Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Activity of Flaccidoxide-13-acetate against HA22T and HepG2 Cells
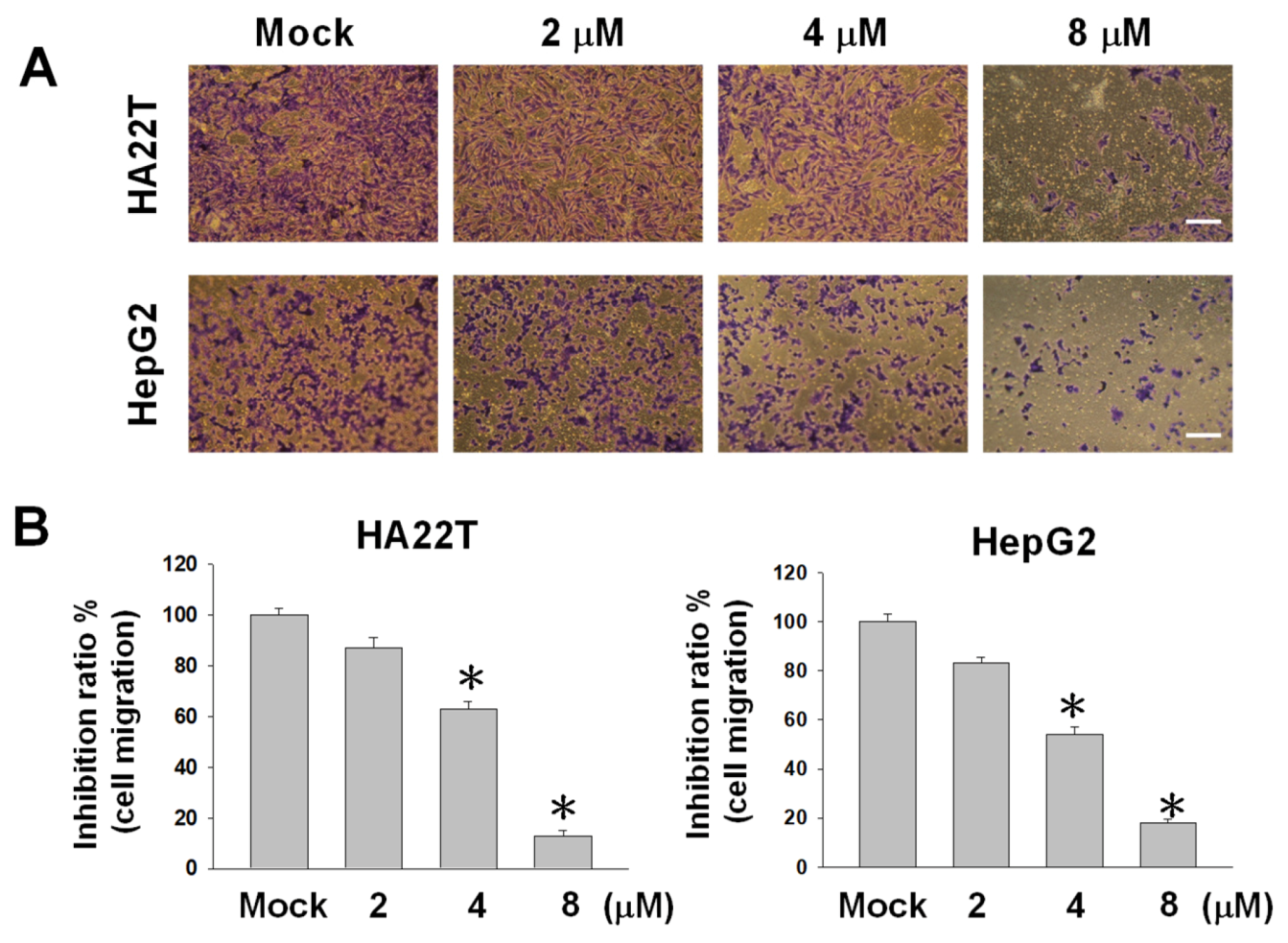
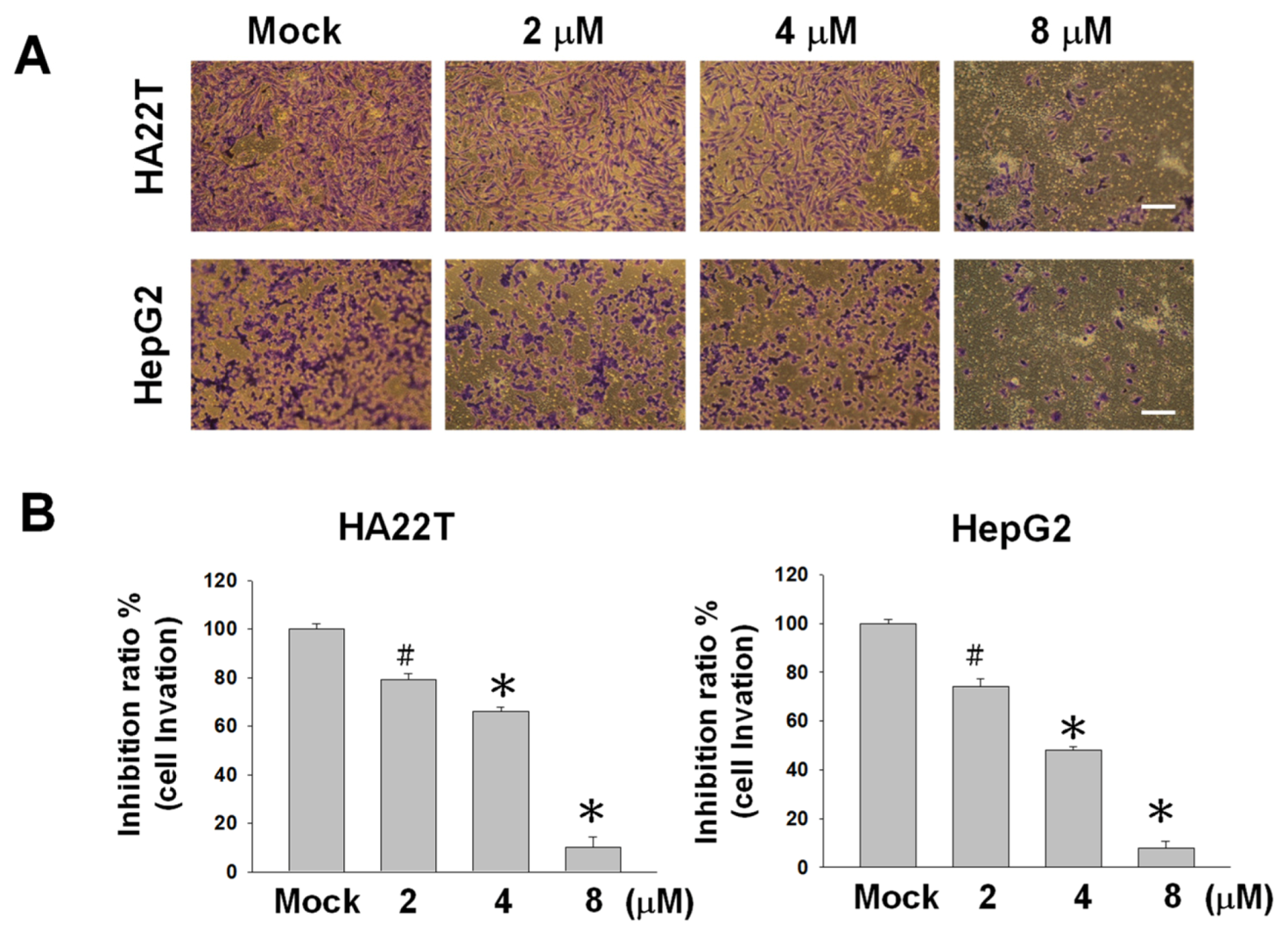
2.2. Flaccidoxide-13-acetate Reduces Cellular Migration and Invasion in HA22T and HepG2 Cells
2.3. Flaccidoxide-13-acetate Reduced the uPA and MMP-2/-9 Activities and Regulated the Expressions of MMP-2/-9, uPA, TIMP-1, and TIMP-2 in HA22T and HepG2 Cells
2.4. Flaccidoxide-13-acetate Inhibited the FAK/PI3K/Akt/mTOR Signaling Pathway
2.5. Flaccidoxide-13-acetate Inhibited the Epithelial-to-Mesenchymal Transition (EMT)
3. Discussion
4. Methods
4.1. Materials and Chemical Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Migration Assay
4.5. Cell Invasion Assay
4.6. Gelatin Zymography Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balogh, J.; Victor, D., III; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; London, W.T. The global epidemiology of hepatocellular carcinoma: Present and future. Clin. Liver Dis. 2011, 15, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.X.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Hou, J.L.; Chutaputti, A. Hepatocellular carcinoma in the Asia pacific region. J. Gastroenterol. Hepatol. 2009, 24, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Stournaras, E.; Neokosmidis, G.; Stogiannou, D.; Protopapas, A.; Tziomalos, K. Effects of antiviral treatment on the risk of hepatocellular cancer in patients with chronic viral hepatitis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1277–1282. [Google Scholar] [CrossRef]
- Viveiros, P.; Riaz, A.; Lewandowski, R.J.; Mahalingam, D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers 2019, 11, 1085. [Google Scholar] [CrossRef]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma (-) a Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Sung, P.-J.; Duh, C.-Y.; Chen, B.-W.; Sheu, J.-H.; Yang, N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Su, T.-R.; Dai, G.-F.; Su, J.-H.; Liu, C.-I. Flaccidoxide-13-Acetate-Induced Apoptosis in Human Bladder Cancer Cells is through Activation of p38/JNK, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress Regulated Pathway. Mar. Drugs 2019, 17, 287. [Google Scholar] [CrossRef]
- Plaks, V.; Koopman, C.D.; Werb, Z. Cancer. Circulating tumor cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Van Dam, P.; Van den Eynden, G.; Rutten, A.; Wuyts, H.; Pouillon, L.; Peeters, M.; Pauwels, P.; Van Laere, S.; Van Dam, P. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br. J. Cancer 2014, 110, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.-A.; Wu, W.-T.; Dai, G.-F.; Su, J.-H.; Liu, C.-I.; Su, T.-R.; Wu, Y.-J. Flaccidoxide-13-Acetate Extracted from the Soft Coral Cladiella kashmani Reduces Human Bladder Cancer Cell Migration and Invasion through Reducing Activation of the FAK/PI3K/AKT/mTOR Signaling Pathway. Molecules 2018, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Lin, Y.; O-Lee, T.; Chou, C.; Lee, T.; Liu, T.; P’eng, F.; Chen, T.; Hu, C. Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol. Cell. Biol. 1983, 3, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.B.; Howe, C.C.; Aden, D.P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980, 209, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Feng, X.; Zhan, Y.; Wang, R.; Zheng, S.; Liu, W.; Zeng, X. Matrix metalloproteinase-2 promotes alphavbeta3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS ONE 2012, 7, e41591. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.B.; Westphal, J.R.; van Muijen, G.N.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Invest. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.; Lloret, P.; Idoate, M.; Inoges, S. Expression and serum levels of MMP-2 and MMP-9 during human melanoma progression. Clin. Exp. Dermatol. 2005, 30, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, L.; Liu, L.; Geng, J.; Zhou, Y.; Chen, L. β-Elemene inhibits the metastasis of B16F10 melanoma cells by downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9. Melanoma Res. 2014, 24, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Alpízar-Alpízar, W.; Christensen, I.J.; Santoni-Rugiu, E.; Skarstein, A.; Ovrebo, K.; Illemann, M.; Laerum, O.D. Urokinase plasminogen activator receptor on invasive cancer cells: A prognostic factor in distal gastric adenocarcinoma. Int. J. Cancer 2012, 131, 329–336. [Google Scholar]
- Sonoda, Y.; Hada, N.; Kaneda, T.; Suzuki, T.; Ohshio, T.; Takeda, T.; Kasahara, T. A synthetic glycosphingolipid-induced antiproliferative effect in melanoma cells is associated with suppression of FAK, Akt, and Erk activation. Biol. Pharm. Bull. 2008, 31, 1279–1283. [Google Scholar] [CrossRef]
- Thang, N.D.; Yajima, I.; Kumasaka, M.Y.; Iida, M.; Suzuki, T.; Kato, M. Deltex-3-like (DTX3L) stimulates metastasis of melanoma through FAK/PI3K/AKT but not MEK/ERK pathway. Oncotarget 2015, 6, 14290–14299. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Xia, H.; Nho, R.S.; Kahm, J.; Kleidon, J.; Henke, C.A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem. 2004, 279, 33024–33034. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Radisky, D.C. Epithelial-mesenchymal transition. J. Cell. Sci. 2005, 118, 4325–4326. [Google Scholar] [CrossRef]
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; De Herreros, A.G. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell. Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Bolós, V.; Peinado, H.; Pérez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J. Cell. Sci. 2003, 116, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell. Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-J.; Wei, W.-C.; Dai, G.-F.; Su, J.-H.; Tseng, Y.-H.; Tsai, T.-C. Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma. Mar. Drugs 2020, 18, 314. https://doi.org/10.3390/md18060314
Wu Y-J, Wei W-C, Dai G-F, Su J-H, Tseng Y-H, Tsai T-C. Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma. Marine Drugs. 2020; 18(6):314. https://doi.org/10.3390/md18060314
Chicago/Turabian StyleWu, Yu-Jen, Wen-Chi Wei, Guo-Fong Dai, Jui-Hsin Su, Yu-Hwei Tseng, and Tsung-Chang Tsai. 2020. "Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma" Marine Drugs 18, no. 6: 314. https://doi.org/10.3390/md18060314
APA StyleWu, Y.-J., Wei, W.-C., Dai, G.-F., Su, J.-H., Tseng, Y.-H., & Tsai, T.-C. (2020). Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma. Marine Drugs, 18(6), 314. https://doi.org/10.3390/md18060314



