The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases
Abstract
:1. Introduction
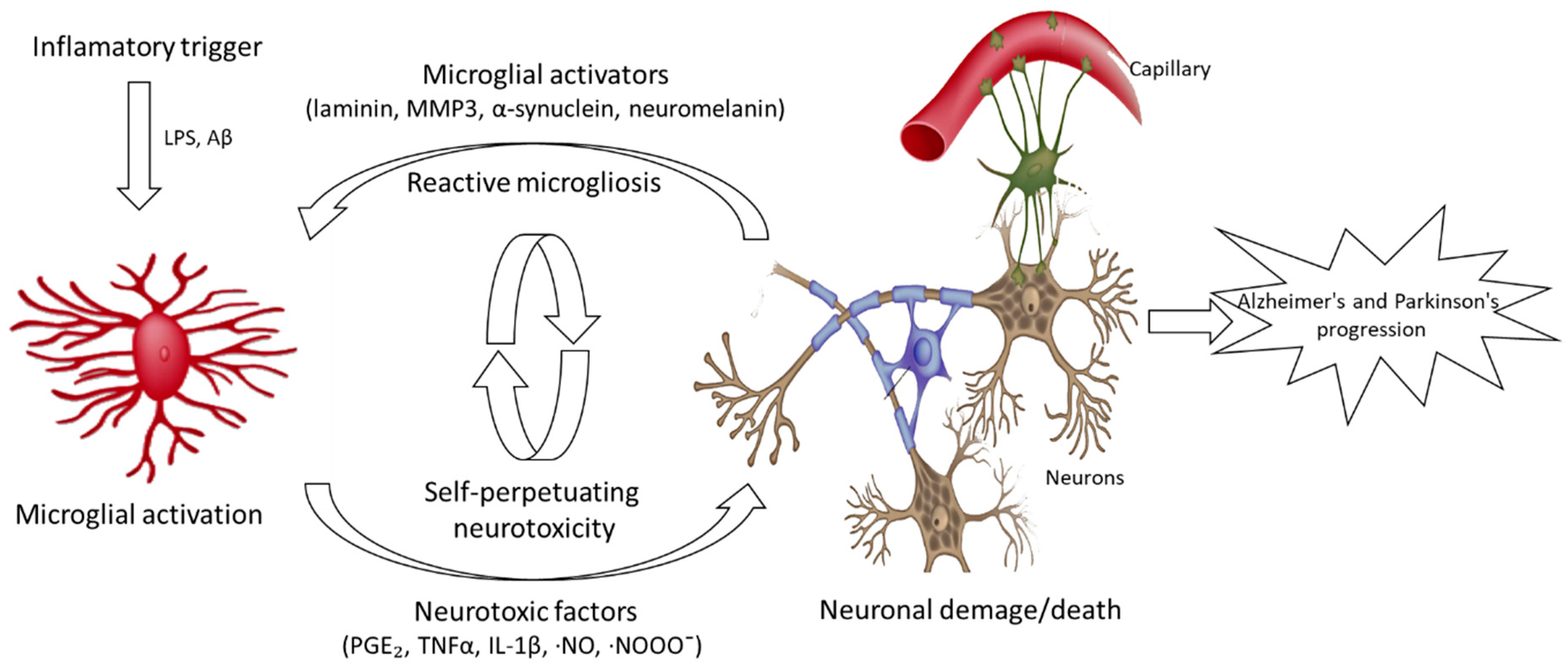
2. Alzheimer’s and Parkinson’s Diseases
3. Seaweeds and Their Neurophysiological Activities
| Species | Extraction Methods | Compounds of Interest and Fractions | Activity | References | |
|---|---|---|---|---|---|
| Phaeophyceae (brown seaweeds) | |||||
| Agarum clathratum subsp. yakishiriense | Ethanol extract at 60 °C for 2 h | The ethanol extract was suspended in distilled water and subjected to a series of partitioning with n-hexane, dichloromethane, ethyl acetate and n-butanol; the mass of crude extract (95% EtOH) and fractions was 840.46 mg | In vivo (animal models) neuronal protection from ischemic injury | [52] | |
| Alaria esculenta | Extracted with methanol/water (1:1) at 50 °C with stirring for 2 h at room temperature | Fractions | The fraction below 5 kDa decreased the melting point of α-synuclein, whereas the fraction above 10 kDa raised the melting point. Both of these fractions were found to inhibit the formation of amyloid aggregates by α-synuclein, in vitro | [53] | |
| Cystoseira humilis | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 50% 10 mg mL−1 | [54] | |
| Gongolaria nodicaulis (as C. nodicaulis) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 64.4% In vitro BuChE inhibitory capacity: 110% 10 mg mL−1 | [54] | |
| Ericaria selaginoides (as Cystoseira tamariscifolia) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 85% In vitro BuChE inhibitory capacity: 86% 10 mg mL−1 | [54] | |
| Gongolaria usneoides (as Cystoseira usneoides) | Methanolic extract | Fraction | In vitro AChE inhibitory capacity: 47% 10 mg mL-1 | [54] | |
| Dictyopteris undulata | Zonarol was prepared as a 10 mM stock solution in dimethyl sulfoxide (DMSO) | Sesquiterpene: Zonarol | Activates the Nrf2/ARE pathway, induces phase-2 enzymes, and protects neuronal cells from oxidative stress, in vitro | [55] | |
| Dictyota humifusa | Methanolic extract | Extract | Inhibiting AChE IC50 = 4.8 mg mL−1, in vitro | [56] | |
| Ecklonia bicyclis and E. bicyclis (as Eisenia bicyclis) | MeOH extract | Phlorotannins | Suppression of BACE-1 enzyme activity IC50 = 5.35 μM, in vitro | [57] | |
| Ethyl acetate extraction | Phlorotannins | Decreased Aβ-induced cell death IC50 = 800 µM, in vivo | [58] | ||
| Ethanolic extract | Phlorotannins | Protection from retinal neuronal death, in vivo | [59] | ||
| Ethanolic extract | Phlorotannins | In vitro inhibitory properties against AChE, BChE, and total ROS with inhibition percentages (%) of 68.01, 95.72, and 73.20 at concentrations of 25 μg mL−1, respectively | [60] | ||
| Ecklonia cava | The seaweed (1 kg) was extracted with 95% ethanol (10 L) for 2 h in a water bath at 50 °C | Phlorotannins: Dieckol and phlorofucofuroeckol | Improvement of memory and possible involvement of the AChE inhibition, in vivo | [61] | |
| Ethanolic extract | Phlorotannin: Triphlorethol-A | Anti-oxidative activity: Scavenging activity against ROS and DPPH via activation of ERK protein, in vivo | [62] | ||
| Methanolic extract | Phlorotnnins | In vitro scavenging activity against hydroxyl, superoxide, and peroxyl radicals IC50 = 392.5, 115.2 and 128.9 µM, respectively | [63] | ||
| Enzymatic extract | Phlorotannins | The phlorotannin-rich fraction significantly potentiated the pentobarbital-induced sleep at >50 mg kg−1, in vivo | [64] | ||
| 80% MeOHextract | Phlorotannins | Neuroprotective effects against H2O2-induced oxidative stress in murine hippocampal HT22 cells IC50 = 50 µM, in vivo | [65] | ||
| Ethanolic extract | Phloroglucinol | Reduce the toxicity ROS induced by hydrogen peroxide IC50 = 10 µg mL−1, in vivo | [66] | ||
| Ethanolic extract | Phlorotannin: 8,8’-Bieckol | Phlorotannin: 8,8’-Bieckol reduce COX-2, NO, and prostaglandin E2 (PGE2) IC50 = 100 μM, in vivo | [67] | ||
| Ethanolic extract | Extract | Extracts have potential analgesic effects in the case of postoperative pain and neuropathic pain, in vivo | [68] | ||
| Ethanolic extract | Phlorotannin (eckol) | Inhibiting BuChE IC50 = 29 μM, in vitro and in vivo model studies | [69] | ||
| Ethanolic extract | Phlorotannin (7-phloroeckol) | Inhibiting BuChE IC50 = 0.95 μM, in vitro and in vivo model studies | [69] | ||
| E. kurome | Provided by Marine Drug and FoodInstitute, Ocean University of China China | Acidic oligosaccharide sugar chain (AOSC) | Blocking the fibril formation of Aβ IC50 = 100 µg mL−1, in vitro | [70] | |
| E. maxima | Crude extract was sequentially extracted with n-hexane, dichloromethane, ethyl acetate, and finally n-butanol | Phlorotannins | IC50 values for the solvent fractions ranged from 62.61 to 150.8 μg mL−1, with the ethyl acetate fraction having the best inhibitory activity, in vitro | [71] | |
| E. stolonifera | Ethanolic extract | Phlorotannin (dieckol) | Inhibiting AChE 17.11 μM, in vitro | [72] | |
| Ethanolic extract | Phlorotannin (eckstolonol) | Inhibiting AChE and BuChE IC50 = 42.66 and 230.27 μM, in vitro | [72] | ||
| Ethanolic extract | Phlorotannin (eckol) | Inhibiting AChE IC50 = 20.56 μM, in vitro | [72] | ||
| Ethanolic extract | Phlorotannin (2-phloroeckol) | Inhibiting AChE IC50 = 38.13 μM, in vitro | [72] | ||
| Ethanolic extract | Phlorotannin (7-phloroeckol) | Inhibiting AChE and BuChE IC50 = 4.89 and 136.71 μM, in vitro | [72] | ||
| Ethanolic extract | Phlorotannin (phlorofucofuroeckol A) | Inhibiting AChE and BuChE IC50 = 4.89 and 136.71 μM, in vitro | [72] | ||
| Ethanolic extract | Sterol (fucosterol) | Inhibiting AChE IC50 = 421.72 μM, in vitro | [72] | ||
| Methanolic extract | Phlorotannins | Inhibiting AChE IC50 = 108.11 mg mL−1, in vitro | [73] | ||
| Fucus vesiculosus | Fucoidan (Sigma) | Fucoidan | Fucoidan completely blocks microglial uptake of fDNA at only 40 ng mL−1, in vivo | [74] | |
| Fucoidan (Sigma) | Fucoidan | In vitro anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals, and lipid peroxidation IC50 = 0.058, 0.157 and 1.250 mg mL−1, respectively | [75] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan has protective effect via inducible nitric oxide synthase (iNOS), in vivo | [76] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan inhibits TNF-alpha- and IFN-gamma-stimulated NO production via p38 MAPK, AP-1, JAK/STAT, and IRF-1, in vivo | [77] | ||
| Fucoidan (Sigma) | Fucoidan | Fucoidan inhibits beta amyloid induced microglial clustering at 10 µM, in vivo | [78] | ||
| Extracted using 70% acetone | Phlorotnnins | Suppressing the overproduction of intracellular ROS induced by hydrogen peroxide IC50 = 0.068 mg mL−1, in vivo | [79] | ||
| Ishige okamurae | Methanolic extraction | Phlorotannin: 6,6ʹ-Bieckol | Inhibiting AChE IC50 = 46.42 μM, in vitro | [73] | |
| Methanolic extraction | Phlorotannin: Diphlorethohydroxycarmalol (DPHC) | In vivo neuroprotection against hydrogen peroxide (H2O2)-induced oxidative stress in murine hippocampal neuronal cells IC50 = 50 µM | [80] | ||
| Marginariella boryana | Sequential extractions with H2SO4 and HCl | Sulfated fucans | Prevents the accumulation of Aβ | [81] | |
| Padina australis | Dichloromethane extract | Extracts | Inhibiting AChE IC50 = 0.149 mg mL−1, in vitro | [82] | |
| P. gymnospora | Methanolic extract | Bioassay-guided fractionation of the active n-hexane and ethyl acetate (EtOAc) soluble fractions | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [72] | |
| Methanolic extract | Extracts | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [83] | ||
| Acetone extracts | Extrats | IC50 value <10 μg mL−1 for both AChE and BuChE, in vitro | [84] | ||
| P. tetrastromatica | Acetone extract | Fucoxanthin | Anti-oxidative activity: Reduce lipid peroxidation in rats IC50 = 0.83 μM, in vivo | [85] | |
| Chloroform and ethanol extracts | Extract | Chloroform extract at 600 mg Kg−1 showed significant anticonvulsant activity, in vivo | [86] | ||
| Papenfussiella lutea | Sequential extractions with H2SO4 and HCl | Sesquiterpenes | Inhibiting AChE IC50 = 48–65 μM, in vivo | [81] | |
| Saccharina japonica | Fucoidan | Fucoidan | Protective effect in MPTP-induced neurotoxicity. In addition, reduce behavioural deficits and cell death and increase dopamine IC50 = 25 mg kg−1, once per day in mice, in vivo and in vitro | [31] | |
| Extracted from seaweeds commercially cultured in Qingdao, China | Fucoidan | Inhibiting microglia which inhibits LPS-induced NO production via suppression of p38 and ERK phosphorylation IC50 = 125 μg mL−1, in vivo | [87] | ||
| Fucoidan (CY110115) was obtained from Ci Yuan Biotechnology Co., Ltd., Xi’an, China. The purity of the chemical was more than 98.0%. | Fucoidan | Anti-oxidative activity: Reduce the toxicity of H2O2 in PC12 cells via activation of PI3K/Akt pathway IC50 = 60 µg mL−1, in vivo | [88] | ||
| Ethanolic extract | Extracts | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 15 μg mL−1, in vitro | [89,90] | ||
| Extracted from seaweeds commercially cultured in Qingdao, China | Fucoidan | Reduced 6-hydroxydopamine (6-OHDA) and reduced the loss of dopaminergic in neurons IC50 = 20 mg kg−1 in rats, in vivo | [91] | ||
| Sargassum fulvellum | MeOH-extract | Pigment: Pheophytin A | Produce neurite outgrowth (from 20 to 100% in the present of 10 ng mL−1 of NGF) and activate IC50 = 3.9 μg mL−1 in PC12 cells, in vivo | [92] | |
| S. macrocarpum | Extracted with chloroform at room temperature | Carotenoids | Promote neurite outgrowth activity to 0.4 in PC12 cells IC50 = 6.25 μg mL−1, in vivo | [93] | |
| Methanol extract | Meroterpenoid: Sargaquinoic acid | Signalling pathway of TrkA-MAP kinase pathway IC50 = 3 μg mL−1, in vivo | [94] | ||
| Methanol extract | Meroterpene: Sargachromenol | Promote survival of PC-12 cells and neurite outgrowth through activation of cAMP and MAP kinase pathways IC50 = 9 μM, in vivo | [95] | ||
| S. micracanthum | Methanol extract | Plastoquinones | Anti-oxidative activity: Lipid peroxidation IC50 = 0.95–44.3 μg mL−1 DPPH IC50 = 3–52.6 μg mL-1, in vivo | [96] | |
| S. fulvellum | Ethanolic extract | Extracts | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 5 μg mL−1, in vivo | [97] | |
| S. fusiforme (as Hijikia fusiformis) | Methanol extract | Fucoxanthin | Anti-oxidative activity: DPPH radical scavenging, in vitro | [98] | |
| Alcohol extract | Fucoidan | Ameliorating learning and memory deficiencies, and otential ingredient on treatment of Alzheimer’s disease, in vivo | [99] | ||
| S. horneri | Ethanol extract | Total sterols and β-sitosterol | Antidepressant effect, in vivo | [100] | |
| S. polycystum | Hexane, dichloromethane, and methanol extracts | Extracts | Inhibiting AChE IC50 = 0.115, 0.180 and 0.162 mg mL−1, respectively, in vitro | [82] | |
| S. sagamianum | MeOH extract | Sesquiterpenes | Inhibiting AChE IC50 = 48–65 μM, in vitro | [101] | |
| MeOH extract | Plastoquinones: Sargaquinoic acid and sargachromenol | Inhibiting AChE IC50 = 23.2 and 32.7 μM, respectively Inhibiting BuChE IC50 = 26 μM (for sargaquinoic), in vitro | [102] | ||
| S. siliquastrum | Extracted with 80% aqueous MeOH | Fucoxanthin | Anti-oxidative activity: Inhibit hydrogen peroxide in Vero cells IC50 = 100 uM, in vivo | [103] | |
| Extracted with CH2Cl2 and MeOH | Meroditerpenoids | These compounds exhibited moderate to significant radical-scavenging activity as well as weak inhibitory activities against sortase A and isocitrate lyase, in vitro | [104] | ||
| Sargassum sp. | Methanolic extract | Extract | Inhibiting AChE IC50 = 1 mg mL−1, in vitro | [83] | |
| S. swartzii (as Sargassum wightii) | Extracted with CH2Cl2 and MeOH | Alginic acid | Polysaccharide inhibition activities to COX-2, lipoxygenase (5-LOX), xanthine oxidase (XO) and myeloperoxidase (MPO) in type II collagen induced arthritic rats IC50 = 100 mg kg−1, in vivo | [105] | |
| Petroleum ether, hexane, benzene, and dichloromethane extracts | Extracts | Inhibiting AChE IC50 = 19.33, 46.81, 27.24, 50.56 µg mL−1, respectively Inhibiting BuChE IC50 = 17.91, 32.75, 12.98, 36.16 µg mL−1, respectively, in vivo | [106] | ||
| S. vulgare | Methanolic extract | Extracts | Inhibiting AChE IC50 = 3.5 mg mL−1, in vitro | [54] | |
| Scytothamnus australis | 6 h with 1% (w/v) H2SO4 at 20 °C, 6 h with 0.2 M HCl at 20 °C, 6 h with 2% CaCl2 at 75 °C | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [81] | |
| Splachnidium rugosum | 6 h with 1% (w/v) H2SO4 at 20 °C, 6 h with 0.2 M HCl at 20 °C, 6 h with 2% CaCl2 at 75 °C | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [81] | |
| Turbinaria decurrens | Dried seaweed powder was depig-mented with acetone followed by hot water extraction at 90–95 °C for 3–4 h | Fucoidan | Potential neuroprotective effect in Parkinson’s deasese, in vivo | [107] | |
| Undaria pinnatifida | Ethanolic extract | Extract | Promoted neurite outgrowth in a dose-dependent manner with optimal concentrations of 5 μg mL−1, in vitro | [90,91] | |
| Ethanolic extract | Extract | Neurogenesis, neuroprotection, anti-inflammatory and anti-Alzheimer’s, in vivo | [108] | ||
| Glycoprotein | Glycoprotein | Neurogenesis, neuroprotection, anti-inflammatory and anti-Alzheimer’s Showed predominantly AChE, BChE, and BACE1 inhibitory activities with IC50 values of 63.56, 99.03 and 73.35 μg mL−1, respectively, in vitro and in vivo | [109] | ||
| Ethanolic extract | Sulfated fucans | Prevents the accumulation of Aβ, in vivo | [81] | ||
| Zonaria spiralis | Ethanolic extract | Phloroglucinol: Spiralisone A and Chromone 6 | Kinases inhibitory to CDK5/p25, CK1δ and GSK3β IC50 = 10.0, <10 and <10 μM, respectively, in vitro | [38] | |
| Rhodophyta (red seaweeds) | |||||
| Amphiroa beauvoisii | 50% Aqueous methanol extract | Phenolic, flavonoid extracts | Inhibiting AChE IC50 = 0.12 mg mL−1, in vitro | [110] | |
| A. bowerbankii | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.3 mg mL−1, in vitro | [56] | |
| A. ephedraea | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.1 mg mL−1, in vitro | [56] | |
| Asparagopsis armata | Methanolic extract | Extracts | AChE inhibitory capacity: 58.4% BuChE inhibitory capacity: 81.4% 10 mg mL−1, in vitro | [54] | |
| Bryothamnion triquetrum | Water extract | Fractions | Protect GT1–7 cells death produced by severe (180 min.) chemical hypoxia/aglycemia insult, in vitro | [21,111] | |
| Chondracanthus acicularis | Alcaline extraction | Lambda-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.046, 0.357 and 2.267 mg mL−1, respectively, in vitro | [75] | |
| Chondrophycus undulatus (as Laurencia undulata) | Glycerol glycosides: Floridoside | Glycerol glycosides: Floridoside | Suppress pro-inflammatory responses in microglia by markedly inhibiting the production of nitric oxide (NO) and reactive oxygen species (ROS) IC50 = 10 μM | [112] | |
| Chondrus crispus | Methanolic extract | Floridoside and d-Isofloridoside | Extract-mediated protection against Parkinson’s disease pathology, in vitro and in vivo | [47] | |
| Eucheuma denticulatum | Alcaline extraction | Iota-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.332, 0.281 and 0.830 mg mL−1, respectively, in vitro | [75] | |
| Gelidiella acerosa | Petroleum ether, hexane, benzene, dichloromethane, chloroform, ethyl acetate, acetone, methanol, and water extracts | Extracts | Inhibiting AChE Benzene extract, IC50 = 434.61 μg mL−1 Ethyl acetate, IC50 = 444.44 μg mL−1 Inhibiting BuChE Benzene extract, IC50 = 163.01 μg mL−1 Chloroform extract, IC50 = 375 μg mL−1, in vitro | [113] | |
| Petroleum ether and successively extracted with benzene | Phytol | In vitro and in vivo antioxidant activities (25–125 μg mL−1) with an IC50 value of 95.27 μg mL−1 and cholinesterase inhibitory potential (5–25 μg mL−1) with IC50 values of 2.704 and 5.798 μg mL−1 for AChE and BuChE, respectively, in vitro | [114] | ||
| Gelidium amansii | Ethanol extract | Extract | Neurogenesis (synaptogenesis promotion), in vitro and in vivo | [90,115] | |
| G. foliaceum | 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.16 mg mL−1, in vitro | [110] | |
| Gloiopeltis furcata | 2-(3-Hydroxy-5-oxotetrahydrofuran-3-yl) acetic acid, glutaric acid, succinic acid, nicotinic acid, (E)-4-hydroxyhex-2-enoic acid, cholesterol, 7-hydroxycholesterol, uridine, glycerol, phlorotannin, fatty acids | Inhibiting AChE 1.4–12.50 μg mL−1 Inhibiting BuChE 6.56–75.25 μg mL−1, in vitro | [116] | ||
| Hydropuntia edulis (as Gracilaria edulis) | Methanolic extract | Extract | Inhibiting AChE IC50 = 3 mg mL−1, in vitro | [83] | |
| Methanolic extract | Extract | Inhibiting AChE IC50 = 3 mg mL−1, in vitro | [72] | ||
| Gracilaria gracilis | Methanolic extract | Extract | Inhibiting AChE IC50 = 1.5 mg mL−1, in vitro | [83] | |
| Gracilariopsis chorda | Ethanolic extract | Extract | Neuronal cell viability and cell cytotoxicity testing revealed that the ethanol extract afforded the most neuroprotection at a concentration of 15 µmL−1, at which G. chorda extract significantly increased cell viability to 119.0–3.2%, and decreased cell death to 80.5–10.3%, in vivo | [46] | |
| G. chorda | Ethanolic extract | Extract | Extract concentration-dependently increased neurite outgrowth, with an optimal concentration of 30 mu g mL−1, in vivo | [117] | |
| Hypnea valentine | Methanolic extract | Extract | Inhibiting AChE IC50 = 2.6 mg mL−1, in vitro | [83] | |
| Methanolic extract | Extracts | Inhibiting AChE IC50 = 2.6 mg mL−1, in vitro | [118] | ||
| Kappaphycus alvarezii | Alcaline extraction | Kappa-carrageenan | Anti-oxidative activity: Inhibit superoxide radicals, hydroxyl radicals and lipid peroxidation IC50 = 0.112, 0.335 and 0.323 mg mL−1, respectively, in vitro | [75] | |
| Ethanolic extract | Extracts | Promotes neurite outgrowth in hippocampal neurons, in vivo | [119] | ||
| Ochtodes secundiramea | Dichloromethane/methanol extract | Halogenated monoterpenes | Extract showed 48% AChE inhibition at 400 µg mL−1, in vitro | [120] | |
| Porphyra/Pyropia sp. (Korean purple laver) | In vitro digestion | Phycoerythrobilin | Antioxidant activity IC50 = 0.048 mmol g−1, in vitro | [121] | |
| Neopyropia yezoensis (as Porphyra yezoensis) | Ethanolic extract | Extract | Increased neurite outgrowth at an optimal concentration of 15 µg mL−1, in vivo | [122] | |
| Rhodomela confervoides | Ethyl acetate extract | Bromophenols | Antioxidant activity IC50 = 5.22–23.60 µM, in vitro | [123] | |
| Rhodomelopsis africana | 50% aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.12 mg mL−1, in vitro | [110] | |
| Chlorophyta (green seaweeds) | |||||
| Caulerpa racemosa | Methanolic extract | Extract | Inhibiting AChE IC50 = 5.5 mg mL−1, in vitro | [56] | |
| Extracted with MeOH and partitioned between H2O and hexane, chloroform, ethyl acetate and n-butanol. | Alkaloid: Caulerpin | Inhibition of nociception 100 μM kg−1 in Swiss albino mice, in vivo | [124] | ||
| Ethanol extract | Bisindole alkaloid (racemosin A) | Increase 5.5% of cell viability in SH-SY5Y cells (neuroblast from neural tissue) IC50 = 10 µM, in vivo | [125] | ||
| Ethanol extract | Bisindole alkaloid (racemosin B) | Increase 14.6% of cell viability in SH-SY5Y cells (neuroblast from neural tissue) IC50 = 10 µM, in vivo | [125] | ||
| Hexane, dichloromethane and methanol extracts | Extracts | Inhibiting AChE IC50 = 0.086, 0.089 and 0.095 mg mL−1, respectively Inhibiting BuChE IC50 = 0.156, >0.2 and 0.118 mg mL−1, respectively, in vitro | [82] | ||
| Ethanol extract | Terpenoid (α-tocospirone) | 13.85% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [126] | ||
| Ethanol extract | Sterol (23E)-3β-hydroxystigmasta-5,23-dien28-one | 11.31% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [126] | ||
| Ethanol extract | Sterol (22E)-3β-hydroxycholesta-5,22-dien24-one | 15.98% increases in cell viability in SH-SY5Y cells IC50 = 10 μM, in vivo | [126] | ||
| Cladophora vagabunda (as Cladophora fascicularis) | Methanolic extract | Extract | Inhibiting AChE IC50 = 2 mg mL−1, in vitro | [83] | |
| Codium capitatum | Methanolic extract | Extract | Inhibiting AChE IC50 = 7.8 mg mL−1, in vitro | [56] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.11 mg mL−1, in vitro | [110] | ||
| C. duthieae | 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.14 mg mL−1, in vitro | [110] | |
| C. fragile | 80% aqueous methanol extract | Sterol: Clerosterol | Exhibit reducing activity to COX-2, iNOS and TNF-α IC50 = 3 μg mL−1, in vitro and in vivo | [127] | |
| Halimeda incrassata | Water extracts | Extracts | Neuroprotective and antioxidant properties, in vitro and in vivo | [21] | |
| H. cuneata | Methanolic extract | Extracts | Inhibiting AChE IC50 = 5.7 mg mL−1, in vitro | [56] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.07 mg mL-1, in vitro | [110] | ||
| Ulva australis (as Ulva pertusa) | Water at 125 °C for 4 h; polysaccharides were precipitated by the addition of 4000 mL of 95% (v/v) ethanol | Sulfated polysaccharide (ulvan) | Scavenging activity for superoxide radicals, in vitro | [128] | |
| U. fasciata | Methanolic extract | Extracts | Inhibiting AChE IC50 = 4.8 mg mL−1, in vitro | [56] | |
| 50% Aqueous methanol extract | Phenolic and Flavonoid compouds | Inhibiting AChE IC50 = 0.13 mg mL−1, in vitro | [110] | ||
| U. prolifera (as Enteromorpha prolifera) | 95% ethanol extract | Pheophorbide A | Antioxidant activity IC50 = 71.9 µM, in vitro | [129] | |
| U. reticulata | Methanolic extract | Extract | Inhibiting AChE IC50 = 10 mg mL−1, in vitro | [83] | |
| Methanolic extract | Extract | Inhibiting AChE IC50 = 10 mg mL−1, in vitro | [118] | ||
4. Multiple Sclerosis, Other Chronic Diseases, and the Seaweed Diet
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M. Alzheimer disease (2010). In Textbook of Geriatric Medicine and Gerontology–Brocklehurst’s, 8th ed.; Fillit, H.M., Rockwood, K., Woodhouse, K., Eds.; Saunders/Elservier: Philadelphia, PA, USA, 2017; 1168p. [Google Scholar]
- FCG—Fundação Calouste Gulbenkian. O Cérebro e as Doenças Neurodegenerativas; Dossier Ciência em Cena, Gulbenkian Descobrir, Maratona da Saúde: Lisboa, Portugal, 2015; 30p, Available online: https://content.gulbenkian.pt/wp-content/uploads/sites/16/2018/04/24100926/Dossie_2015_Neurodegenerativas.pdf (accessed on 12 January 2021).
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.-A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Neurological activities of seaweeds and their extracts (Chapter 11), In Therapeutic and Nutritional Uses of Algae, 1st ed.; Pereira, L., Ed.; Science Publishers’ (SP), An Imprint of CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 485–502. [Google Scholar] [CrossRef]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; d’Angelo, M.; Cimini, A. Benefits under the Sea: The role of marine compounds in neurodegenerative disorders. Mar. Drugs 2021, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Déléris, P.; Nazih, H.; Bard, J.M. Seaweeds in human health (Chapter 10). In Seaweed in Health and Disease Prevention, 1st ed.; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK, 2016; pp. 319–367. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Lessing, D.; Bonini, N.M. Maintaining the brain: Insight into human neurodegeneration from Drosophila mutants. Nat. Rev. Genet. 2009, 10, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood-Kaczmar, A.; Gandhi, S.; Wood, N. Understanding the molecular causes of Parkinson’s disease. Trends Mol. Med. 2006, 12, 521–528. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef]
- García-Ayllón, M.-S.; Cauli, O.; Silveyra, M.-X.; Rodrigo, R.; Candela, A.; Compañ, A.; Jover, R.; Pérez-Mateo, M.; Martínez, S.; Felipo, V. Brain cholinergic impairment in liver failure. Brain 2008, 131, 2946–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.; Zeisel, J.M.; Bennett, K. World Alzheimer Report 2020: Design Dignity Dementia: Dementia-Related Design and the Built Environment, 1st ed.; Alzheimer’s Disease International: London, UK, 2020; Volume 1, 248p, Available online: https://www.alzint.org/u/WorldAlzheimerReport2020Vol1.pdf (accessed on 12 January 2021).
- Cavdar, H.; Senturk, M.; Guney, M.; Durdagi, S.; Kayik, G.; Supuran, C.T.; Ekinci, D. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzym. Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, J.L. Mechanisms of cell injury by activated oxygen species. Environ. Health Perspect. 1994, 102, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimpler, M.M.; Rauen, U.; Schmidt, T.; Möröy, T.; De Groot, H. Protection against hydrogen peroxide cytotoxicity in Rat-1 fibroblasts provided by the oncoprotein BCL-2: Maintenance of calcium homoeostasis is secondary to the effect of BCL-2 on cellular glutathione. Biochem. J. 1999, 340, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Pôrto, W.G. Radicais livres e neurodegeneração. Entendimento fisiológico: Base para nova terapia? Rev. Neurociências 2001, 9, 70–76. [Google Scholar] [CrossRef]
- Fallarero, A.; Loikkanen, J.J.; Männistö, P.T.; Castañeda, O.; Vidal, A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G. Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1–7 mouse hypothalamic immortalized cells. Phytomedicine 2003, 10, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Usigli, H.A. Parkinson Disease—Professional Version. MSD Manual; Global Medical Knowledge: Kenilworth, NJ, USA, 2019; Available online: https://www.msdmanuals.com/en-pt/professional/neurologic-disorders/movement-and-cerebellar-disorders/parkinson-disease (accessed on 12 January 2021).
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Pereira, L. Nutritional composition of the main edible algae (Chapter 2). In Therapeutic and Nutritional Uses of Algae, 1st ed.; Pereira, L., Ed.; Science Publishers’ (SP), An Imprint of CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 65–127. ISBN 9781498755382. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, R.; Fonseca, R.; Pereira, L. Illustrated Guide to the Macroalgae of Buarcos Bay, Figueira da Foz, Portugal; MARE UC, DCV, FCT: Coimbra, Portugal, 2020; 128p. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-derived oligosaccharide inhibits neuroinflammation and promotes microglial phagocytosis of β-amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Langston, J.; Forno, L.; Tetrud, J.; Reeves, S.; Kaplan, J.; Karluk, D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999, 46, 598–605. [Google Scholar] [CrossRef]
- Gerlach, M.; Maetzler, W.; Broich, K.; Hampel, H.; Rems, L.; Reum, T.; Riederer, P.; Stöffler, A.; Streffer, J.; Berg, D. Biomarker candidates of neurodegeneration in Parkinson’s disease for the evaluation of disease-modifying therapeutics. J. Neural Transm. 2012, 119, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Wischik, C.; Crowther, R.; Walker, J.; Klug, A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsel, D.N.; Hyman, A.; Cobb, N.H.; Kirschner, M. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 1992, 3, 1141–1154. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, N.; Funakoshi, T.; Sato-Harada, R.; Kanai, Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 1996, 132, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Santa-Maria, I.; Perez, M.; Hernandez, F.; Moreno, F. Tau phosphorylation, aggregation, and cell toxicity. J. Biomed. Biotechnol 2006, 2006, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selkoe, D.J. Alzheimer’s disease: Genotypes, phenotypes, and treatments. Science 1997, 275, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Seereeram, A.; Noble, W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Expert Rev. Neurother. 2009, 9, 1647–1666. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, X.; Conte, M.M.; Khalil, Z.; Capon, R.J. Spiralisones A–D: Acylphloroglucinol hemiketals from an Australian marine brown alga, Zonaria spiralis. Org. Biomol. Chem. 2012, 10, 9671–9676. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Cowan, C.M.; Thai, J.; Krajewski, S.; JReed, J.C.; Nicholson, D.W.; Kaufmann, S.H.; Roskams, A.J. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J. Neurosci. 2001, 21, 7099–7109. [Google Scholar] [CrossRef]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef]
- Dörschmann, P.; Klettner, A. Fucoidans as potential therapeutics for age-related macular degeneration—current evidence from in vitro research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Najam, R.; Ahmed, S.P.; Azhar, I. Pharmacological activities of Hypnea musciformis. Afr. J. Biomed. Res. 2010, 13, 69–74. [Google Scholar]
- Mohibbullah, M.; Hannan, M.A.; Choi, J.Y.; Bhuiyan, M.M.H.; Hong, Y.K.; Choi, J.S.; Choi, I.S.; Moon, I.S. The edible marine alga Gracilariopsis chorda alleviates hypoxia/reoxygenation-induced oxidative stress in cultured hippocampal neurons. J. Med. Food 2015, 18, 960–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Banskota, A.H.; Critchley, A.T.; Hafting, J.; Prithiviraj, B. Neuroprotective effects of the cultivated Chondrus crispus in a C. elegans model of Parkinson’s disease. Mar. Drugs 2015, 13, 2250–2266. [Google Scholar] [CrossRef] [Green Version]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.; Moon, I.S.; Hong, Y.K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson’s disease model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral Aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef] [Green Version]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.H.; Yoo, K.Y.; Park, J.H.; Yan, B.C.; Ahn, J.H.; Lee, J.C.; Kwon, H.M.; Kim, J.D.; Kim, Y.M.; You, S.G.; et al. Comparison of neuroprotective effects of extract and fractions from Agarum clathratum against experimentally induced transient cerebral ischemic damage. Pharm. Biol. 2014, 52, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giffin, J.C.; Richards, R.C.; Craft, C.; Jahan, N.; Leggiadro, C.; Chopin, T.; Szemerda, M.; MacKinnon, S.L.; Ewart, K.V. An extract of the marine alga Alaria esculenta modulates α-synuclein folding and amyloid formation. Neurosci. Lett. 2017, 644, 87–93. [Google Scholar] [CrossRef]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a Human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Stirk, W.A.; Reinecke, D.L.; Staden, J.V. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Jung, H.A.; Oh, S.H.; Choi, J.S. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible seaweed, Eisenia bicyclis, protects retinal ganglion cells death caused by oxidative stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharm. Res. 2015, 38, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Cho, S.; Han, D.; Kim, S.B.; Yoon, M.; Yang, H.; Jin, Y.H.; Jo, J.; Yong, H.; Lee, S.H.; Jeon, Y.J.; et al. Depressive effects on the central nervous system and underlying mechanism of the enzymatic extract and its phlorotannin-rich fraction from Ecklonia cava edible brown seaweed. Biosci. Biotechnol. Biochem. 2012, 76, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Kang, K.A.; Lee, N.H.; Hyun, J.W.; Kim, H.-S. Phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells. J. Pharmacol. Sci. 2012, 119, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-I.; Jung, S.-H.; Lee, K.-T.; Choi, J.-H. 8,8’-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-kB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Lim, D.W.; Cho, S.; Han, D.; Kim, Y.T. The edible brown seaweed Ecklonia cava reduces hypersensitivity in postoperative and neuropathic pain models in rats. Molecules 2014, 19, 7669–7678. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.W.; Lee, H.S.; Shin, H.C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for Alzheimer’s disease therapy from Ecklonia cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Geng, M.; Li, J.; Xin, X.; Wang, J.; Tang, M.; Zhang, J.; Zhang, X.; Ding, J. Acidic oligosaccharide sugar chain, a marine-derived acidic oligosaccharide, inhibits the cytotoxicity and aggregation of amyloid beta protein. J. Pharm. Sci. 2004, 95, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Staden, J.V. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Lee, S.-H.; Kim, S.-K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Liu, D.; Woodward, S.; Barger, S.W.; Mrak, R.E.; Griffin, W.S. Microglial activation by uptake of fDNA via a scavenger receptor. J. Neuroimmunol. 2004, 147, 50–55. [Google Scholar] [CrossRef] [Green Version]
- De Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; da Silva, F.R.F.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-R.; Do, H.; Lee, S.-R.; Sohn, E.-R.; Pyo, S.; Son, E. Effects of fucoidan on neuronal cell proliferation: Association with NO production through the iNOS pathway. J. Food Nutr. Sci. 2007, 12, 74–78. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 2009, 21, 671–679. [Google Scholar] [CrossRef]
- Huang, W.C.; Yen, F.C.; Shiao, Y.J.; Shie, F.S.; Chan, J.L.; Yang, C.N.; Sung, Y.L.; Huang, F.L.; Tsay, H.J. Enlargement of Abeta aggregates through chemokine-dependent microglial clustering. Neurosci. Res. 2009, 63, 280–287. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 1251–1257. [Google Scholar] [CrossRef]
- Natarajan, S.; Shanmugiahthevar, K.P.; Kasi, P.D. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: Seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar). Nat. Prod. Res. 2009, 23, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Malar, D.S.; Sathya, S.; Devi, K.P. Antiaggregation potential of Padina gymnospora against the toxic Alzheimer’s beta-amyloid peptide 25–35 and cholinesterase inhibitory property of its bioactive compounds. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangeetha, R.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.R.; Harle, U.N.; Ittadwar, A.M. Insignificant anticonvulsant activity of Padina tetrastromatica (brown macroalgae) in mice. J. Pharm. Negat. Results 2016, 7, 33–36. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide activated primary microglia. Clin. Exp. Pharmacol. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Hannan, M.A.; Mohibbullah, M.; Hong, Y.-K.; Nam, J.H.; Moon, I.S. Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Mohibbullah, M.; Hwang, S.Y.; Lee, K.; Kim, Y.C.; Hong, Y.K.; Moon, I.S. Differential neuritogenic activities of two edible brown macroalgae, Undaria pinnatifida and Saccharina japonica. Am. J. Chin. Med. 2014, 42, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; He, Y.; Zheng, Y.; Zhang, W.J.; Wang, Q.; Jia, Y.J.; Song, H.L.; An, H.T.; Zhang, H.B.; Qian, Y.J. Therapeutic effects of fucoidan in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: Role of NADPH oxidase-1. CNS Neurosci. Ther. 2014, 20, 1036–1044. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Tsang, C.; Sagara, A.; Kamei, Y. Structure-activity relationship of a neurite outgrowth-promoting substance purified from the brown alga, Sargassum macrocarpum, and its analogues on PC12D cells. J. Appl. Phycol. 2001, 13, 349–357. [Google Scholar] [CrossRef]
- Kamei, Y.; Tsang, C.K. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int. J. Dev. Neurosci. 2003, 21, 255–262. [Google Scholar] [CrossRef]
- Tsang, C.; Ina, A.; Goto, T.; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.S.; Chowdhury, M.T.H.; Choi, J.-S.; Choi, I.S.; Moon, I.S. A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 535–544. [Google Scholar] [CrossRef]
- Yan, X.J.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zheng, L.; Qi, L.; Wang, S.; Guan, L.; Xia, Y.; Cai, J. Structural features and potent antidepressant effects of total sterols and β-sitosterol extracted from Sargassum horneri. Mar. Drugs 2016, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Park, S.H.; Kim, E.S.; Choi, B.W.; Ryu, S.Y.; Lee, B.H. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch. Pharm. Res. 2003, 26, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Rhu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum, lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.-B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef]
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol. 2013, 51, 1401–1410. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, R.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Mohibbullah, M.; Hannan, M.A.; Hong, Y.-K.; Choi, J.-S.; Choi, I.S.; Moon, S. Undaria pinnatifida promotes spinogenesis and synaptogenesis and potentiates functional presynaptic plasticity in hippocampal neurons. Am. J. Chin. Med. 2015, 43, 529–542. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Moon, I.S.; Kim, J.M.; Kong, I.S. Anti-Alzheimers and antiinflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Subrtova, M.; Dolezal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Fallarero, A.; Peltoketo, A.; Loikkanen, J.; Tammela, P.; Vidal, A.; Vuorela, P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2006, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.M.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Assessment of anticholinesterase activity of Gelidiella acerosa: Implications for its therapeutic potential against Alzheimer’s disease. Evid. Based Complement. Alternat. Med. 2012, 497242, 8. [Google Scholar] [CrossRef] [Green Version]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Kang, J.-K.; Hong, Y.-K.; Lee, H.S.; Choi, J.-S.; Choi, I.S.; Moon, I.S. The Marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother. Res. 2013, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohibbullah, M.; Hannan, M.A.; Park, I.S.; Moon, I.S.; Hong, Y.K. The edible red seaweed Gracilariopsis chorda promotes axodendritic architectural complexity in hippocampal neurons. J. Med. Food 2016, 19, 638–644. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar marine biosphere reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J. Appl. Phycol. 2016, 28, 2515–2522. [Google Scholar] [CrossRef]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Revista Brasileira de Farmacognosia 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food Sci. Technol. 2010, 16, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Mohibbullah, M.; Bhuiyan, M.M.H.; Hannan, M.A.; Getachew, P.; Hong, Y.K.; Choi, J.S.; Choi, I.S. The edible red alga Porphyra yezoensis promotes neuronal survival and cytoarchitecture in primary hippocampal neurons. Cell. Mol. Neurobiol. 2016, 36, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.-M.; Gloer, J.B.; Wang, B.-G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- De Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.C.; de Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; de Miranda, V.P.; de Araújo-Júnior, J.X.; Chaves, M.C.O.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-Q.; Mao, S.-C.; Zhang, H.-Y.; Yu, X.-Q.; Feng, M.-T.; Wang, B.; Feng, L.-H.; Guo, Y.-W. Racemosins A and B, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. [Google Scholar] [CrossRef]
- Yang, P.; Liu, D.-Q.; Liang, T.-J.; Li, J.; Zhang, H.-Y.; Liu, A.-H.; Guo, Y.-W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.; Park, C.-I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X. Antioxidant activity of different sulphate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2015, 37, 195–199. [Google Scholar] [CrossRef]
- Cho, M.; Lee, K.-S.; Kang, I.-J.; Won, M.-H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef]
- Grisante, A.I.; Stanich, P. Esclerose múltipla: Aspectos nutricionais e o papel dos nutrientes específicos. ConScientiaeSaúde 2006, 5, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Valado, A.; Leitao, M.J.; Martinho, A.; Pascoal, R.; Cerqueira, J.; Correia, I.; Batista, S.; Sousa, L.; Baldeiras, I. Multiple Sclerosis: Association of gelatinase B/matrix metalloproteinase-9 with risk and clinical course the disease. Mult. Scler. Relat. Disord. 2017, 11, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of two popular eating plans within the Multiple Sclerosis community: Low saturated fat and modified paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Swank, R.L. Treatment of Multiple Sclerosis with low-fat diet. AMA Arch. Neurol. Psychiatry 1953, 69, 91–103. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in Multiple Sclerosis treated with interferon beta-1b. Oxid. Med. Cell Longev. 2013, 709493. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses, 1st ed.; Pomin, V.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 15–47. ISBN -13. [Google Scholar]
- Pereira, L. Edible Seaweeds of the World, 1st ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; 463p, ISBN 9781498730471. [Google Scholar]
- Pereira, L. Biodiversity and description of the main algae with bioactive properties. In Therapeutic and Nutritional Uses of Algae, 1st ed.; Chapter 1; Pereira, L., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 1–64. ISBN 9781498755382. [Google Scholar] [CrossRef]
- Mišurcová, L.; Ambrožová, J.; Samek, D. Seaweed lipids as nutraceuticals. In Advances in Food and Nutrition Research, 1st ed.; Chapter 27; Se-Kwon, K., Ed.; Academic Press: Burlington, NJ, USA, 2011; Volume 64, pp. 339–355. [Google Scholar] [CrossRef]
- Pereira, L.; Correia, F. Algas Marinhas da Costa Portuguesa—Ecologia, Biodiversidade e Utilizações, 1st ed.; Nota de Rodapé Edições: Paris, France, 2015; pp. 196–201. ISBN 978-989-20-5754-5. [Google Scholar]
- Aragão, M.J. Plantas e Algas Medicinais, 1st ed.; Livros Horizonte: Lisboa, Portugal, 2009; pp. 153–174. ISBN 9789722416580. [Google Scholar]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty acid patterns of the kelps Saccharina latissima, Saccorhiza polyschides and Laminaria ochroleuca: Influence of changing environmental conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural deep eutectic solvents as alternatives for extracting phlorotannins from brown algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Valado, A.; Pereira, M.; Caseiro, A.; Figueiredo, J.P.; Loureiro, H.; Almeida, C.; Cotas, J.; Pereira, L. Effect of carrageenans on vegetable jelly in humans with hypercholesterolemia. Mar. Drugs 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, N.; Rosa, G.P.; Ferraz, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Fatty acid composition, TLC screening, ATR-FTIR analysis, anti-cholinesterase activity, and in vitro cytotoxicity to A549 tumor cell line of extracts of 3 macroalgae collected in Madeira. J. Appl. Phycol. 2020, 32, 759–771. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals, 1st ed; EFSA: Parma, Italy, 2006; 480p, Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 12 January 2021).
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of Multiple Sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo-Moyano, B.; Benito-Leon, J.; Mitchell, A.J.; Hernandez-Gallego, J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in Multiple Sclerosis. Neuroepidemiology 2013, 40, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, H.D.; Yamamoto, Y.; Zolfaghari, R.; Rosales, F.J.; Dietz, J.; Shimada, T.; Li, N.; Ross, A.C. Regulation of hepatic vitamin A storage in a rat model of controlled vitamin A status during aging. J. Nutr. 2000, 130, 1280–1286. [Google Scholar] [CrossRef]
- Roenigk, H.H. Liver toxicity of retinoid therapy. J. Am. Acad. Dermatol. 1989, 19, 199–208. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A deficiency and the lung. Nutrients 2008, 10, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LTS. Health and Food Technology. Home Economics, Learning and Teaching, Resource Management; Learning & Teaching Scotland: Scotland, UK, 2005; 170p, Available online: http://www.sqa.org.uk/files/nq/HE_Health_and_Food_Technology_Higher.pdf (accessed on 12 January 2021)ISBN -13978-1843990888.
- Škrovánková, S. Seaweed vitamins as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 357–369. [Google Scholar] [CrossRef] [PubMed]
- OHT. Seaweed: A Rich Source of Vitamins and Bioactive Compounds; Ocean Harvest Technology: Galway, Ireland, 2013; Available online: https://oceanharvesttechnology.wordpress.com/2013/02/25/seaweed-a-rich-source-of-vitamins-and-bioactive-compounds/ (accessed on 12 January 2021).
- García, I.; Castroviejo, R.; Neira, C. Las Algas en Galicia: Alimentación y Otros Usos, 1st ed.; Consellería de Pesca, Marisqueo e Acuicultura—Xunta de Galícia: La Coruña, Spain, 1993; 231p, ISBN 84-453-0718-5. [Google Scholar]
- Morrissey, J.; Kraan, S.; Guiry, M.D. A Guide to Commercially Important Seaweeds on the Irish Coast, 1st ed; Bord Iascaigh Mhara/Irish Fisheries Board: Dublin, Ireland, 2001; pp. 5–40. [Google Scholar]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lloréns, J.L.P.; Carero, I.H.; Oñate, J.J.V.; Murillo, F.G.B.; León, A. Las Algas se Comen: Un Periplo por La Biologia, La Historia, Las Curiosidades y La Gastronomia, 1st ed.; Editorial UCA: Universidade de Cádiz, Cádiz, Spain, 2016; 336p, ISBN 978-84-9828-567-3. [Google Scholar]
- Sáa, C.F. Atlantic Sea Vegetables—Nutrition and Health: Properties, Recipes, Description, 1st ed.; Algamar: Redondela-Pontevedra, Spain, 2002; 300p. [Google Scholar]
- Quirós, A.R.B.; Ron, C.; López-Hernández, J.; Lage-Yusty, M.A. Determination of folates in seaweeds by high-performance liquid chromatography. J. Liq. Chromatogr. A 2004, 1032, 135–139. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, S.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweed. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Thennarasan, S. Murugesan Biochecmial composition of marine brown alga Lobophora variegata from Mandapam in the south east coast of Tamil Nadu. Int. J. Phytopharm. 2015, 5, 25–29. [Google Scholar] [CrossRef]
- Kolb, N.; Vallorani, L.; Milanovi, N.; Stocchi, V. Evaluation of marine algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata, L. japonica) as food supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Tsuchiya, Y. Physiological studies on the vitamin C content of marine algae. Tohoku J. Agric. Res. 1950, 1, 97–102. [Google Scholar]
- Noda, H. Health benefits and nutritional properties of Nori. J. Appl. Phycol. 1993, 5, 255–258. [Google Scholar] [CrossRef]
- Watanabe, F.; Takenaka, S.; Katsura, H.; Miyamoto, E.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Characterization of a vitamin B12 compound in the edible purple laver, Porphyra yezoensis. Biosci. Biotechnol. Biochem. 2000, 64, 2712–2715. [Google Scholar] [CrossRef]

| Species/Vitamins | A | B1 | B2 | B3 | B5 | B6 | B8 | B12 | C | D | E | Folic Acid | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyta | |||||||||||||
| Ulva lactuca | 0.017 | <0.024 | 0.533 | 98 * | - | - | - | 6 * | <0.242 | - | - | - | [155,156] |
| U. rigida | 9581 | 0.47 | 0.199 | <0.5 | 1.70 | <0.1 | 0.012 | 6 | 9.42 | - | 19.7 | 0.108 | [157] |
| Phaeophyceae | |||||||||||||
| Alaria esculenta | - | 0.6 | 0.3–2.7 | 11 | - | 6 | - | 5 | 100–500 * | - | - | - | [156,158] |
| Ascophyllum nodosum | - | 1.5 | 0.6 | 1.9 | - | <0.1 | - | <0.1 | 52 | - | - | 15 | [158] |
| Fucus vesiculosus | 0.307 | 0.02 | 0.035 | - | - | - | - | - | 14.124 | - | - | - | [155,159] |
| Himanthalia elongata | 0.079–0.3 | 0.020–0.3 | 0.020–4.5 | - | - | - | - | - | 28.56–66 | - | 5.8 | 0. 176–0.258 | [155,158,159,160] |
| Laminaria digitata | - | 0.3–1.250 | 0.138–0.8 | 2.6–6.12 | - | 6.41 | 6.41 | 0.0005 | 16–35.5 | - | 3.43–4.7 | - | [157,161] |
| L. ochroleuca | 0.041 | 0.058 | 0.212 | - | - | - | - | - | 0.353 | - | - | 0.479 | [155,160] |
| Lobophora variegata | 10.340 | 0.3771 | 0.3491 | 4.0162 | 1.36 | 0.3040 | - | 0.119 | 23.430 | 0.6442 | 2.13 | 1.983 | [162] |
| Saccharina japonica | 0.481 | 0.2 | 0.85 | 1.58 | 0.09 | - | - | - | - | - | [163] | ||
| S. latissima | 0.04–0.4 | 0.05–0.2 | 0.21–0.4 | 1.7 | - | 0.2 | - | 0.0003-0.2 | 0.35 | 18 | 1.6 | [158,159] | |
| Undaria pinnatifida | 0.04–0.22 | 0.17–0.30 | 0.23–1.4 | 2.56 | - | 0.18 | - | 0.0036 | 5.29 | 1.4–2.5 | 0.479 | [155,159,160,163] | |
| Rhodophyta | |||||||||||||
| Chondrus crispus | <0.1 | <0.1 | 2.5 | 3.2 | - | 0.4 | - | 0.6–4 * | 10–13 * | 16 | - | 4.7 | [156,158,164] |
| Gracilaria spp. | - | - | - | - | - | - | - | - | 16–149 ** | - | - | - | [165] |
| Palmaria palmata | 1.59–3.7 | 0.073–1.56 | 0.51–1.91 | 1.89–2.6 | - | 6.8–8.99 | - | 0.009–3.5 | 6.34–34.5 | - | 2.2–13.9 | 0.267–3.5 | [156,158,159,160] |
| Porphyra umbilicalis | 3.65 | 0.144 | 0.36 | - | - | - | - | 0.029 | 4.214 | 60 | - | 0.363 | [155,159,160] |
| Neopyropia yezoensis | 16000 *** | 0.129 | 0.382 | 11.0 | - | - | - | 0.052 | - | - | - | - | [166,167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. https://doi.org/10.3390/md19030128
Pereira L, Valado A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Marine Drugs. 2021; 19(3):128. https://doi.org/10.3390/md19030128
Chicago/Turabian StylePereira, Leonel, and Ana Valado. 2021. "The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases" Marine Drugs 19, no. 3: 128. https://doi.org/10.3390/md19030128







