| 10 | − | 36.0 | 24 | 1.41 m 1.17 m | 34.0 |
| 11 | 1.64 m 1.59 m | 21.2 | 25 | 1.82 m | 32.8 |
| 12 | 2.10 m 1.36 m | 41.1 | 26 | 4.11 dd (10.6, 5.4) 3.96 dd (10.6, 6.8) | 69.1 |
| 13 | − | 43.9 | 27 | 0.94 d (6.8) | 17.0 |
| 14 | 1.45 t (10.4) | 61.0 |
| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1′ | − | 173.3 | − | 173.3 | − | 173.1 | − | 173.3 |
| 2′ | 2.43 t (7.4) | 33.7 | 2.40 t (7.5) | 34.3 | 2.43 t (7.2) | 33.6 | 2.40 t (7.5) | 34.2 |
| 3′ | 1.80 m | 25.2 | 1.70 m | 25.1 | 1.80 m | 25.2 | 1.71 m | 25.1 |
| 4′ | 2.17 m | 26.7 | 2.17 q (7.2) | 26.7 | ||||
| 5′ | 5.44 m | 128.9 | 5.46 m | 128.9 | ||||
| 6′ | 5.51 m | 130.9 | 5.51 m | 130.9 | 2.11 m | 27.3 | ||
| 7′ | 2.10 m | 27.4 | 2.10 m | 27.4 | 5.50 m | 130.2 | ||
| 10′ | 2.11 m | 27.4 | 2.11 m | 27.3 | 2.11 m | 27.4 | 5.48 m | 129.7 |
| 11′ | 5.48 m | 129.8 | 5.49 m | 130.0 | 5.49 m | 129.8 | 2.11 m | 27.1 |
| 12′ | 5.49 m | 130.1 | 5.49 m | 130.0 | 5.50 m | 130.1 | ||
| 13′ | 2.11 m | 27.1 | 2.11 m | 27.1 | 2.11 m | 27.1 | ||
| 16′ or 18′ | 1.25 m | 31.7 | 1.25 m | 31.8 | 1.25 m | 31.9 | 1.25 m | 31.8 |
| 17′ or 19′ | 1.28 m | 22.7 | 1.28 m | 22.8 | 1.28 m | 22.7 | 1.28 m | 22.8 |
| 18′ or 20′ | 0.88 t (6.9) | 14.0 | 0.87 t (6.9) | 14.0 | 0.88 t (7.0) | 14.0 | 0.89 t (6.9) | 14.0 |
| Fatty Acids | Content, % | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 16:0 | 14.91 | 9.36 | 11.83 | 5.88 |
| Δ7-16:1 | 5.11 | 0.75 | 5.06 | 1.07 |
| 18:0 | 11.23 | 8.92 | 8.13 | 8.03 |
| Δ5-18:1 | 1.62 | 4.62 | 17.05 | 0.57 |
| Δ9-18:1 | 5.97 | 6.53 | 6.65 | 4.83 |
| Δ11-18:1 | 4.06 | 51.16 | 3.92 | 2.05 |
| Δ5,11-18:2 | 53.31 | 3.73 | 6.14 | |
| Δ7-20:1 | 8.86 | 3.50 | 66.67 | |
| Δ9-20:1 | 0.63 | 3.88 | ||
| Δ5,11-20:2 | 3.78 | 9.79 | 39.51 | 0.87 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyarenko, T.V.; Kicha, A.A.; Malyarenko, O.S.; Zakharenko, V.M.; Kotlyarov, I.P.; Kalinovsky, A.I.; Popov, R.S.; Svetashev, V.I.; Ivanchina, N.V. New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster patagonicus and Their Anticancer Activity. Mar. Drugs 2020, 18, 260. https://doi.org/10.3390/md18050260
Malyarenko TV, Kicha AA, Malyarenko OS, Zakharenko VM, Kotlyarov IP, Kalinovsky AI, Popov RS, Svetashev VI, Ivanchina NV. New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster patagonicus and Their Anticancer Activity. Marine Drugs. 2020; 18(5):260. https://doi.org/10.3390/md18050260
Chicago/Turabian StyleMalyarenko, Timofey V., Alla A. Kicha, Olesya S. Malyarenko, Viktor M. Zakharenko, Ivan P. Kotlyarov, Anatoly I. Kalinovsky, Roman S. Popov, Vasily I. Svetashev, and Natalia V. Ivanchina. 2020. "New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster patagonicus and Their Anticancer Activity" Marine Drugs 18, no. 5: 260. https://doi.org/10.3390/md18050260
APA StyleMalyarenko, T. V., Kicha, A. A., Malyarenko, O. S., Zakharenko, V. M., Kotlyarov, I. P., Kalinovsky, A. I., Popov, R. S., Svetashev, V. I., & Ivanchina, N. V. (2020). New Conjugates of Polyhydroxysteroids with Long-Chain Fatty Acids from the Deep-Water Far Eastern Starfish Ceramaster patagonicus and Their Anticancer Activity. Marine Drugs, 18(5), 260. https://doi.org/10.3390/md18050260