Secondary Metabolites with Nitric Oxide Inhibition from Marine-Derived Fungus Alternaria sp. 5102
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction, Isolation, and Characterization
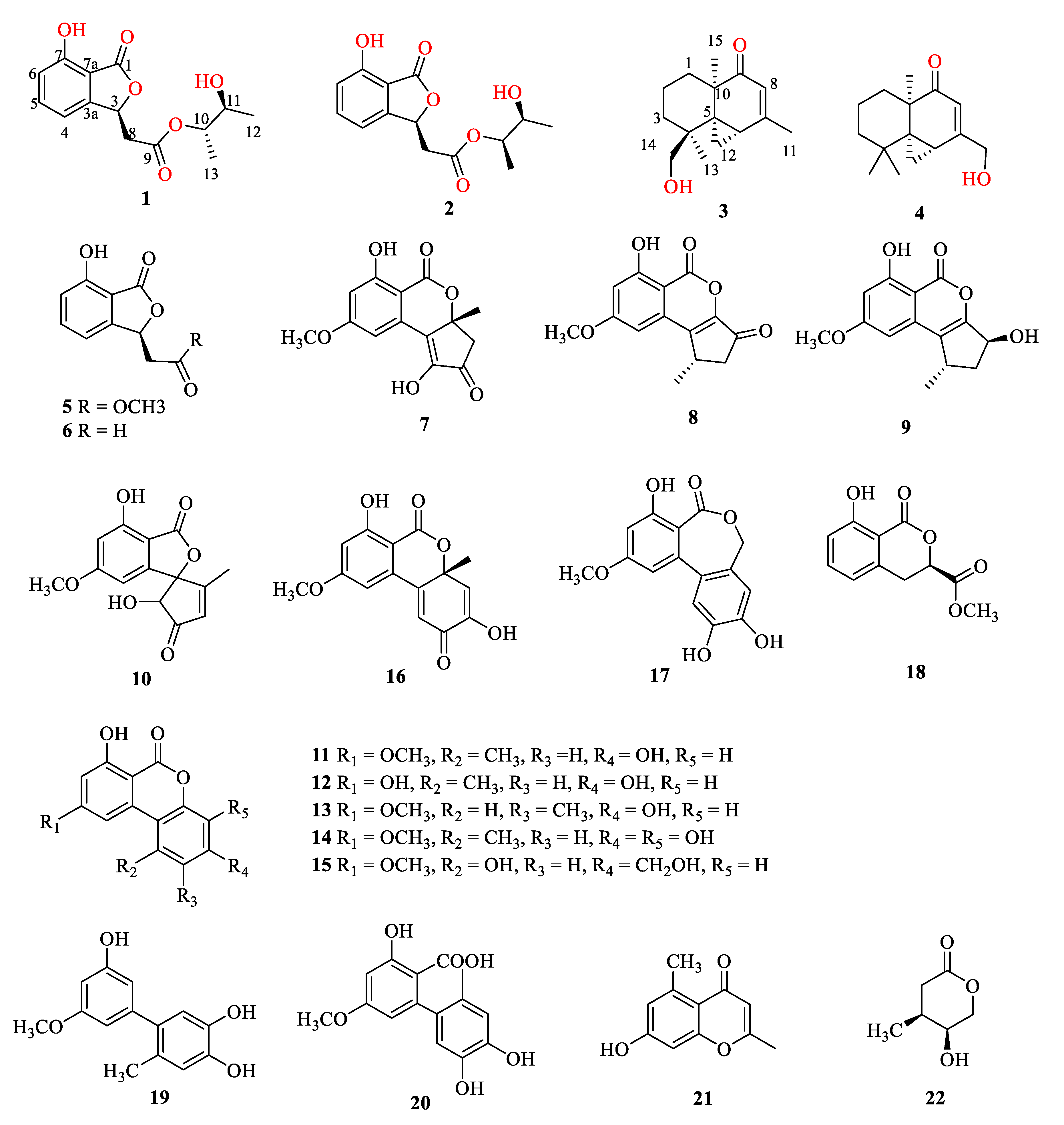
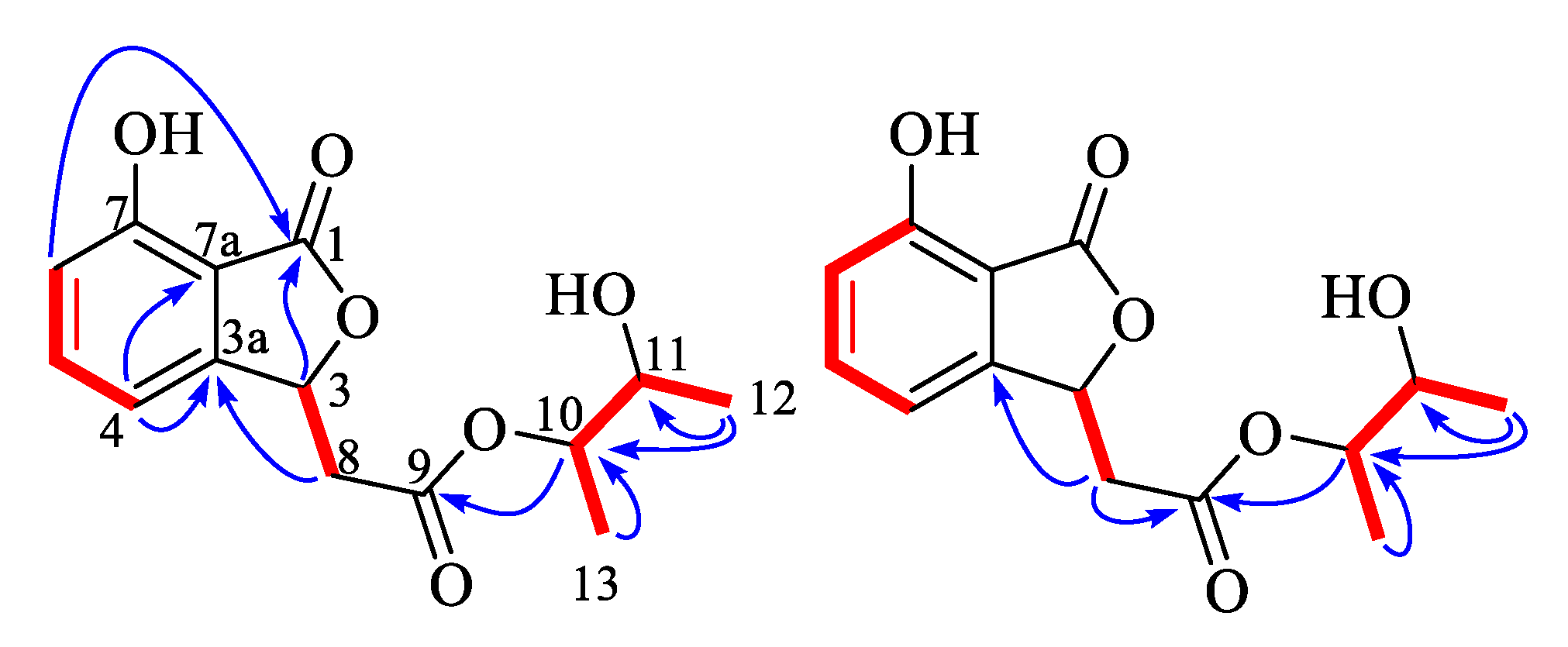
3.3.1. Alternabenzofuran A (1)
3.3.2. Alternabenzofuran B (2)
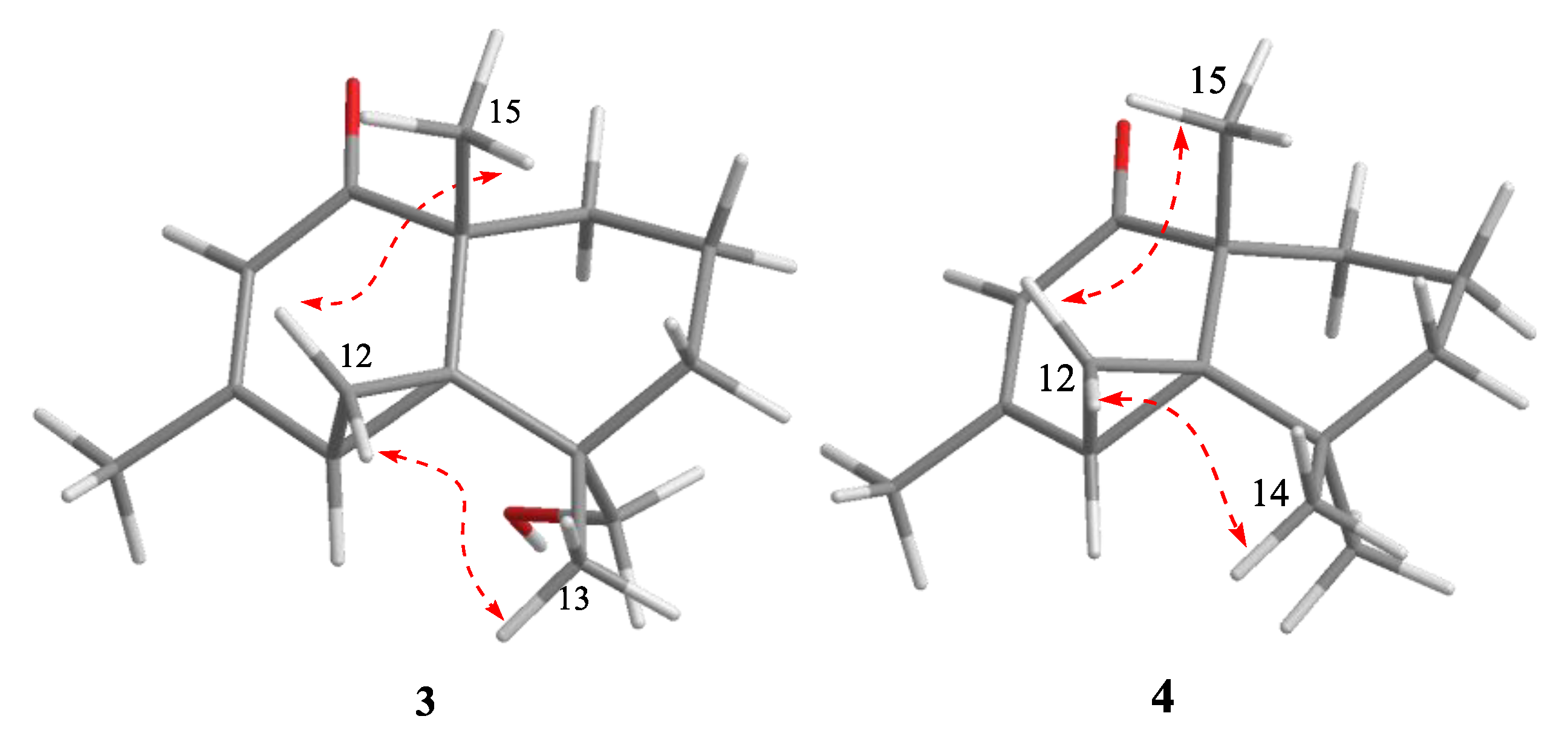
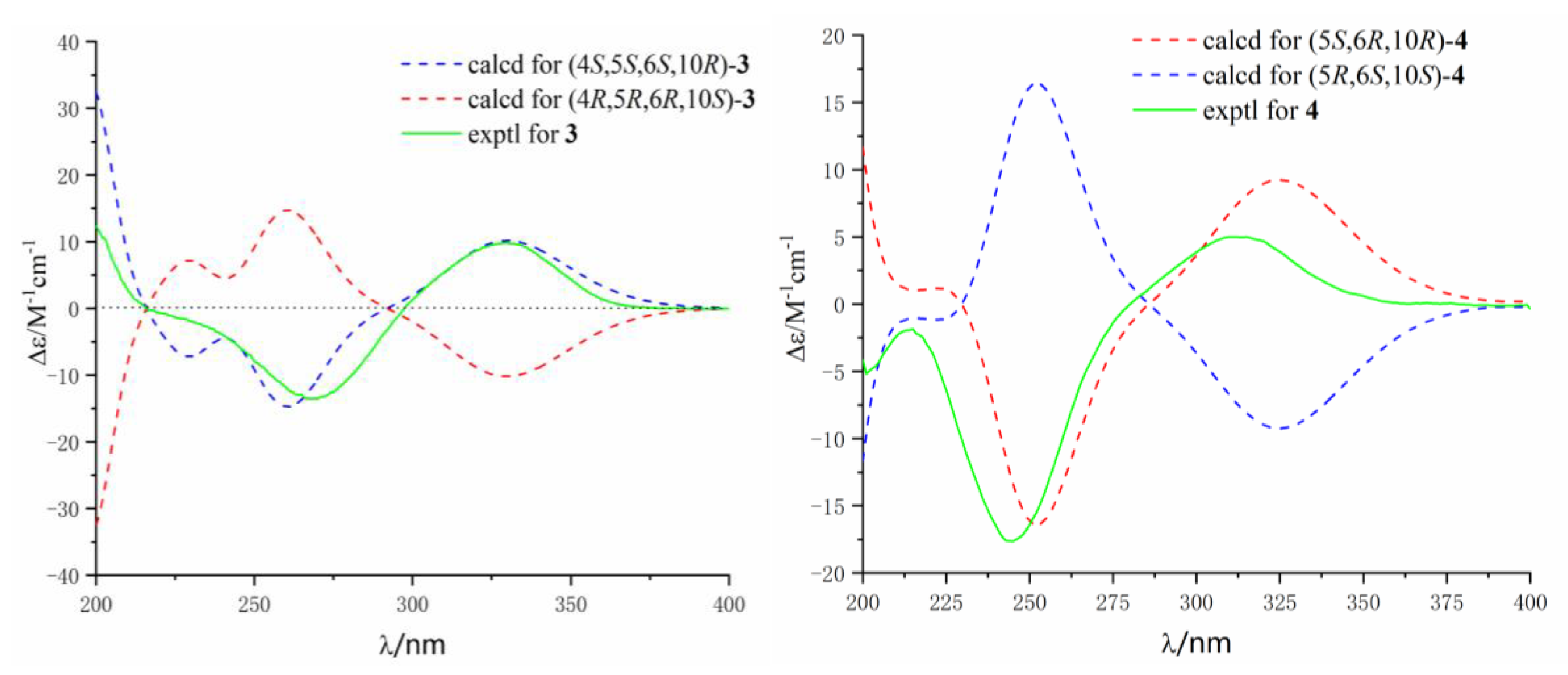
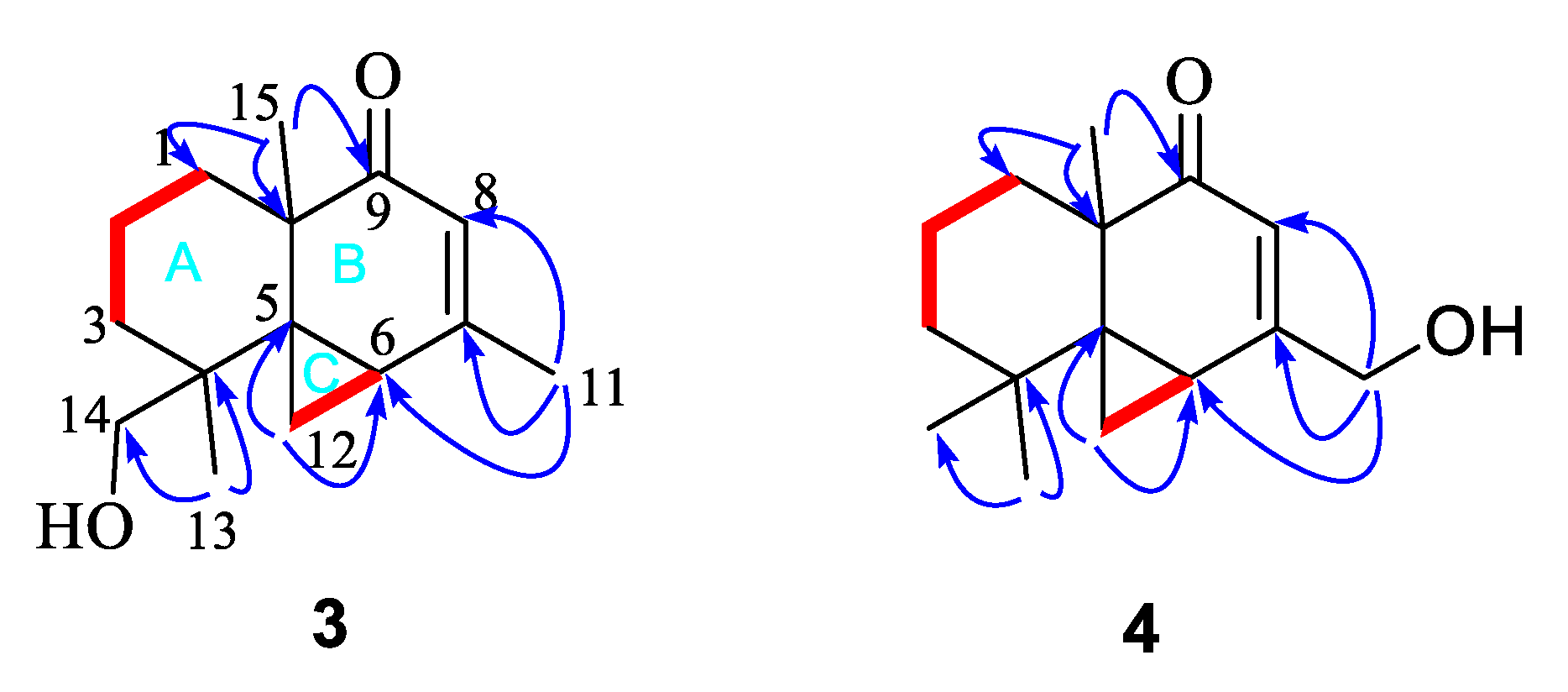
3.3.3. Alternaterpenoid A (3)
3.3.4. Alternaterpenoid B (4)
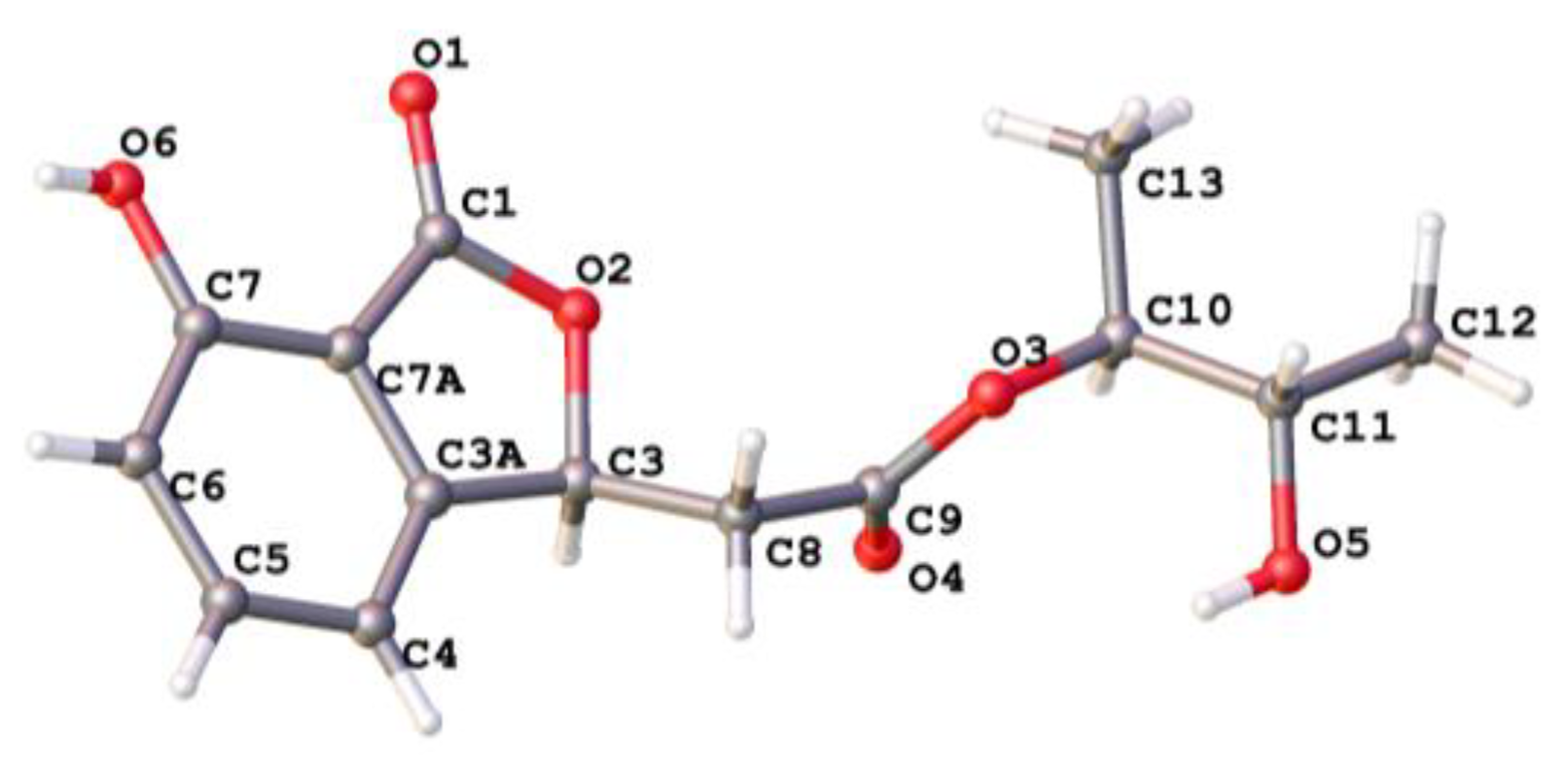
3.4. X-ray Crystallographic Analysis of Compound 1
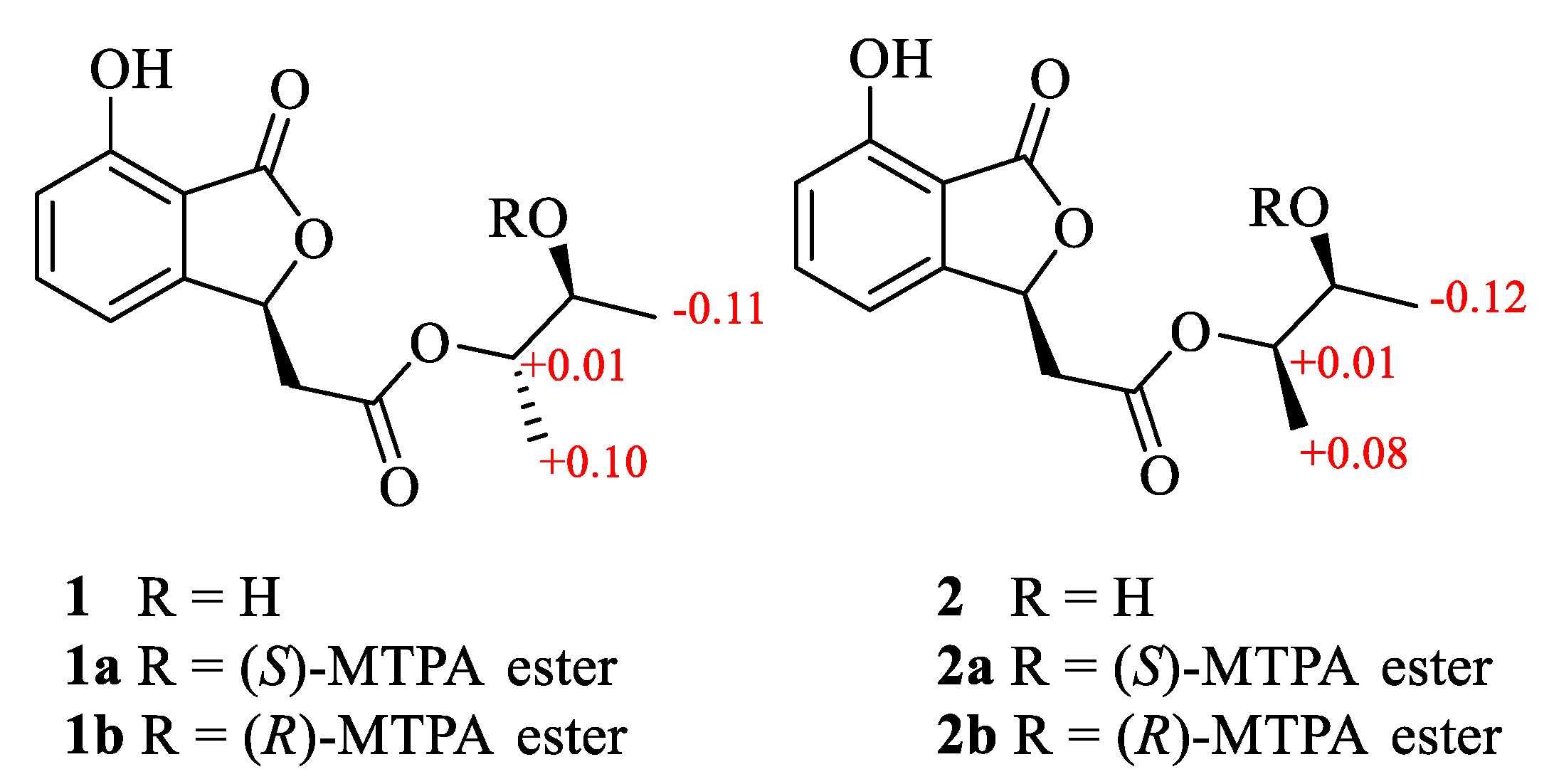
3.5. Preparation of (S)-MTPA Ester and (R)-MTPA Ester
3.5.1. (S)-MTPA Ester (1a) and (R)-MTPA Ester (1b)
3.5.2. (S)-MTPA Ester (2a) and (R)-MTPA Ester (2b)
3.6. Calculation of the ECD Spectra
3.7. Cell Viability Assay and Anti-Inflammatory Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, S.; Li, J.; Liu, L. The biological and chemical diversity of tetramic acid compounds from marine-derived microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Zhang, D.; Zhou, B.; Ma, Z. Inhibitors of BRD4 protein from a marine-derived fungus Alternaria sp. NH-F6. Mar. Drugs 2017, 15, 76. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mao, W.-J.; Yan, M.-X.; Liu, X.; Wang, S.-Y.; Xia, Z.; Xiao, B.; Cao, S.-J.; Yang, B.-Q.; Li, J. Purification, chemical characterization, and bioactivity of an extracellular polysaccharide produced by the marine sponge endogenous fungus Alternaria sp. SP-32. Mar. Biotech. 2016, 18, 301–313. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Miao, F.-P.; Fang, S.-T.; Liu, X.-H.; Yin, X.-L.; Ji, N.-Y. Sesteralterin and Tricycloalterfurenes A–D: Terpenes with Rarely Occurring Frameworks from the Marine-Alga-Epiphytic Fungus Alternaria alternata k21-1. J. Nat. Prod. 2017, 80, 2524–2529. [Google Scholar] [CrossRef]
- Huang, C.-H.; Pan, J.-H.; Chen, B.; Yu, M.; Huang, H.-B.; Zhu, X.; Lu, Y.-J.; She, Z.-G.; Lin, Y.-C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9-6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Shao, C.-L.; Guo, Z.-Y.; Chen, J.-F.; Deng, D.-S.; Yang, K.-L.; Chen, Y.-Y.; Fu, X.-M.; She, Z.-G.; Lin, Y.-C. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Zhang, X.; Zheng, H.; Zheng, Z.; Nong, X.; Liang, X.; Ma, X.; Qi, S. Novel anthraquinone derivatives as inhibitors of protein tyrosine phosphatases and indoleamine 2, 3-dioxygenase 1 from the deep-sea derived fungus Alternaria tenuissima DFFSCS013. Org. Chem. Front. 2019, 6, 3252–3258. [Google Scholar] [CrossRef]
- Wang, H.-L.; Li, R.; Li, J.; He, J.; Cao, Z.-Y.; Kurtaán, T.; Mándi, A.; Zheng, G.-L.; Zhang, W. Alternarin A, a Drimane Meroterpenoid, Suppresses Neuronal Excitability from the Coral-Associated Fungi Alternaria sp. ZH-15. Org. Lett. 2020, 22, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Liu, H.; Long, Y.; Chen, D.; Lu, Y.; She, Z. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. Org. Biomol. Chem. 2017, 15, 6338–6341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Deng, Y.; Lin, X.; Chen, B.; Li, J.; Liu, H.; Chen, S.; Liu, L. Anti-inflammatory mono- and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, M.; Chen, B.; Salaenoi, J.; Niaz, S.-I.; He, J.; Liu, L. Penicamide A, a unique N, N′-ketal quinazolinone alkaloid from ascidian-derived fungus Penicillium sp. 4829. Mar. Drugs 2019, 17, 522. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Liu, X.; Jiang, M.; Luo, G.; Wu, Z.; Chen, B.; Li, J.; Liu, L.; Chen, S. Anti-Inflammatory Cembrane-Type Diterpenoids and Prostaglandins from Soft Coral Lobophytum sarcophytoides. Mar. Drugs 2019, 17, 481. [Google Scholar] [CrossRef] [Green Version]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef]
- Hooft, R.W.; Straver, L.H.; Spek, A.L. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J. Appl. Crystal. 2008, 41, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher's method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.-I.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and anti-inflammatory meroterpenoids: Stachybonoids A–F from the crinoid-derived fungus Stachybotrys chartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.J.; Tantillo, D.J. Branching out from the bisabolyl cation. Unifying mechanistic pathways to barbatene, bazzanene, chamigrene, chamipinene, cumacrene, cuprenene, dunniene, isobazzanene, iso-γ-bisabolene, isochamigrene, laurene, microbiotene, sesquithujene, sesquisabinene, thujopsene, trichodiene, and widdradiene sesquiterpenes. J. Am. Chem. Soc. 2014, 136, 2450–2463. [Google Scholar] [PubMed]
- Wang, Y.; Liu, H.-X.; Chen, Y.-C.; Sun, Z.-H.; Li, H.-H.; Li, S.-N.; Yan, M.-L.; Zhang, W.-M. Two new metabolites from the endophytic fungus Alternaria sp. A744 derived from Morinda officinalis. Molecules 2017, 22, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, P.J.; Sperry, J.; Brimble, M.A. Heteroatom-directed reverse Wacker oxidations. Synthesis of the reported structure of (−)-herbaric acid. J. Org. Chem. 2010, 75, 7388–7392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Zhai, R.; Liu, Z.; Huang, X.; She, Z. Altenusin derivatives from mangrove endophytic fungus Alternaria sp. SK6YW3L. RSC Adv. 2016, 6, 72127–72132. [Google Scholar] [CrossRef]
- Ye, F.; Chen, G.-D.; He, J.-W.; Li, X.-X.; Sun, X.; Guo, L.-D.; Li, Y.; Gao, H. Xinshengin, the first altenusin with tetracyclic skeleton core from Phialophora spp. Tetrahedron Lett. 2013, 54, 4551–4554. [Google Scholar] [CrossRef]
- Hildebrand, A.A.; Kohn, B.N.; Pfeiffer, E.; Wefers, D.; Metzler, M.; Bunzel, M. Conjugation of the mycotoxins alternariol and alternariol monomethyl ether in tobacco suspension cells. J. Agri. Food Chem. 2015, 63, 4728–4736. [Google Scholar] [CrossRef]
- Sun, J.; Awakawa, T.; Noguchi, H.; Abe, I. Induced production of mycotoxins in an endophytic fungus from the medicinal plant Datura stramonium L. Bioorg. Med. Chem. Lett. 2012, 22, 6397–6400. [Google Scholar] [CrossRef]
- He, J.-W.; Chen, G.-D.; Gao, H.; Yang, F.; Li, X.-X.; Peng, T.; Guo, L.-D.; Yao, X.-S. Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 2012, 83, 1087–1091. [Google Scholar] [CrossRef]
- Zhang, J.-c.; Chen, G.-Y.; Li, X.-Z.; Hu, M.; Wang, B.-Y.; Ruan, B.-H.; Zhou, H.; Zhao, L.-X.; Zhou, J.; Ding, Z.-T. Phytotoxic, antibacterial, and antioxidant activities of mycotoxins and other metabolites from Trichoderma sp. Nat. Prod. Res. 2017, 31, 2745–2752. [Google Scholar] [CrossRef]
- Shi, Y.-N.; Pusch, S.; Shi, Y.-M.; Richter, C.; Macia-Vicente, J.G.; Schwalbe, H.; Kaiser, M.; Opatz, T.; Bode, H.B. (±)-Alternarlactones A and B, Two Antiparasitic Alternariol-like Dimers from the Fungus Alternaria alternata P1210 Isolated from the Halophyte Salicornia sp. J. Org. Chem. 2019, 84, 11203–11209. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Deshmukh, S.K.; Reddy, M.S.; Prasad, R.; Goel, M. Endolichenic fungi: A hidden source of bioactive metabolites. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Bao, L.; Yang, X.-L.; Guo, H.; Yang, R.-N.; Ren, B.; Zhang, L.-X.; Dai, H.-Q.; Guo, L.-D.; Liu, H.-W. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia 2012, 83, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, X.; Wang, P.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Perylenequione derivatives with anticancer activities isolated from the marine sponge-derived fungus, Alternaria sp. SCSIO41014. Mar. Drugs 2018, 16, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, D.; Zhou, M.; Lin, T.; Chen, J.; Wang, G.; Huang, Y.; Jiang, X.; Tian, W.; Chen, H. Cytotoxic Components from Hypericum elodeoides Targeting RXRα and Inducing HeLa Cell Apoptosis through Caspase-8 Activation and PARP Cleavage. J. Nat. Prod. 2019, 82, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Shin, Y.J.; Choi, S.U.; Lee, K.R. New cytotoxic δ-valerolactones from Cornus walteri. Bull. Korean Chem. Soc. 2011, 32, 2443–2445. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine natural products with anti-inflammatory activity. Appl. Microbiol. Biol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Xie, C.; Xia, J.; Luo, Z.; Shao, Z.; Yang, X. New anti-inflammatory guaianes from the Atlantic hydrotherm-derived fungus Graphostroma sp. MCCC 3A00421. Sci. Rep. 2018, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Ding, M.; Liu, W.; Huang, X.; Liu, Z.; Lu, Y.; Liu, H.; She, Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry 2018, 146, 8–15. [Google Scholar] [CrossRef]
- Liu, M.; Sun, W.; Wang, J.; He, Y.; Zhang, J.; Li, F.; Qi, C.; Zhu, H.; Xue, Y.; Hu, Z.; et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.N.; Anguera, M.; Jensen, P.R.; Fenical, W.; Kock, M. Oxepinamides A–C and Fumiquinazolines H-I: Bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chem. A Eur. J. 2000, 6, 1355–1360. [Google Scholar] [CrossRef]
- Ko, W.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.S.; Kim, Y.C.; Oh, H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Biol. Interact. 2016, 244, 16–26. [Google Scholar] [CrossRef]
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | 171.5, C | 171.6, C | ||
| 3 | 78.3, CH | 5.90, t (6.6) | 78.3, CH | 5,87, t (6.5) |
| 3a | 149.0, C | 149.0, C | ||
| 4 | 116.2, CH | 6.96, dd (2.5, 7.3) | 116.2, CH | 6.96, dd (4.6, 7.0) |
| 5 | 137.4, CH | 7.56, t (7.9) | 137.4, CH | 7.56, t (7.8) |
| 6 | 113.4, CH | 6.96, dd (2.5, 7.3) | 113.5, CH | 6.96, dd (4.6, 7.0) |
| 7 | 156.7, C | 156.8, C | ||
| 7a | 111.1, C | 111.1, C | ||
| 8 | 39.7, CH2 | 2.95, m | 39.7, CH2 | 2.94, m |
| 9 | 168.8, C | 168.9, C | ||
| 10 | 76.3, CH | 4.86, m | 76.2, CH | 4.85, m |
| 11 | 70.1, CH | 3.73, m | 70.0, CH | 3.75, m |
| 12 | 19.1, CH3 | 1.18, d (6.4) | 19.2, CH3 | 1.19, d (6.3) |
| 13 | 16.4, CH3 | 1.25, d (6.4) | 16.3, CH3 | 1.20, d (6.3) |
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | 34.1, CH2 | 1.39, m 1.29, m | 34.2, CH2 | 1.77, m 1.45, m |
| 2 | 37.3, CH2 | 1.54, td (1.6, 3.3) 1.32, td (1.6, 3.4) | 40.0, CH2 | 1.81, m 1.57, m |
| 3 | 17.6, CH2 | 1.83, dt (3.5, 13.6) 1.61, t (3.6) | 17.6, CH2 | 1.60, m 1.79, m |
| 4 | 34.0, C | 51.0, C | ||
| 5 | 36.7, C | 42.9, C | ||
| 6 | 25.0, CH | 1.97, dd (4.7, 9.1) | 39.3, CH | 1.37, m |
| 7 | 164.7, C | 164.6, C | ||
| 8 | 118.0, CH | 5.53, s | 123.1, CH | 6.00, s |
| 9 | 202.5, C | 209.9, C | ||
| 10 | 46.8, C | 41.4, C | ||
| 11 | 24.6, CH3 | 2.09, s | 71.3, CH2 | 3.73, d (11.4) 3.32, d (11.4) |
| 12 | 27.5, CH2 | 1.69, d (4.1) 0.05, t (4.3) | 33.1, CH2 | 1.94, m 1.84, m |
| 13 | 22.6, CH3 | 1.18, s | 30.4, CH3 | 1.16, s |
| 14 | 72.1, CH2 | 3.22, d (11.3) 3.14, d (11.3) | 27.0, CH3 | 1.15, s |
| 15 | 21.9, CH3 | 1.45, s | 27.4, CH3 | 1.34, s |
| Compounds | IC50 (μM) | CC50 (μM) a | SI b |
|---|---|---|---|
| 1 | >50 | >100 | |
| 18.7 ± 2.35 | >100 | ||
| 2.4 ± 0.79 | 24.9 ± 1.2 | 10.4 | |
| 4 | ≈50 | >100 | |
| 41.1 ± 4.78 | >100 | ||
| 5.2 ± 1.96 | 20.6 ± 2.3 | 4.0 | |
| 8 | >50 | >100 | |
| 9 | 1.3 ± 0.10 | >100 | |
| 10 | 23.9 ± 3.30 | 27.2 ± 1.6 | 1.1 |
| 11 | 39.0 ± 1.92 | >100 | |
| 12 | 16.6 ± 1.60 | 50.4 ± 2.1 | 3.0 |
| 13 | 24.5 ± 4.51 | 48.2 ± 2.6 | 2.0 |
| 14 | 5.9 ± 0.48 | >100 | |
| 15 | 26.3 ± 3.99 | 48.7 ± 1.8 | 1.9 |
| 17 | 16.2 ± 2.62 | >100 | |
| 18 | 24.5 ± 1.06 | 28.3 ± 2.5 | 1.2 |
| 25.4 ± 3.03 | >100 | ||
| 14.9 ± 1.92 | 17.3 ± 2.2 | 1.2 | |
| 22 | 14.9 ± 1.92 | 46.4 ± 1.7 | 3.1 |
| Indometacin c | 35.8 ± 5.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Deng, Y.; Yan, C.; Wu, Z.; Guo, H.; Liu, L.; Liu, H. Secondary Metabolites with Nitric Oxide Inhibition from Marine-Derived Fungus Alternaria sp. 5102. Mar. Drugs 2020, 18, 426. https://doi.org/10.3390/md18080426
Chen S, Deng Y, Yan C, Wu Z, Guo H, Liu L, Liu H. Secondary Metabolites with Nitric Oxide Inhibition from Marine-Derived Fungus Alternaria sp. 5102. Marine Drugs. 2020; 18(8):426. https://doi.org/10.3390/md18080426
Chicago/Turabian StyleChen, Senhua, Yanlian Deng, Chong Yan, Zhenger Wu, Heng Guo, Lan Liu, and Hongju Liu. 2020. "Secondary Metabolites with Nitric Oxide Inhibition from Marine-Derived Fungus Alternaria sp. 5102" Marine Drugs 18, no. 8: 426. https://doi.org/10.3390/md18080426





