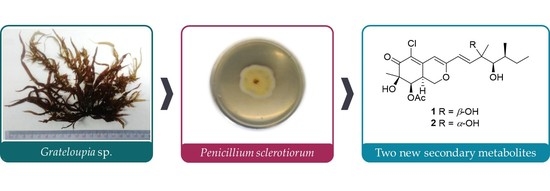
Anti-Inflammatory Azaphilones from the Edible Alga-Derived Fungus Penicillium sclerotiorum
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of New Compounds 1 and 2
2.2. Cytotoxicity of Compounds 1–7
2.3. Anti-Inflammatory Activity of Compounds 1–7
2.4. Anti-Fibrotic Activity of Compounds 1–7
3. Discussion
4. Materials and Methods
4.1. General
4.2. Alga Material
4.3. Separation and Identification of Fungal Material
4.4. Extraction and Isolation
4.5. Spectroscopic Data
4.6. Cytotoxicity Assays
4.7. Western Blot Assay
4.8. NFκB and Smad Transcriptional Activity Assays
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Flewelling, A.J.; Currie, J.; Gray, C.A.; Johnson, J.A. Endophytes from marine macroalgae: Promising sources of novel natural products. Curr. Sci. India 2015, 109, 88–111. [Google Scholar]
- Bernard, M.; Rousvoal, S.; Jacquemin, B.; Ballenghien, M.; Peters, A.F.; Leblanc, C. qPCR-based relative quantification of the brown algal endophyte Laminarionema elsbetiae in Saccharina latissima: Variation and dynamics of host-endophyte interactions. J. Appl. Phycol. 2018, 30, 2901–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Su, M.; Song, S.-J.; Jung, J.H. Marine-derived Penicillium species as producers of cytotoxic metabolites. Mar. Drugs 2017, 15, 329. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.-L.; Zhou, Z.-Y.; Yang, T.; Yao, C.; Wu, L.-W.; Li, G.-Y. Azaphilone alkaloids with anti-inflammatory activity from fungus Penicillium sclerotiorum cib-411. J. Agric. Food. Chem. 2019, 67, 2175–2182. [Google Scholar] [CrossRef]
- Jia, Q.; Du, Y.; Wang, C.; Wang, Y.; Zhu, T.; Zhu, W. Azaphilones from the Marine sponge-derived fungus Penicillium sclerotiorum OUCMDZ-3839. Mar. Drugs 2019, 17, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Qiu, P.; Liu, H.; Li, J.; Shao, C.; Yan, T.; Cao, W.; She, Z. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg. Chem. 2019, 88, 102973. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Ruan, Q.; Pan, W.; Tang, Y.; Zhao, Z.; Cui, H. New polyketides and diterpenoid derivatives from the fungusPenicilliumsclerotiorumGZU-XW03-2 and their anti-inflammatory activity. Fitoterapia 2020, 143, 104561. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hao, J.-D.; Ning, X.-Y.; Wu, J.-S.; Zhao, D.-L.; Kong, C.-J.; Shao, C.-L.; Wang, C.-Y. Penicilazaphilones D and E: Two new azaphilones from a sponge-derived strain of the fungus Penicillium sclerotiorum. RSC Adv. 2018, 8, 4348–4353. [Google Scholar] [CrossRef] [Green Version]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for anti-fibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Pittet, J.F.; Griffiths, M.J.; Geiser, T.; Kaminski, N.; Dalton, S.L.; Huang, X.; Brown, L.A.; Gotwals, P.J.; Koteliansky, V.E.; Matthay, M.A.; et al. TGF-β is a critical mediator of acute lung injury. J. Clin. Investig. 2001, 107, 1537–1544. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.-B.; Wu, Y.; Tang, J.; Jiao, W.-H.; Li, L.; Sun, F.; Wang, S.-P.; Yang, F.; Lin, H.-W. Azaphilone and isocoumarin derivatives from the sponge-derived fungus Eupenicillium sp. 6A-9. Tetrahedron. Lett. 2018, 59, 3345–3348. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Chidananda, C.; Sattur, A.P. Sclerotiorin, a novel inhibitor of lipoxygenase from Penicillium frequentans. J. Agric. Food Chem. 2007, 55, 2879–2883. [Google Scholar] [CrossRef]
- Arai, N.; Shiomi, K.; Tomoda, H.; Tabata, N.; Yang, D.J.; Masuma, R.; Kawakubo, T.; Omura, S. Isochromophilones III-VI, inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium multicolor FO-3216. J. Antibiot. 1995, 48, 696–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Wadhwa, M. Therapeutic use of specific tumour necrosis factor inhibitors in inflammatory diseases including COVID-19. Biomed. Pharmacother. 2021, 140, 111785. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Jope, R.S.; Cheng, Y.; Lowell, J.A.; Worthen, R.J.; Sitbon, Y.H.; Beurel, E. Stressed and inflamed, can GSK3 be blamed? Trends Biochem. Sci. 2017, 42, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Rhee, M.H.; Kim, E.; Cho, J.Y. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediat. Inflamm. 2012, 2012, 416036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Hu, H.-H.; Chang, F.-R.; Tsai, J.-Y.; Kuo, C.-Y.; Wu, Y.-C.; Wu, C.-C. Different effects of 4β-hydroxywithanolide E and withaferin A, two withanolides from Solanaceae plants, on the Akt signaling pathway in human breast cancer cells. Phytomedicine 2019, 53, 213–222. [Google Scholar] [CrossRef] [PubMed]







| 1 a | 2 a | |||
|---|---|---|---|---|
| δH (J in Hz) | δc, Type | δH (J in Hz) | δc, Type | |
| 1α | 4.47, dd (10.8, 4.8) | 67.5, CH2 | 4.49, dd (10.9, 4.8) | 67.6, CH2 |
| 1β | 3.81, m | 3.83, dd (13.1, 10.9) | ||
| 3 | 161.0, C | 161.0, C | ||
| 4 | 6.10, s | 102.6, CH | 6.11, s | 102.6, CH |
| 4a | 144.3, C | 144.3, C | ||
| 5 | 117.4, C | 117.3, C | ||
| 6 | 192.5, C | 192.5, C | ||
| 7 | 76.3, C | 76.2, C | ||
| 8 | 5.52, d (2.5) | 74.4, CH | 5.52, d (3.1) | 74.4, CH |
| 8a | 3.23, m | 36.9, CH | 3.25, m | 36.9, CH |
| 9 | 6.30, d (15.5) | 112.6, CH | 6.32, d (15.5) | 112.2, CH |
| 10 | 6.55, d (15.5) | 144.5, CH | 6.51, d (15.5) | 141.6, CH |
| 11 | 76.0, C | 76.2, C | ||
| 12 | 3.49, brs | 78.5, CH | 3.52, d (2.0) | 79.2, CH |
| 13 | 1.70, m | 35.5, CH | 1.55, m | 36.6, CH |
| 14 | 1.41, m | 28.8, CH2 | 1.42, m | 28.9, CH2 |
| 1.33, m | 1.31, m | |||
| 15 | 0.92, t (7.0) | 12.0, CH3 | 0.91, t (7.4) | 12.0, CH3 |
| 16 | 0.97,d (6.8) | 13.5, CH3 | 0.88, d (7.0) | 13.3, CH3 |
| 17 | 1.32, s | 23.7, CH3 | 1.37, s | 27.0, CH3 |
| 18 | 1.45, s | 24.6, CH3 | 1.45, s | 24.6, CH3 |
| 19 | 170.7, C | 170.7, C | ||
| 20 | 2.02, s | 20.7, CH3 | 2.02, s | 20.7, CH3 |
| Compounds | A549 | CL1-5 | MCF-7 | MDA-MB-231 | HCT15 | HCT116 | SH-SY5Y | WI-38 |
|---|---|---|---|---|---|---|---|---|
| 1 | >100 | >100 | >100 | >100 | >100 | >100 | 35.6 ± 3.1 | >100 |
| 2 | >100 | >100 | >100 | >100 | >100 | >100 | 73.2 ± 1.5 | >100 |
| 3 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 | >100 | >100 | >100 | 95.2 ± 4.1 | >100 |
| 5 | >100 | >100 | >100 | >100 | 87.2 ± 4.7 | 36.0 ± 0.8 | >100 | >100 |
| 6 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 7 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| doxorubicin | 3.7 ± 0.1 | 3.6 ± 0.1 | 1.2 ± 0.1 | 3.4 ± 0.1 | 1.8 ± 0.5 | 0.5 ± 0.0 | 0.36 ± 0.0 | >10 |
| Compounds | A549 | CL1-5 | MCF-7 | MDA-MB-231 | HCT15 | HCT116 | SH-SY5Y | WI-38 |
|---|---|---|---|---|---|---|---|---|
| 1 | >100 | 89.3 ± 4.9 | >100 | 62.4 ± 4.0 | 88.2 ± 0.7 | 66.0 ± 3.8 | 26.8 ± 4.2 | >100 |
| 2 | >100 | >100 | >100 | 79.4 ± 0.6 | 94.2 ± 3.2 | 72.1 ± 2.5 | >100 | >100 |
| 3 | >100 | 78.4 ± 5.0 | 68.8 ± 3.5 | 40.4 ± 2.8 | 76.1 ± 1.8 | 47.3 ± 2.0 | >100 | >100 |
| 4 | >100 | 77.6 ± 3.0 | 80.4 ± 5.1 | 45.1 ± 0.8 | 76.9 ± 0.8 | 56.3 ± 0.6 | >100 | >100 |
| 5 | 44.3 ± 1.3 | 60.3 ± 3.8 | 52.1 ± 9.0 | 44.4 ± 0.6 | 78.6 ± 2.0 | 37.5 ± 0.8 | >100 | >100 |
| 6 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 7 | >100 | 84.3 ± 4.3 | 92.8 ± 2.7 | 47.4 ± 1.2 | 61.0 ± 2.5 | 54.1 ± 2.0 | >100 | >100 |
| doxorubicin | 1.3 ± 0.1 | 2.2 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 1.2 ± 0.3 | 0.38 ± 0.0 | 0.9 ± 0.3 | >10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-C.; Ke, T.-Y.; Ko, Y.-C.; Lin, J.-J.; Chang, J.-S.; Cheng, Y.-B. Anti-Inflammatory Azaphilones from the Edible Alga-Derived Fungus Penicillium sclerotiorum. Mar. Drugs 2021, 19, 529. https://doi.org/10.3390/md19100529
Wang H-C, Ke T-Y, Ko Y-C, Lin J-J, Chang J-S, Cheng Y-B. Anti-Inflammatory Azaphilones from the Edible Alga-Derived Fungus Penicillium sclerotiorum. Marine Drugs. 2021; 19(10):529. https://doi.org/10.3390/md19100529
Chicago/Turabian StyleWang, Hui-Chun, Tzu-Yi Ke, Ya-Chen Ko, Jue-Jun Lin, Jui-Sheng Chang, and Yuan-Bin Cheng. 2021. "Anti-Inflammatory Azaphilones from the Edible Alga-Derived Fungus Penicillium sclerotiorum" Marine Drugs 19, no. 10: 529. https://doi.org/10.3390/md19100529