Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea
Abstract
:1. Introduction
2. Nutritional Profile of Fish
3. Fish as Nutraceuticals
4. Background of Fish Bioactive Compounds and Fishery By-Products
5. Global Fish Consumption and its Nutraceutical Market: Current Scenario and Future Trends
6. Importance and Necessity of Fish Bioactive Components as Dietary Intake
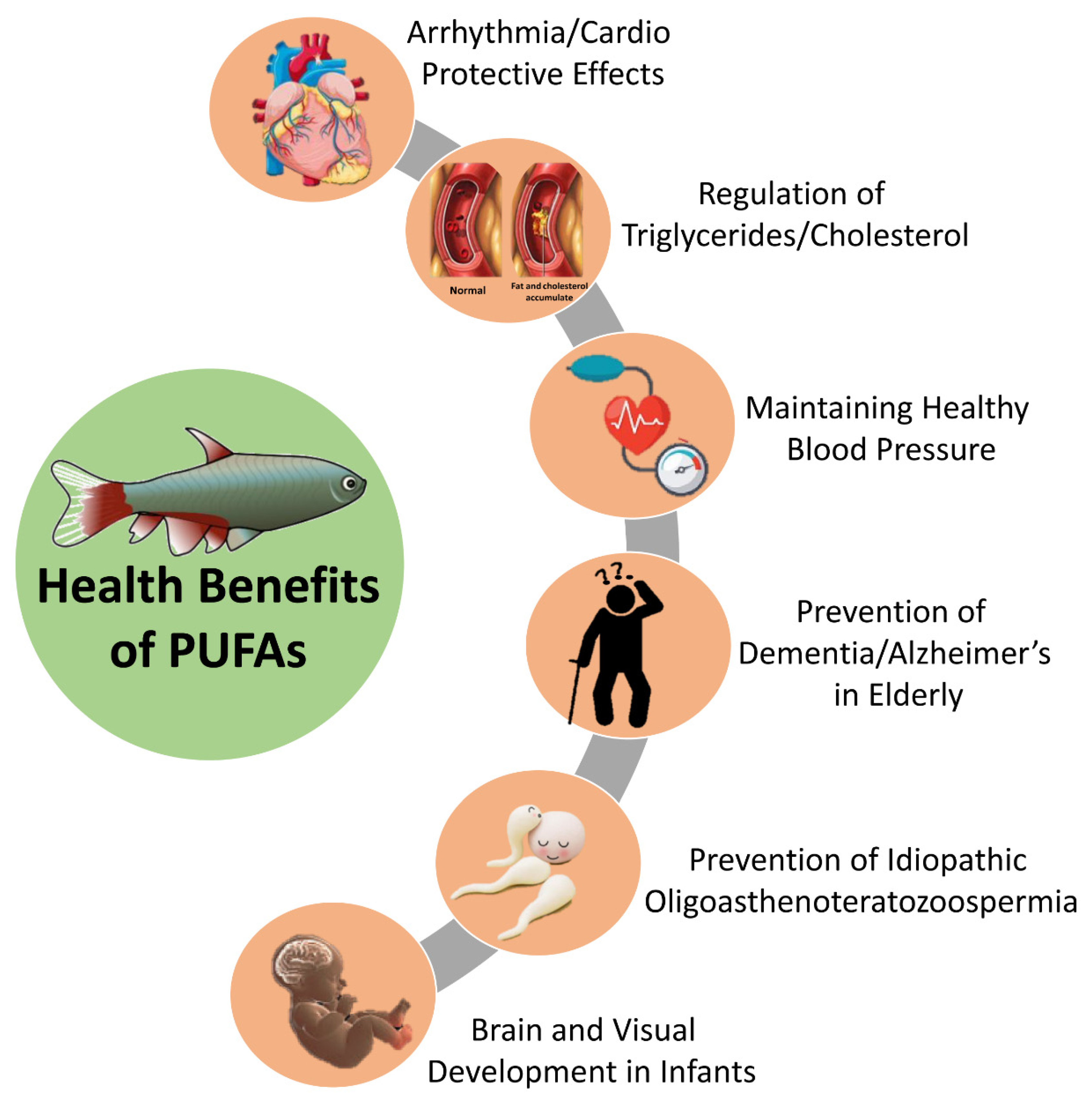
6.1. Fatty Acids: Power of Omega
6.2. Proteins and Amino Acids
6.3. Vitamins, Minerals, and Trace Elements
6.4. Carotenoids
6.5. Bioactive Peptides and Protein Hydrolysate
6.6. Chitin and Chitosan
6.7. Chondroitin, Glucosamine, and Hyaluronic Acid
6.8. Gelatin and Collagen
6.9. Squalene
7. Challenges and Complications
8. Future Perspectives and Conclusions
Author Contributions
Conflicts of Interest
References
- Paital, B. Nutraceutical values of fish demand their ecological genetic studies: A short review. J. Basic Appl. Zool. 2018, 79, 16. [Google Scholar] [CrossRef]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Mar. Drugs 2016, 14, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Population Nutrient Intake Goals for Preventing Diet-Related Chronic Diseases. Available online: https://www.who.int/nutrition/topics/5_population_nutrient/en/index13.html (accessed on 17 March 2020).
- American Heart Association (AHA). Eating Fish Twice a Week Reduces Heart Stroke Risk. Available online: https://www.heart.org/en/news/2018/05/25/eating-fish-twice-a-week-reduces-heart-stroke-risk (accessed on 17 March 2020).
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Syama Dayal, J.; Venkateshwarlu, G.; et al. DHA and EPA Content and Fatty Acid Profile of 39 Food Fishes from India. Biomed. Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood consumption and components for health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O.; Pandey, G.; Amitha, A. A review on role of fish in human nutrition with special emphasis to essential fatty acid. Int. J. Fish. Aquat. Stud. 2018, 6, 427–430. [Google Scholar]
- Suleria, H.A.; Osborne, S.; Masci, P.; Gobe, G. Marine-Based Nutraceuticals: An Innovative Trend in the Food and Supplement Industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Ranjan, A. Comparative Study on Macro and Micro Nutrient Profiling of Selected Freshwater, Brackish Water and Marine Water Food Fishes Available in Kerala, India. Food Nutr. Sci. 2016, 1, 1–7. [Google Scholar]
- Marques, I.; Botelho, G.; Guiné, R. Comparative study on nutritional composition of fish available in Portugal. Nutr. Food Sci. 2019, 49, 925–941. [Google Scholar] [CrossRef]
- Roos, N.C.; Chamnan, C.; Loeung, D.; Jakobsen, J.; Thilsted, S.H. Freshwater fish as a dietary source of vitamin A in Cambodia. Food Chem. 2007, 103, 1104–1111. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Proximate Composition of 5 Parts and Whole Fish. Available online: http://www.fao.org/3/ae581e/ae581e09.htm#bm9 (accessed on 30 April 2020).
- Nerhus, I.; Wik Markhus, M.; Nilsen, B.M.; Øyen, J.; Maage, A.; Ødegård, E.R.; Kolden Midtbø, L.; Frantzen, S.; Kögel, T.; Eide Graff, I.; et al. Iodine content of six fish species, Norwegian dairy products and hen’s egg. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, C.; Bird, P. Risk assessment for the consumption of fish with elevated selenium levels. NSW Public Health Bull. 2003, 14, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Ashraf, S.A.; Ahmad, F.; Ansari, J.; Siddiquee, M.R.A. Nutraceutical Market and its Regulation. American J. Food Technol. 2011, 6, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.A. Nutraceutical is the need of hour. World J. Pharm. Pharm. Sci. 2013, 2, 2516–2525. [Google Scholar]
- Devadasan, K. Fish - Based Pharmaceuticals and Nutraceuticals and their Applications. Fish. Chimes 2004, 24, 1. [Google Scholar]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Alshammari, E.; Patel, M.; Sachidanandan, M.; Kumar, P.; Adnan, M. Potential Evaluation and Health Fostering Intrinsic Traits of Novel Probiotic Strain Enterococcus durans F3 Isolated from the Gut of Fresh Water Fish Catla catla. Food Sci. Anim. Resour. 2019, 39, 844–861. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Patel, M.; Hadi, S. Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 2017, 5, e3085. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagchi, D. Nutraceutical and Functional Food Regulations in the United States and Around the World. Second Edition 2014. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Karwande, V.; Borade, R. Phytochemicals of Nutraceutical Importance; Scitus Academics LLC.: New York, NY, USA, 2015. [Google Scholar]
- Mandadi, R.; Mohd, A.; Mousa, A.; Mohd, S.; Mitesh, P. Evaluation of Anticancer, Antibacterial and Antioxidant Properties of a Medicinally Treasured Fern Tectaria coadunata with its Phytoconstituents Analysis by HR-LCMS. Anti Cancer Agents Med. Chem. 2020, 20, 1–12. [Google Scholar]
- Adnan, M. Bioactive potential of essential oil extracted from the leaves of Eucalyptus globulus (Myrtaceae). J. Pharmacogn. Phytochem. 2019, 8, 213–216. [Google Scholar]
- Adnan, M.; Ashraf, S.A.; Khan, S.; Alshammari, E.; Awadelkareem, A.M. Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA J. Food 2017, 15, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Adnan, M.; Alreshidi, M.M.; Patel, M.; Soni, A.; Redman, W. Immune responses in liver and spleen against Plasmodium yoelii pre-erythrocytic stages in Swiss mice model. J. Adv. Res. 2020, 24, 29–41. [Google Scholar] [CrossRef]
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and Functional Food as Future Food: A Review. Der Pharm. Lett. 2010, 2, 1106–1116. [Google Scholar]
- Adnan, M.; Khan, S.; Al-Shammari, E.; Patel, M.; Saeed, M.; Hadi, S. In pursuit of cancer metastasis therapy by bacteria and its biofilms: History or future. Med. Hypotheses 2017, 100, 78–81. [Google Scholar] [CrossRef]
- Yao, J.-J.; Zhao, Y.-L.; Wang, Q.; Zhou, Z.-L.; Hu, X.-C.; Duan, X.-W.; An, C.-G. Biochemical compositions and digestive enzyme activities during the embryonic development of prawn, Macrobrachium rosenbergii. Aquaculture 2006, 253, 573–582. [Google Scholar] [CrossRef]
- Adnan, M.; Alshammari, E.; Patel, M.; Amir Ashraf, S.; Khan, S.; Hadi, S. Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: Necessity for green chemistry. PeerJ 2018, 6, e5049. [Google Scholar] [CrossRef]
- Kundam, D.N. Israel Okpunyi Acham, and Abraham Tartenger Girgih. Asian Food Sci. J. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Soni, A.; Tiwari, V.; Puri, S.K. Assessment of real-time method to detect liver parasite burden under different experimental conditions in mice infected with Plasmodium yoelii sporozoites. Microb. Pathog. 2015, 89, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.R.; Datta, S.; Mahapatra, B.K.; Sardar, P. Bioactive compounds from fishery resources—A boon for human health. Presented at International Conference on Aquatic Resources and Sustainable Management, Kolkata, India, 17 February 2016. [Google Scholar]
- Muzaifa, M.; Safriani, N.; Zakaria, F. Production of protein hydrolysates from fish by-product prepared by enzymatic hydrolysis. Int. J. Bioflux. Soc. 2012, 5, 36–39. [Google Scholar]
- Anais, P.; Raul, P.G.; Jean-Pascal, B. By-products from Fish Processing: Focus on French Industry. In Utilization of Fish Waste; CRC Press: Cleveland, OH, USA, 2013; Chapter: 1; pp. 1–25. [Google Scholar]
- Caruso, G. Fishery Wastes and By-products: A Resource to Be Valorised. J. Fish. Sci. 2016, 10, 12–15. [Google Scholar]
- Mahro, B.; Timm, M. Potential of Biowaste from the Food Industry as a Biomass Resource. Eng. Life Sci. 2007, 7, 457–468. [Google Scholar] [CrossRef]
- Wang, C.-H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Reclamation of Fishery Processing Waste: A Mini-Review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [Green Version]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n- 3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Gomez Candela, C.; Bermejo Lopez, L.M.; Loria Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Johnson, M.A.C.B. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycom. Lipidom. 2014, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Kessler, A.T.; Raja, A. Biochemistry, histidine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Holvik, K.; Iversen, P.O.; Vaktskjold, A. Risk assessment of “other substances”—L-histidine. VKM Rep. 2016, 24. [Google Scholar] [CrossRef]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino Acids 2014, 2014, 269797. [Google Scholar] [CrossRef] [PubMed]
- Hesse, H.; Kreft, O.; Maimann, S.; Zeh, M.; Hoefgen, R. Current understanding of the regulation of methionine biosynthesis in plants. J. Exp. Bot. 2004, 55, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, B.; Sultana, T.; Sultana, S.; Ahmed, Z.; Mahboob, S. Study on impact of habitat degradation on proximate composition and amino acid profile of Indian major carps from different habitats. Saudi J. Biol. Sci. 2018, 25, 755–759. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. (Elite Ed.) 2011, 3, 1192–1200. [Google Scholar] [CrossRef]
- Jongkees, B.J.; Hommel, B.; Kuhn, S.; Colzato, L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review. J. Psychiatr. Res. 2015, 70, 50–57. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Chantar, M.L.; Vazquez-Chantada, M.; Ariz, U.; Martinez, N.; Varela, M.; Luka, Z.; Capdevila, A.; Rodriguez, J.; Aransay, A.M.; Matthiesen, R.; et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008, 47, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Martins, D.A.; Rocha, F.; Castanheira, F.; Mendes, A.; Pousao-Ferreira, P.; Bandarra, N.; Coutinho, J.; Morais, S.; Yufera, M.; Conceicao, L.E.; et al. Effects of dietary arachidonic acid on cortisol production and gene expression in stress response in Senegalese sole (Solea senegalensis) post-larvae. Fish. Physiol. Biochem. 2013, 39, 1223–1238. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, V.; Wicklow, B.; Wittmeier, K.; Hay, J.; MacIntosh, A.; Venugopal, N.; Ryner, L.; Berard, L.; McGavock, J. A clinically relevant method to screen for hepatic steatosis in overweight adolescents: A cross sectional study. BMC Pediatr. 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic acid as anticancer agent: An overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Ikelle, L.; Naash, M.I.; Al-Ubaidi, M.R. The Intersection of Serine Metabolism and Cellular Dysfunction in Retinal Degeneration. Cells 2020, 9, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobreva, D.; Merdzhanova, A.; Stancheva1, M.; Terziyski, D.; Panayotova, V. Black Sea fish and shellfish as essential source of vitamin B12. Int. J. Sci Rep. 2018, 4, 199–203. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Perera, C.O.; Yen, G.M. Functional Properties of Carotenoids in Human Health. Int. J. Food Prop. 2007, 10, 201–230. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. (Indore) 2016, 4, 411–427. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudeva, S.; Squillante, A.; Tholen, J. Nutritional Supplementation and Nutraceuticals as Used in the Treatment of Osteoarthritis and their Ability to Alter Mechanical Properties of Cartilage. EC Nutr. 2017, 9, 6–14. [Google Scholar]
- Gruenwald, J.; Petzold, E.; Busch, R.; Petzold, H.P.; Graubaum, H.J. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv. Ther. 2009, 26, 858–871. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Mirghani, M.E.S.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ. 2013, 11, 73–79. [Google Scholar]
- Alfaro, A.D.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Irwandi, J.; Faridayanti, S.; Mohamed, E.S.M.; Hamzah, M.S.; Torla, H.H.; Che Man, Y.B. Extraction and characterization of gelatin from different marine fish species in Malaysia. Int. Food Res. J. 2009, 16, 381–389. [Google Scholar]
- Jeevithan, E.; Zhao, Q.; Bao, B.; Wu, W. Biomedical and Pharmaceutical Application of Fish Collagen and Gelatin: A Review. J. Nutr. Ther. 2013, 2, 218–227. [Google Scholar]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Da´vila-Ortiz, G.; Martınez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Popa, O. Narcisa Elena Bsbeanu, Ioana Popa, Sultana Nita and Cristina Elena Dinu-Pârvu, Methods for Obtaining and Determination of Squalene from Natural Sources. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopicova, Z.; Vavreinova, S. Occurrence of squalene and cholesterol in various species of Czech freshwater fish. Czech. J. Food Sci. 2007, 25, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Guillen, J.; Natale, F.; Carvalho, N.; Casey, J.; Hofherr, J.; Druon, J.N.; Fiore, G.; Gibin, M.; Zanzi, A.; Martinsohn, J.T. Global seafood consumption footprint. Ambio 2019, 48, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture. Food and Agriculture Organization Rome. 200, 2016. Available online: http://www.fao.org/3/a-i5555e.pdf (accessed on 30 April 2020).
- Grand View Research. Global Fish Oil Market. Available online: https://www.grandviewresearch.com/press-release/global-fish-oil-market (accessed on 17 March 2020).
- Grand View Research. Global Nutraceuticals Market. Available online: https://www.grandviewresearch.com/press-release/global-nutraceuticals-market (accessed on 17 March 2020).
- Statista. Global Fish Consumption by Region. Available online: https://www.statista.com/statistics/1026312/global-fish-consumption-by-region (accessed on 17 March 2020).
- Abbas, K.A.; Saeed, M.A.E.; Bakar, J. Fatty acids in fish and beef and their nutritional values: A review. J. Food Agric. Environ. 2009, 7, 37–42. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [Green Version]
- Mahan, L.K.; Escott-Stump, S. Krause’s Food, Nutrition, and Diet Therapy, 10th ed.; W.B. Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, D.; Pantuso, T. Omega-3 fatty acids: An update emphasizing clinical use. Agro. Food Ind. Hi. Tech. 2012, 23, 10–13. [Google Scholar]
- Sati, A.; Bhatt, P. Review on therapeutic effects mediated by omega-3 fatty acids in Alzheimer’s disease. Asian J. Pharm. Clin. Res. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, J.; Siddiqui, A.J.; Goyal, M.; Prakash, K.; Soni, A.; Puri, S.K. Repetitive live sporozoites inoculation under arteether chemoprophylaxis confers protection against subsequent sporozoite challenge in rodent malaria model. Acta. Trop. 2016, 158, 130–138. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Siddiqui, A.J.; Goyal, M.; Prakash, K.; Soni, A.; Puri, S.K.; Srivastava, M. Host immune response is severely compromised during lethal Plasmodium vinckei infection. Parasitol. Res. 2015, 114, 3445–3457. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.; Ljung, H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319858901. [Google Scholar] [CrossRef] [PubMed]
- Balami, S.; Sharma, A.; Karn, R. Significance of Nutritional Value of Fish for Human Health. Malays. J. Halal Res. J. (MJHR) 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kaarniranta, K.; Salminen, A.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Age-related macular degeneration (AMD): Alzheimer’s disease in the eye? J. Alzheimers Dis. 2011, 24, 615–631. [Google Scholar] [CrossRef] [Green Version]
- Aucoin, M.; Cooley, K.; Knee, C.; Fritz, H.; Balneaves, L.G.; Breau, R.; Fergusson, D.; Skidmore, B.; Wong, R.; Seely, D. Fish-Derived Omega-3 Fatty Acids and Prostate Cancer: A Systematic Review. Integr. Cancer Ther. 2017, 16, 32–62. [Google Scholar] [CrossRef]
- Torpy, J.M.; Lynm, C.; Glass, R.M. Eating Fish: Health Benefits and Risks. JAMA 2006, 296, 1926. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; McLntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2012, FAO Fisheries and Aquaculture Department. Rome, FAO. Available online: http://www.fao.org/docrep/016/i2727e/i2727e.pdf (accessed on 17 March 2020).
- Njinkouea, J.M.; Gouado, I.; Tchoumbougnang, F.; Yanga Ngueguim, J.H.; Ndinteh, D.T.; Fomogne-Fodjo, C.Y.; Schweigert, F.J. Proximate composition, mineral content and fatty acid profile of two marine fishes from Cameroonian coast: Pseudotolithus typus (Bleeker, 1863) and Pseudotolithus elongatus (Bowdich, 1825). NFS J. 2016, 4, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.M.; Waagbo, R.; Espe, M. Functional amino acids in fish nutrition, health and welfare. Front. Biosci. (Elite Ed.) 2016, 8, 143–169. [Google Scholar]
- Faustino, J.F.; Ribeiro-Silva, A.; Dalto, R.F.; Souza, M.M.; Furtado, J.M.; Rocha Gde, M.; Alves, M.; Rocha, E.M. Vitamin A and the eye: an old tale for modern times. Arq. Bras. Oftalmol. 2016, 79, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogobe, O.; Mosepele, K.; Masamba, W.R.L. Essential mineral content of common fish species in Chanoga, Okavango Delta, Botswana. Afr. J. Food Sci. 2015, 9, 480–486. [Google Scholar]
- Jiang, D.; Hu, Z.; Liu, F.; Zhang, R.; Duo, B.; Fu, J.; Cui, Y.; Li, M. Heavy metals levels in fish from aquaculture farms and risk assessment in Lhasa, Tibetan Autonomous Region of China. Ecotoxicology 2014, 23, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, V. Nutrients and Nutraceuticals from Seafood. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–45. [Google Scholar]
- Gupta, S.K.; Jha, A.K.; Pal, A.K.; Venkateshwarlu, G. Use of natural carotenoids for pigmentation in fishes. Nat. Prod. Radiance 2007, 6, 46–49. [Google Scholar]
- Vilchez, C.; Forjan, E.; Cuaresma, M.; Bedmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits: A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Le Gouic, A.V.; Harnedy, P.A.; FitzGerald, R.J. Bioactive Peptides from Fish Protein By-Products. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 355–388. [Google Scholar]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. (NY) 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Takarina, N.D.; Fanani, A.A. Characterization of Chitin and Chitosan Synthesized from Red Snapper (Lutjanus sp.) Scale’s Waste; American Institute of Physics Conference Series; American Institute of Physics: College Park, MD, USA, 2017; p. 030108. [Google Scholar]
- Pandharipande, S.; Jana, R.; Ramteke, A. Synthesis and Characterization of Chitosan from Fish Scales. Int. J. Sci. Eng. Technol. Res. (IJSETR) 2018, 7, 287–291. [Google Scholar]
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Proced. Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Wichers, H.J.; Govers, C. Beneficial Health Effects of Chitin and Chitosan. In Chitin and Chitosan; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 145–167. [Google Scholar]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Biomedical and Nutraceutical Applications of Chitin and Chitosan. In High Value Fermentation Products; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 319–349. [Google Scholar]
- Pokhrel, S.; Yadav, P.N.; Adhikari, R. Applications of Chitin and Chitosan in Industry and Medical Science: A Review. Nepal J. Sci. Technol. 2015, 16, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng. Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Structure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year duration. Osteoarthr. Cartil. 2010, 18 (Suppl. 1), S28–S31. [Google Scholar] [CrossRef] [Green Version]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers (Basel) 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr Res. 2014, 72, 137–176. [Google Scholar]
- Ibrahim, N.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef] [Green Version]
- Popa, I.; Băbeanu, E.; Niță, S.; Popa, A. Squalene-Natural resources and applications. Farmacia 2014, 62, 840–862. [Google Scholar]
- PREEZ, H.D. Squalene – antioxidant of the future? South Afr. J. Nat. Med. 2007, 33, 106–112. [Google Scholar]
- McPherson, B.F.; Miller, R.L.; Haag, K.H.; Bradner, A. Water Quality in Southern Florida, 1996–1998. U.S. Geol. Surv. Circ. 2000, 1207, 32. [Google Scholar]
- Mozaffarian, D.; Rimm, E.B. Fish Intake, Contaminants, and Human Health: Evaluating the Risks and the Benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wijngaarden, E.; Thurston, S.W.; Myers, G.J.; Harrington, D.; Cory-Slechta, D.A.; Strain, J.J.; Watson, G.E.; Zareba, G.; Love, T.; Henderson, J.; et al. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol. Teratol. 2017, 59, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oken, E.; Choi, A.L.; Karagas, M.R.; Marien, K.; Rheinberger, C.M.; Schoeny, R.; Sunderland, E.; Korrick, S. Which fish should I eat? Perspectives influencing fish consumption choices. Environ. Health Persp. 2012, 120, 790–798. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Advice About Eating Fish. Available online: https://www.fda.gov/food/consumers/advice-about-eating-fish (accessed on 17 March 2020).
- U.S. Department of Agriculture; U.S. Department of Health and Human Services (HHS, U., USDA and HHS). Dietary Guidelines for Americans; National Academies Press: Washington, DC, USA, 2015.
- American Heart Association (AHA). Fish 101. 2014. Available online: http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Fish-101_UCM_305986_Article.jsp (accessed on 17 March 2020).



| Nutraceutical/Bioactive Components | Fish Species Rich in Specific Bioactive Components | Therapeutic Efficacy and Clinical Implications | References |
|---|---|---|---|
| Fatty Acids | |||
| Omega 3 | Mackerel, Spiny dogfish, Black halibut, Salmon, Sardines | Anti-inflammatory, cardio-protective effects, visual and neurodevelopment, different cancers (breast, colorectal, prostate, etc.), asthma, inflammatory bowel disease, rheumatoid arthritis and osteoporosis, improve insulin sensitivity | [9,42,43,44,45] |
| Omega 6 | Arctic Char, Sardine, Fried Calamari, Anchovies | Reduce risk of cardiovascular problems, ameliorate diseases such as arthritis and hypertension, increases vascular adhesion molecule-1 expression, oxidation, platelet aggregation, vasoconstriction, eicosanoid synthesis | [9,43,45] |
| Amino Acids | |||
| Arginine | Oncorhynchus mykiss | Required for the detoxification of ammonia, nutritionally essential for spermatogenesis, embryonic survival, fetal and neonatal growth, as well as maintenance of vascular tone and hemodynamics | [46] |
| Histidine | Rastrelliger kanagurta, Catla catla | Precursor for several hormones (thyrotropin-release hormone), critical metabolite for renal functions, neurotransmission, gastric secretion and immune system, antioxidant and anti-inflammatory properties, important for the regulation and metabolism of trace elements as well as precursor of histamine | [47,48] |
| Isoleucine | Oncorhynchusmykiss, Labeo rohita | Help in muscle formation and proper growth | [49] |
| Lysine | Stolephorus waitei, Rastrelliger kanagurta, S. commersonii | Needed for optimal growth and to act as an immunomodulator, prevention and treatment of cold sores | [49] |
| Methionine | Stolephorus waitei, Tor putitora | Used at multiple levels in cellular metabolism, as a protein constituent, in the initiation of mRNA translation, and as a regulatory molecule in the form of S-adenosylmethionine | [50] |
| Phenylalanine | Cirrhinus mrigala, Catla catla | Precursor for tyrosine | [51] |
| Threonine | Thunnus albacores, Nemipterus japonicus | Plays a critical role in the maintenance of intestinal mucosal integrity and barrier function | [52] |
| Tyrosine | Oncorhynchus mykiss, Tor putitora | Precursor of dopamine and norepinephrine | [53] |
| Valine | Nemipterus japonicas, Cirrhinus mrigala | Protein synthesis, glucose homeostasis, anti-obesity, and nutrient-sensitive signaling pathways | [54] |
| Glutamine | Cirrhinus mrigala, Catla catla, Labeo rohita | Act as substrate for nucleotide synthesis (purines, pyrimidines, and amino sugars), nicotinamide adenine dinucleotide phosphate (NADPH), antioxidants, and many other biosynthetic pathways involved in the maintenance of cellular integrity and function | [55] |
| Glycine | Cirrhinus mrigala, Catla catla, Labeo rohita | Help in regulation of gene expression, protein configuration and activity, and several biological functions, such as glutathione synthesis. Low plasma glycine concentrations have been consistently reported in association with obesity and type 2 diabetes | [56,57] |
| Proline | Oncorhynchus mykiss, Tor putitora | Important role in differentiation of cells as well as fetus and associated with extra-embryonic membrane and development | [58] |
| Alanine | Polypedates maculates | Helps in biosynthesis of proteins, serves as an important carbon source for hepatic gluconeogenesis | [59] |
| Aspartic acid | Labeo niloticus | Treatment for chronic fatigue due to the role it plays in generating cellular energy | [59] |
| Glutamic acid | Labeo niloticus | Surfactants, buffer, chelating agents, flavor enhancer, agriculture, acts as fuel, immune function | [59,60] |
| Leucine | Lethrinus harak | Leucine promotes energy metabolism (glucose uptake, mitochondrial biogenesis, and fatty acid oxidation) to provide energy for protein synthesis, while inhibiting protein degradation | [59,61] |
| Serine | Mugil cephalus | Cellular homeostasis, proliferation, and differentiation | [59,62] |
| Vitamins | |||
| Vitamin A | Amblypharyngodon mola | Growth promoter, helps in poor eyesight, helps in bone growth | [3,6] |
| Vitamin D | Amblypharyngodon mola, Sardinella longiceps | Rickets, osteomalacia, improve bone density | [3,13] |
| Vitamin B Complex | Black sea fish, Shellfish | Responsible for converting food to energy in the cells of the body and helps with the function of nerve tissue | [6,63] |
| Minerals | |||
| Iron | Sperata seenghala, Rita rita | Help in synthesis of hemoglobin in red blood cells | [13] |
| Zinc | Sperata seenghala, Rita rita | Important role in growth and development as well in the proper functioning of the immune system and for healthy skin. Helps in cell division, cell growth, wound healing, and breakdown of carbohydrates. Essential for senses of smell and taste | [6] |
| Calcium | Xenentodon cancila, Gudusia chapra | Essential for strong bones (formation and mineralization) and for the normal functioning of muscles and the nervous system | [6] |
| Iodine | - | Important for hormones that regulate body metabolism, and in children it is required for growth and normal mental development | [6] |
| Carotenoids | |||
| Astaxanthine, Beta carotene, Zeaxanthin and lutein | Freshwater fish, red fishes, and other fishes | Antioxidant, cancer, neurological disorder, cardiovascular, anti-atherogenic, ophthalmology, psoriasis, preservative, cosmetics | [64,65] |
| Other Bioactive Components | |||
| Bioactive Peptides | Fish protein, fish by-products, and muscle | Antihypertensive, antioxidant, antimicrobials, and anti-proliferative effects | [66,67] |
| Chitin and Chitosan | Fish waste product (fish scale) | Wound healing accelerator, reduces blood cholesterol levels, anti-ulcer agent, anti-ageing, cosmetics, ophthalmology | [68,69,70] |
| Chondroitin | Fish waste product | Osteoarthritis, dietary supplement | [71] |
| Glucosamine | Fish waste product | Anti-inflammatory, osteoarthritis, dietary supplement | [71,72] |
| Gelatin | Fish waste product | Pharmaceutical industries, food industries, microencapsulation of vitamin, stabilizer in dairy products, cosmetics | [73,74,75,76] |
| Collagen | Fish waste product | In osteoarthritis, hypertension, tissue engineering, antioxidant, anti-hypertensive, and anti-skin ageing | [9,76] |
| Squalene | Scardinius erthrophthalmus, Tinca tinca | Cardio-protective, antioxidant, anti-bacterial, antifungal, and anticancer | [77,78,79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. https://doi.org/10.3390/md18050265
Ashraf SA, Adnan M, Patel M, Siddiqui AJ, Sachidanandan M, Snoussi M, Hadi S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Marine Drugs. 2020; 18(5):265. https://doi.org/10.3390/md18050265
Chicago/Turabian StyleAshraf, Syed Amir, Mohd Adnan, Mitesh Patel, Arif Jamal Siddiqui, Manojkumar Sachidanandan, Mejdi Snoussi, and Sibte Hadi. 2020. "Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea" Marine Drugs 18, no. 5: 265. https://doi.org/10.3390/md18050265










