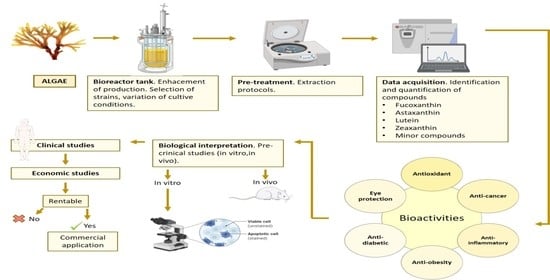
Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids
Abstract
1. Introduction
2. Main Xanthophylls Present in Algae
2.1. Fucoxanthin
2.2. Astaxanthin
2.3. Lutein
2.4. Zeaxanthin
2.5. Minor Carotenoids
2.5.1. β-Cryptoxanthin
2.5.2. Siphonaxanthin
2.5.3. Saproxanthin
2.5.4. Myxol
2.5.5. Diatoxanthin
2.5.6. Diadinoxanthin
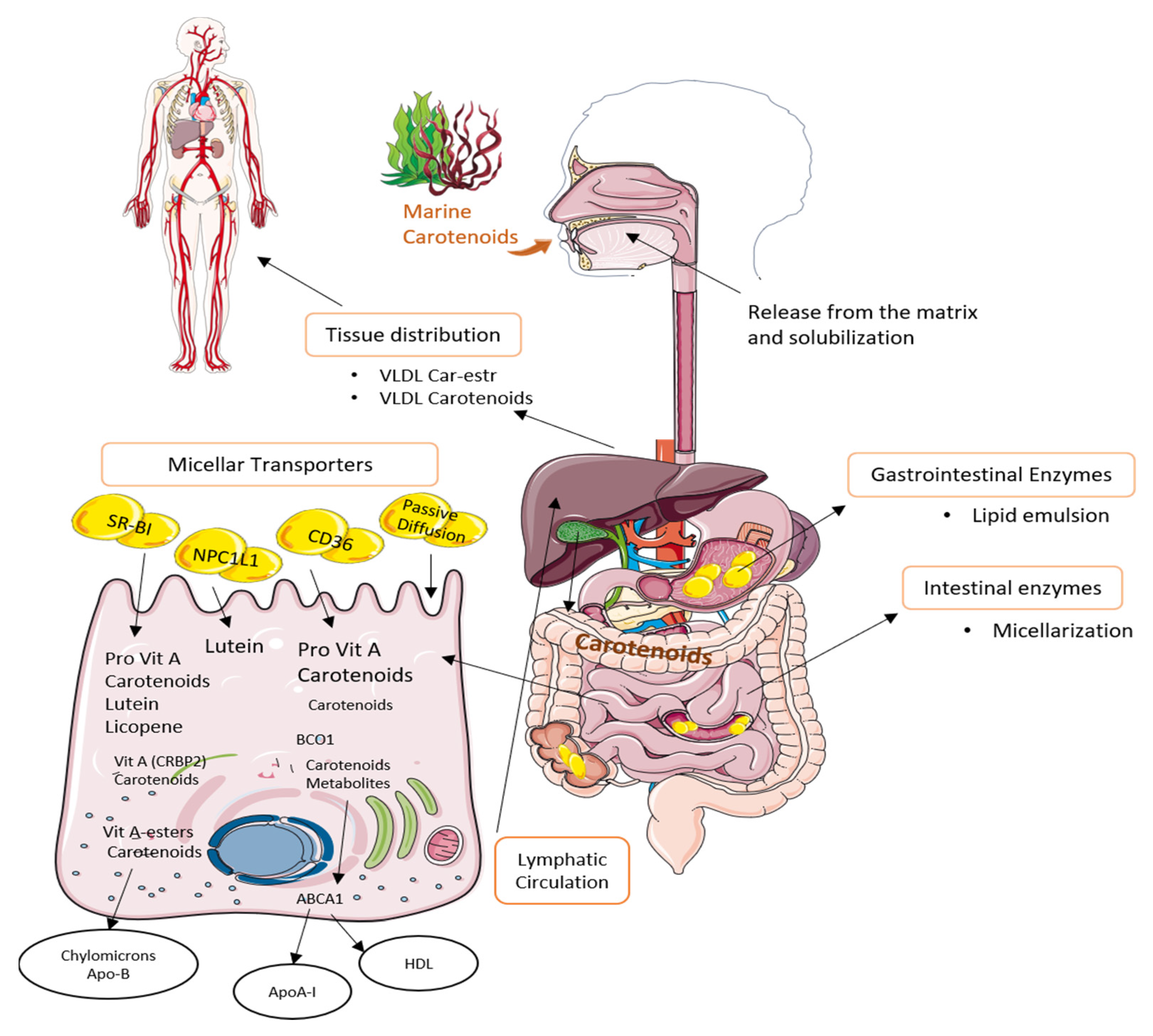
3. Mechanism of Action of Xanthophylls
3.1. Metabolism
3.2. Bioavailability and Bioaccessibility
3.2.1. Fucoxanthin
3.2.2. Astaxanthin
3.2.3. β-Cryptoxanthin
3.2.4. Zeaxanthin
3.3. Experimental Studies
3.3.1. Observation In Vitro
3.3.2. Observation In Vivo
3.3.3. Observational and Epidemiological Studies
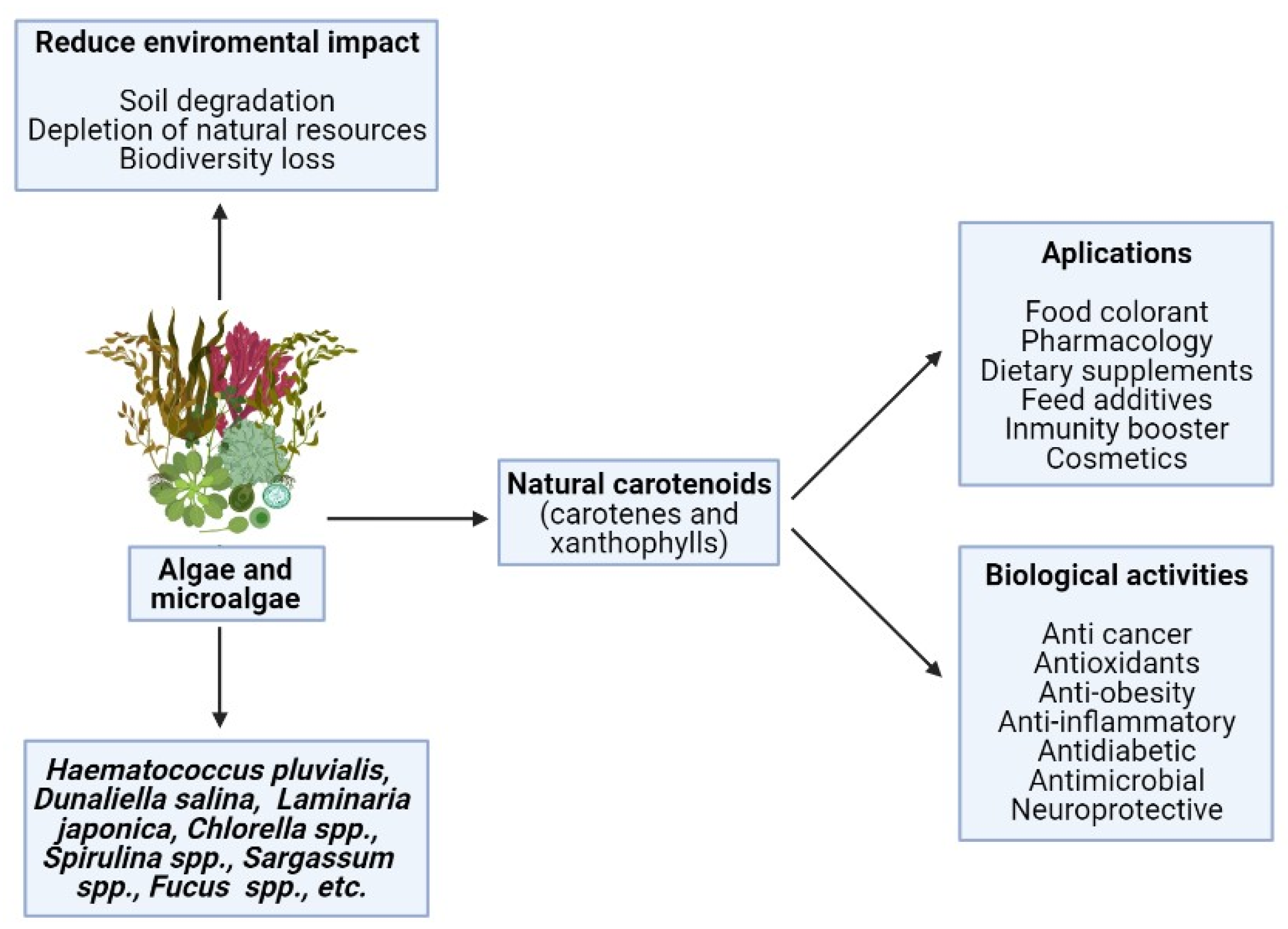
4. Algae as Source of Carotenoids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for Bioactive Compounds from Algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and Its Deacetylated Product, Fucoxanthinol, Induce Apoptosis of Primary Effusion Lymphomas. Cancer Lett. 2011. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of Fucoxanthin from Raw Macroalgae Excluding Drying and Cell Wall Disruption by Liquefied Dimethyl Ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria Bifurcata: A Key Macro-Alga as a Source of Bioactive Compounds and Functional Ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- AGRIOS, G.N. Plant diseases caused by parasitic higher plants, invasive climbing plants, and parasitic green algae. In Plant Pathology; Springer: San Diego, CA, USA, 2005; pp. 705–722. [Google Scholar]
- Ibañez, E.; Cifuentes, A. Benefits of Using Algae as Natural Sources of Functional Ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological Activities of Two Macroalgae from Adriatic Coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 1–34. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological Importance of Marine Algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, Old Sustainable Food and Fashion Nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Andersen, R.A. Diversity of Eukaryotic Algae. Biodivers. Conserv. 1992, 1, 267–292. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative Assessment of Antioxidant Properties of Natural Colorants and Phytochemicals: Carotenoids, Flavonoids, Phenols and Indigoids. The Role of β-Carotene in Antioxidant Functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef]
- Bolhassani, A. Cancer Chemoprevention by Natural Carotenoids as an Efficient Strategy. Anticancer. Agents Med. Chem. 2015, 15, 1026–1031. [Google Scholar] [CrossRef]
- Garewal, H. Antioxidants in Oral Cancer Prevention. Am. J. Clin. Nutr. 1995, 62, 1410S–1416S. [Google Scholar] [CrossRef]
- Kim, J.; Leite, J.; DeOgburn, R.; Smyth, J.; Clark, R.; Fernandez, M. A Lutein-Enriched Diet Prevents Cholesterol Accumulation and Decreases Oxidized LDL and Inflammatory Cytokines in the Aorta of Guinea Pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin Inhibits the Inflammatory Response by Suppressing the Activation of NF-ΚB and MAPKs in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Products Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sirisha, V.L. Algal Carotenoids: Understanding Their Structure, Distribution and Potential Applications in Human Health. Encycl. Mar. Biotechnol. 2020, 33–64. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, G.C.; Zhang, M.; Tseng, C.K. Isolation of Fucoxanthin from the Rhizoid of Laminaria Japonica Aresch. J. Integr. Plant Biol. 2005, 47, 1009–1015. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Gi Lee, S.; Nam, J.O. Therapeutic Effect of Seaweed Derived Xanthophyl Carotenoid on Obesity Management; Overview of the Last Decade. Int. J. Mol. Sci. 2020, 21, 2502. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Cysewski, G. Commercial Potential for Haematococcus Microalgae as a Natural Source of Astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Vanitha, A.; Rajesha, J.; Swamy, M.M.; Sowmya, P.R.; Ravishankar, G.A. In Vivo Antioxidant Activity of Carotenoids from Dunaliella Salina - A Green Microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional Properties of Carotenoids Originating from Algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Esteban, R.; Martínez, B.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Carotenoid Composition in Rhodophyta: Insights into Xanthophyll Regulation in Corallina Elongata. Eur. J. Phycol. 2009, 44, 221–230. [Google Scholar] [CrossRef]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; Von Holst, C. Supercritical Fluid Extraction for Liquid Chromatographic Determination of Carotenoids in Spirulina Pacifica Algae: A Chemometric Approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a Green Algal Carotenoid, as a Novel Functional Compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Graham, J.E.; Bryant, D.A. The Biosynthetic Pathway for Myxol-2′ Fucoside (Myxoxanthophyll) in the Cyanobacterium Synechococcus Sp. Strain PCC 7002. J. Bacteriol. 2009, 191, 3292–3300. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Joel, J. Carotenoids Market by Type (Astaxanthin, Beta-Carotene, Lutein, Lycopene, Canthaxanthin, Zeaxanthin, and Others) for Feed, Food, Supplements, Cosmetics, and Pharmaceuticals-Global Industry Perspective, Comprehensive Analysis, Size, Share, Growth, Segmen. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html (accessed on 12 February 2021).
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic Pigment Production with Microalgae: Biological Constraints and Opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, B.; Chua, E.T.; Zhao, X.; Lu, K.; Ho, S.H.; Shi, X.; Liu, L.; Xie, Y.; Lu, Y.; et al. Comprehensive Utilization of Marine Microalgae for Enhanced Co-Production of Multiple Compounds. Mar. Drugs 2020, 18, 467. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Enzymatic Extraction of Fucoxanthin from Brown Seaweeds. Int. J. Food Sci. Technol. 2018, 53, 2195–2204. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of Extraction Methods for Selected Carotenoids from Macroalgae and the Assessment of Their Seasonal/Spatial Variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of Fucoxanthin from Edible Brown Algae by Microwave-Assisted Extraction Coupled with High-Speed Countercurrent Chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Chen, Y.C.; Cheng, C.Y.; Liu, C.T.; Sue, Y.M.; Chen, T.H.; Hsu, Y.H.; Huang, N.J.; Chen, C.H. Combined Protective Effects of Oligo-Fucoidan, Fucoxanthin, and L-Carnitine on the Kidneys of Chronic Kidney Disease Mice. Eur. J. Pharmacol. 2021, 892, 173708. [Google Scholar] [CrossRef]
- Sugimura, R.; Suda, M.; Sho, A.; Takahashi, T.; Sashima, T.; Abe, M.; Hosokawa, M.; Miyashita, K. Stability of Fucoxanthin in Dried Undaria Pinnatifida (Wakame) and Baked Products (Scones) Containing Wakame Powder. Food Sci. Technol. Res. 2012, 18, 687–693. [Google Scholar] [CrossRef]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Raguraman, V.; Abraham, S.L.; MubarakAli, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling Rapid Extraction of Fucoxanthin from Padina Tetrastromatica: Purification, Characterization and Biomedical Application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal Variations of Total Lipids, Fatty Acid Composition, and Fucoxanthin Contents of Sargassum Horneri (Turner) and Cystoseira Hakodatensis (Yendo) from the Northern Seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of Dietary Fucoxanthin from Himanthalia Elongata Brown Seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Gonçalves de Oliveira-Júnior, R.; Grougnet, R.; Bodet, P.E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated Pigment Composition of Tisochrysis Lutea and Purification of Fucoxanthin Using Centrifugal Partition Chromatography Coupled to Flash Chromatography for the Chemosensitization of Melanoma Cells. Algal Res. 2020, 51, 102035. [Google Scholar] [CrossRef]
- Robertson, R.C.; Gracia Mateo, M.R.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An Assessment of the Techno-Functional and Sensory Properties of Yoghurt Fortified with a Lipid Extract from the Microalga Pavlova Lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Mok, I.K.; Yoon, J.R.; Pan, C.H.; Kim, S.M. Development, Quantification, Method Validation, and Stability Study of a Novel Fucoxanthin-Fortified Milk. J. Agric. Food Chem. 2016, 64, 6196–6202. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial Astaxanthin Production Derived by Green Alga Haematococcus Pluvialis: A Microalgae Process Model and a Techno-Economic Assessment All through Production Line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the Relative Efficiency of Two- vs. One-Stage Production of Astaxanthin by the Green Alga Haematococcus Pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between Photochemical Activities and Pigment Composition in an Outdoor Culture of Haematococcus Pluvialis during the Shift from the Green to Red Stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Ranga, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of Carotenoids from Green Alga Haematococcus Pluvialis by HPLC and LC-MS (APCI) and Their Antioxidant Properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of Astaxanthin from Microalga Haematococcus Pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. In Advances in Biochemical Engineering/Biotechnology; Springer: Heidelberg, Germany, 2016; Volume 153, pp. 37–58. [Google Scholar]
- Shi, X.M.; Zhang, X.W.; Chen, F. Heterotrophic Production of Biomass and Lutein by Chlorella Protothecoides on Various Nitrogen Sources. Enzyme Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Shi, X.M.; Jiang, Y.; Chen, F. High-Yield Production of Lutein by the Green Microalga Chlorella Protothecoides in Heterotrophic Fed-Batch Culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of Lutein from the Green Microalgae Chlorella Vulgaris by Integrated Use of a New Extraction Protocol and a Multi-Injection High Performance Counter-Current Chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of Culture Conditions on the Productivity and Lutein Content of the New Strain Scenedesmus Almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; García-Encina, P.; Muñoz, R. Influence of Biogas Flow Rate on Biomass Composition during the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.C.; Hong, S.E.; Chang, L.P.; Shen, C.T.; Li, Y.C.; Wu, Y.P.; Jong, T.T.; Shieh, C.J.; Hsu, S.L.; Chang, C.M.J. Separation of Sight-Protecting Zeaxanthin from Nannochloropsis Oculata by Using Supercritical Fluids Extraction Coupled with Elution Chromatography. Sep. Purif. Technol. 2011, 78, 1–8. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Optimization of Pressurized Liquid Extraction of Zeaxanthin from Chlorella Ellipsoidea. J. Appl. Phycol. 2012, 24, 725–730. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid Composition of Marine Red Algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Othman, R.; Noh, N.H.; Hatta, F.A.M.; Jamaludin, M.A. Natural Carotenoid Pigments of 6 Chlorophyta Freshwater Green Algae Species. J. Pharm. Nutr. Sci. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Geisert, M.; Rose, T.; Bauer, W.; Zahn, R.K. Occurrence of Carotenoids and Sporopollenin in Nanochlorum Eucaryotum, a Novel Marine Alga with Unusual Characteristics. BioSystems 1987, 20, 133–142. [Google Scholar] [CrossRef]
- Ricketts, T.R. The Structures of Siphonein and Siphonaxanthin from Codium Fragile. Phytochemistry 1971, 10, 155–160. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in Cultivation, Wastewater Treatment Application, Bioactive Components of Caulerpa Lentillifera and Their Biotechnological Applications. PeerJ 2019, 2019, e6118. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M. Isolation and Purification of All-Trans Diadinoxanthin and All-Trans Diatoxanthin from Diatom Phaeodactylum Tricornutum. J. Appl. Phycol. 2017, 29, 79–87. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of Fucoxanthin and Diadinoxanthin and Function of Initial Pathway Genes in Phaeodactylum Tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella Aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; D’Alcalà, M.R. Circadian Variability in the Photobiology of Phaeodactylum Tricornutum: Pigment Content. J. Plankton Res. 2007, 29, 141–156. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Jiménez-López, C.; Pereira, A.G.; García-Oliveira, P.; Prieto, M.A.; Simal-Gándara, J. Fucoxanthin Extraction from Algae - Properties and Bioactivities. In Proceedings of the Iberphenol. Iberian Congress on Phenolic Compounds, Ourense, Spain, 2 October 2019. [Google Scholar]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and Its Metabolite, Fucoxanthinol, Suppress Adipocyte Differentiation in 3T3-L1 Cells. Int. J. Mol. Med. 2006. [Google Scholar] [CrossRef]
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Characterization of Fucoxanthin and Fucoxanthinol Esters in the Chinese Surf Clam, Mactra Chinensis. J. Agric. Food Chem. 2007, 18, 147–152. [Google Scholar] [CrossRef]
- Willstatter, R.; Page, H. Chlorophyll. XXIV. The Pigments of the Brown Algae. Justus Liebigs Ann. Chem. 1914, 404, 237–271. [Google Scholar]
- Kajikawa, T.; Okumura, S.; Iwashita, T.; Kosumi, D.; Hashimoto, H.; Katsumura, S. Stereocontrolled Total Synthesis of Fucoxanthin and Its Polyene Chain-Modified Derivative. Org. Lett. 2012. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2019, 47, D1388–D1395. [Google Scholar] [CrossRef]
- Soo-Jin You-Jin, H.; Seok-Chun, K.; Sung-Myung, K.; Hahk-Soo, K.; Jong-Pyung, K.; Soo-Hyun, K.; Ki-Wan, L.; Man-Gi, C. Jeon Cytoprotective Effect of Fucoxanthin Isolated from Brown Algae Sargassum Siliquastrum against H2O2-Induced Cell Damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.B. Marine Carotenoids: Bioactivities and Potential Benefits to Human Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Sugawara, T.; Yamashita, K.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Esterification of Xanthophylls by Human Intestinal Caco-2 Cells. Arch. Biochem. Biophys. 2009. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Protective Effect of Fucoxanthin Isolated from Sargassum Siliquastrum on UV-B Induced Cell Damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A Marine Carotenoid Exerting Anti-Cancer Effects by Affecting Multiple Mechanisms. Mar. Drugs 2013, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Lysoglyceroglycolipids Improve the Intestinal Absorption of Micellar Fucoxanthin by Caco-2 Cells. J. Oleo Sci. 2015. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K.R. Use of Macroalgae for Marine Biomass Production and CO2 Remediation: A Review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds. Int. J. Mol. Sci. 2013. [Google Scholar] [CrossRef]
- Market Reports World. Global Fucoxanthin Market Report 2017; Market Reports World: Pune, India, 2017. [Google Scholar]
- Gumus, R.; Urcar Gelen, S.; Koseoglu, S.; Ozkanlar, S.; Ceylan, Z.G.; Imik, H. The Effects of Fucoxanthin Dietary Inclusion on the Growth Performance, Antioxidant Metabolism and Meat Quality of Broilers. Rev. Bras. Cienc. Avic. 2018, 20, 487–496. [Google Scholar] [CrossRef]
- Satomi, Y. Antitumor and Cancer-Preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017, 1562, 1557–1562. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, W.C.; Chu, P.M.; Hsieh, P.L.; Hung, C.H.; Tsai, K.L. Fucoxanthin Protects against OxLDL-Induced Endothelial Damage via Activating the AMPK-Akt-CREB-PGC1α Pathway. Mol. Nutr. Food Res. 2019, 63, 1–10. [Google Scholar] [CrossRef]
- Cianciosi, D.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Reboredo-Rodriguez, P.; Zhang, J.J.; Quiles, J.L.; Nabavi, S.F.; Battino, M.; et al. Targeting Molecular Pathways in Cancer Stem Cells by Natural Bioactive Compounds. Pharmacol. Res. 2018, 135, 150–165. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health Benefits of Fucoxanthin in the Prevention of Chronic Diseases. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Almeida, T.P.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A.A. Cytotoxic Activity of Fucoxanthin, Alone and in Combination with the Cancer Drugs Imatinib and Doxorubicin, in CML Cell Lines. Environ. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A Promising Compound for Human Inflammation-Related Diseases. Life Sci. 2020, 255, 1178503. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Yoon, W.; Kim, K.; Ahn, G.; Kang, S.; Kang, D.; Oh, C.; Jung, W.; Jeon, Y. Evaluation of Anti-Inflammatory Effect of Fucoxanthin Isolated from Brown Algae in Lipopolysaccharide-Stimulated RAW 264. 7 Macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kim, S.M.; Pan, C.H.; Chung, D. Effects of Heating, Aerial Exposure and Illumination on Stability of Fucoxanthin in Canola Oil. Food Chem. 2014, 145, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ishihara, K.; Oyamada, C.; Sato, A.; Fukushi, A.; Arakane, T.; Motoyama, M.; Yamazaki, M.; Mitsumoto, M. Effects of Fucoxanthin Addition to Ground Chicken Breast Meat on Lipid and Colour Stability during Chilled Storage, before and after Cooking. Asian-Australasian J. Anim. Sci. 2008, 21, 1067–1072. [Google Scholar] [CrossRef]
- Hastings, J.; Owen, G.; Dekker, A. ChEBI in 2016: Improved Services and an Expanding Collection of Metabolites. Nucleic Acids Res. 2016, 44, D1214–D1219. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and Other Nutrients from Haematococcus Pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in Microalgae: Pathways, Functions and Biotechnological Implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications - A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Golan, Y. Astaxanthin Production from Microalgae. Microalgae Biotechnol. Food, Heal. High Value Prod. 2020, 175–242. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella Zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of Astaxanthin-Dimethyldisuccinate (Carophyll® Stay-Pink 10%-CWS) for Salmonids, Crustaceans and Other Fish. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical Stability of Astaxanthin Integrated into a Food Matrix: Effects of Food Processing and Methods for Preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as Modulators of Lipid Membrane Physical Properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Goukassian, D.; Pathak, M.A. Dietary Lutein/Zeaxanthin Decreases Ultraviolet B-Induced Epidermal Hyperproliferation and Acute Inflammation in Hairless Mice. J. Invest. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The Carotenoid Pigment Zeaxanthin—A Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Ravikrishnan, R.; Rusia, S.; Ilamurugan, G.; Salunkhe, U.; Deshpande, J.; Shankaranarayanan, J.; Shankaranarayana, M.L.; Soni, M.G. Safety Assessment of Lutein and Zeaxanthin (LutemaxTM 2020): Subchronic Toxicity and Mutagenicity Studies. Food Chem. Toxicol. 2011, 49, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-Inflammatory Potential of Carotenoid Meso-Zeaxanthin and Its Mode of Action. Pharm. Biol. 2015, 53, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and Protective Effects of Natural Carotenoids. Biochim. Biophys. Acta - Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.M. Effects of Lutein and Zeaxanthin on Aspects of Eye Health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Jin, E.; Feth, B.; Melis, A. A Mutant of the Green Alga Dunaliella Salina Constitutively Accumulates Zeaxanthin under All Growth Conditions. Biotechnol. Bioeng. 2003, 81, 115–124. [Google Scholar] [CrossRef]
- Li, X.R.; Tian, G.Q.; Shen, H.J.; Liu, J.Z. Metabolic Engineering of Escherichia Coli to Produce Zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, Metabolism, and Functions of β-Cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a Marine Carotenoid from Green Algae, Effectively Induces Apoptosis in Human Leukemia (HL-60) Cells. Biochim. Biophys. Acta - Gen. Subj. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive Effects of Carotenoids on the Antigeninduced Degranulation in RBL-2H3 Rat Basophilic Leukemia Cells. J. Oleo Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N.; Moreira, C.; Airs, R.; Llewellyn, C.A.; Wiegand, S.; Jogler, C.; Lage, O.M. Pink- and Orange-Pigmented Planctomycetes Produce Saproxanthin-Type Carotenoids Including a Rare C45 Carotenoid. Environ. Microbiol. Rep. 2019, 11, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare Carotenoids, (3R)-Saproxanthin and (3R,2′S)-Myxol, Isolated from Novel Marine Bacteria (Flavobacteriaceae) and Their Antioxidative Activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, S.; Liaaen-Jensen, S.; Siegelman, H.W. The Carotenoids of Blue-Green Algae. Phytochemistry 1971, 10, 3121–3127. [Google Scholar] [CrossRef]
- Marasco, E.K.; Vay, K.; Schmidt-Dannert, C. Identification of Carotenoid Cleavage Dioxygenases from Nostoc Sp. PCC 7120 with Different Cleavage Activities. J. Biol. Chem. 2006, 281, 31583–31593. [Google Scholar] [CrossRef]
- Francis, G.W.; Hertzberg, S.; Andersen, K.; Liaaen-Jensen, S. New Carotenoid Glycosides from Oscillatoria Limosa. Phytochemistry 1970, 9, 629–635. [Google Scholar] [CrossRef]
- Hamidi, M.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Gastineau, R.; Davidovich, N.; Hansen, G.; Rines, J.; Wulff, A.; Kaczmarska, I.; Ehrman, J.; Hermann, D.; Maumus, F.; Hardivillier, Y.; et al. Haslea Ostrearia -like Diatoms. Biodiversity out of the Blue. Adv. Bot. Res. 2014, 71, 441–465. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Gersonde, R.; Medlin, L.K.; Mann, D.G. The Origin and Evolution of the Diatoms. Their Adaptation to a Planktonic Existence. Evol. Prim. Prod. Sea 2007, 207–249. [Google Scholar] [CrossRef]
- Tanno, Y.; Kato, S.; Takahashi, S.; Tamaki, S.; Takaichi, S.; Kodama, Y.; Sonoike, K.; Shinomura, T. Light Dependent Accumulation of β-Carotene Enhances Photo-Acclimation of Euglena Gracilis. J. Photochem. Photobiol. B Biol. 2020, 209, 111950. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Torzillo, G. Synthesis of Antioxidant Carotenoids in Microalgae in Response to Physiological Stress. IntechOpen 2017, 143–157. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Absorption and Metabolism of Xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorption by Caco-2 Human Intestinal Cells and Mice. J. Nutr. 2002. [Google Scholar] [CrossRef]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of Fucoxanthinol into Amarouciaxanthin a in Mice and HepG2 Cells: Formation and Cytotoxicity of Fucoxanthin Metabolites. Drug Metab. Dispos. 2004. [Google Scholar] [CrossRef]
- Yim, M.J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive Effects of Amarouciaxanthin A on 3T3-L1 Adipocyte Differentiation through down-Regulation of PPARγ and C/EBPα MRNA Expression. J. Agric. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The Distribution and Accumulation of Fucoxanthin and Its Metabolites after Oral Administration in Mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, F.; Xia, G.; Liu, H.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X.; et al. Isolation of Fucoxanthin from Sargassum Thunbergii and Preparation of Microcapsules Based on Palm Stearin Solid Lipid Core. Front. Mater. Sci. 2017, 11, 66–74. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, Bioactivity, and Bioaccessibility of Fucoxanthin in Zein-Caseinate Composite Nanoparticles Fabricated at Neutral PH by Antisolvent Precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Dai, J.; Kim, S.M.; Shin, I.S.; Kim, J.D.; Lee, H.Y.; Shin, W.C.; Kim, J.C. Preparation and Stability of Fucoxanthin-Loaded Microemulsions. J. Ind. Eng. Chem. 2014, 20, 2103–2110. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Sun, Q.; Um, B.H.; Park, Y.; McClements, D.J. In Vitro and in Vivo Study of Fucoxanthin Bioavailability from Nanoemulsion-Based Delivery Systems: Impact of Lipid Carrier Type. J. Funct. Foods 2015. [Google Scholar] [CrossRef]
- Ravi, H.; Baskaran, V. Chitosan-Glycolipid Nanocarriers Improve the Bioavailability of Fucoxanthin via up-Regulation of PPARγ and SRB1 and Antioxidant Activity in Rat Model. J. Funct. Foods 2017, 28, 215–226. [Google Scholar] [CrossRef]
- Barros, M.P.; Marin, D.P.; Bolin, A.P.; De Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C.; Guerra, B.A.; Polotow, T.G.; Vardaris, C.; Mattei, R.; et al. Combined Astaxanthin and Fish Oil Supplementation Improves Glutathione-Based Redox Balance in Rat Plasma and Neutrophils. Chem. Biol. Interact. 2012, 197, 58–67. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-Grade Pickering Emulsion as a Novel Astaxanthin Encapsulation System for Making Powder-Based Products: Evaluation of Astaxanthin Stability during Processing, Storage, and Its Bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Xu, Y.; McCleiments, D.J.; Wang, D. Formation, Characterization, and Application of Chitosan/Pectin-Stabilized Multilayer Emulsions as Astaxanthin Delivery Systems. Int. J. Biol. Macromol. 2019, 140, 985–997. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Zhao, Z.; Lin, Q.; Wei, D.; Jiang, Y. Enhancing the Stability of Astaxanthin by Encapsulation in Poly (l-Lactic Acid) Microspheres Using a Supercritical Anti-Solvent Process. Particuology 2019, 44, 54–62. [Google Scholar] [CrossRef]
- Fratter, A.; Biagi, D.; Cicero, A.F.G. Sublingual Delivery of Astaxanthin through a Novel Ascorbyl Palmitate-Based Nanoemulsion: Preliminary Data. Mar. Drugs 2019, 17, 508. [Google Scholar] [CrossRef] [PubMed]
- Ligia Focsan, A.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability — The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focşan, M.; Rugină, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell Disruption of Nannochloropsis Sp. Improves in Vitro Bioaccessibility of Carotenoids and Ω3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and First-Pass Metabolism of Astaxanthin in Rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Wolz, E.; Liechti, H.; Notter, B.; Oesterhelt, G.; Kistler, A. Characterization of Metabolites of Astaxanthin in Primary Cultures of Rat Hepatocytes. Drug Metab. Dispos. 1999, 27, 456–462. [Google Scholar]
- Kistler, A.; Liechti, H.; Pichard, L.; Wolz, E.; Oesterhelt, G.; Hayes, A.; Maurel, P. Metabolism and CYP-Inducer Properties of Astaxanthin in Man and Primary Human Hepatocytes. Arch. Toxicol. 2002, 75, 665–675. [Google Scholar] [CrossRef]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular Mechanisms of the Coordination between Astaxanthin and Fatty Acid Biosynthesis in Haematococcus Pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef]
- Khachik, F.; Steck, A.; Pfander, H. Bioavailability, Metabolism, and Possible Mechanism of Chemoprevention by Lutein and Lycopene in Humans. Food Factors Cancer Prev. 1997, 542–547. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Biofunctionality of Carotenoid Metabolites: An Insight into Qualitative and Quantitative Analysis. In Metabolomics - Fundamentals and Applications; IntechOpen: London, UK, 2016; p. 19. [Google Scholar]
- Khachik, F.; Englert, G.; Beecher, G.R.; Cecil Smith, J. Isolation, Structural Elucidation, and Partial Synthesis of Lutein Dehydration Products in Extracts from Human Plasma. J. Chromatogr. B Biomed. Sci. Appl. 1995, 670, 219–233. [Google Scholar] [CrossRef]
- Giordano, E.; Quadro, L. Lutein, Zeaxanthin and Mammalian Development: Metabolism, Functions and Implications for Health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef]
- Berg, J.; Lin, D. Lutein and Zeaxanthin: An Overview of Metabolism and Eye Health. J. Hum. Nutr. Food Sci. 2014, 2, 1039. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual Knowledge on Food Sources, Intakes, Stability and Bioavailability and Their Protective Role in Humans. Mol. Nutr. Food Res. 2009, 53, 194–218. [Google Scholar] [CrossRef] [PubMed]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In Vitro Bioaccessibility Assessment as a Prediction Tool of Nutritional Efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Helena de Abreu-Martins, H.; Artiga-Artigas, M.; Hilsdorf Piccoli, R.; Martín-Belloso, O.; Salvia-Trujillo, L. The Lipid Type Affects the in Vitro Digestibility and β-Carotene Bioaccessibility of Liquid or Solid Lipid Nanoparticles. Food Chem. 2020, 311, 126024. [Google Scholar] [CrossRef]
- Iddir, M.; Dingeo, G.; Porras Yaruro, J.F.; Hammaz, F.; Borel, P.; Schleeh, T.; Desmarchelier, C.; Larondelle, Y.; Bohn, T. Influence of Soy and Whey Protein, Gelatin and Sodium Caseinate on Carotenoid Bioaccessibility. Food Funct. 2020, 11, 5446–5459. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of Fatty Acyl Composition and Quantity of Triglycerides on Bioaccessibility of Dietary Carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef]
- Bohn, T.; Mcdougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the Gap-Deficits in Our Knowledge of Aspects Impacting the Bioavailability of Phytochemicals and Their Metabolites-a Position Paper Focusing on Carotenoids and Polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Failla, M.L. Bioaccessibility and Intestinal Cell Uptake of Astaxanthin from Salmon and Commercial Supplements. Food Res. Int. 2017, 99, 936–943. [Google Scholar] [CrossRef]
- Tyssandier, V.; Lyan, B.; Borel, P. Main Factors Governing the Transfer of Carotenoids from Emulsion Lipid Droplets to Micelles. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and Sr-Bi Are Involved in Cellular Uptake of Provitamin a Carotenoids by Caco-2 and Hek Cells, and Some of Their Genetic Variants Are Associated with Plasma Concentrations of These Micronutrients in Humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll Carotenoids Are More Bioaccessible from Fruits than Dark Green Vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Borel, P.; Grolier, P.; Armand, M.; Partier, A.; Lafont, H.; Lairon, D.; Azais-Braesco, V. Carotenoids in Biological Emulsions: Solubility, Surface-to-Core Distribution, and Release from Lipid Droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-Related Factors Explaining Interindividual Variability of Carotenoid Bioavailability and Tissue Concentrations in Humans. Mol. Nutr. Food Res. 2017, 61, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of Physicochemical Properties of Carotenoids on Their Bioaccessibility, Intestinal Cell Uptake, and Blood and Tissue Concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Guo, B.; Oliviero, T.; Fogliano, V.; Ma, Y.; Chen, F.; Capuano, E. Gastrointestinal Bioaccessibility and Colonic Fermentation of Fucoxanthin from the Extract of the Microalga Nitzschia Laevis. J. Agric. Food Chem. 2020, 68, 1844–1850. [Google Scholar] [CrossRef]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine Enhances Carotenoid Uptake from Mixed Micelles by Caco-2 Human Intestinal Cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c Levels by Fucoxanthin-Enriched Akamoku Oil Possibly Involves the Thrifty Allele of Uncoupling Protein 1 (UCP1): A Randomised Controlled Trial in Normal-Weight and Obese Japanese Adults. Sapporo Med. J. 2017, 86, 108–109. [Google Scholar] [CrossRef]
- Asai, A.; Yonekura, L.; Nagao, A. Low Bioavailability of Dietary Epoxyxanthophylls in Humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef]
- Mimoun-Benarroch, M.; Hogot, C.; Rhazi, L.; Niamba, C.N.; Depeint, F. The Bioavailability of Astaxanthin Is Dependent on Both the Source and the Isomeric Variants of the Molecule. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2016, 73, 61. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, Cellular Uptake, and Transport of Astaxanthin Isomers and Their Antioxidative Effects in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral Bioavailability of the Antioxidant Astaxanthin in Humans Is Enhanced by Incorporation of Lipid Based Formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Li, D.; Wang, Y.; Chen, Z.; Zou, C.; Liu, W.; Ma, Y.; Cao, M.J.; Liu, G.M. Re-Assembled Oleic Acid-Protein Complexes as Nano-Vehicles for Astaxanthin: Multispectral Analysis and Molecular Docking. Food Hydrocoll. 2020, 103, 105689. [Google Scholar] [CrossRef]
- Olson, J.A. Absorption, Transport, and Metabolism of Carotenoids in Humans. Pure Appl. Chem. 1994, 66, 1011–1016. [Google Scholar] [CrossRef]
- do Nascimento, T.C.; Pinheiro, P.N.; Fernandes, A.S.; Murador, D.C.; Neves, B.V.; de Menezes, C.R.; de Rosso, V.V.; Jacob-Lopes, E.; Zepka, L.Q. Bioaccessibility and Intestinal Uptake of Carotenoids from Microalgae Scenedesmus Obliquus. LWT 2021, 140, 110780. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Borel, P.; Reboul, E.; Caporiccio, B.; Besancon, P.; Amiot, M.J. β-Cryptoxanthin from Citrus Juices: Assessment of Bioaccessibility Using an in Vitro Digestion/Caco-2 Cell Culture Model. Br. J. Nutr. 2007, 97, 883–890. [Google Scholar] [CrossRef]
- Burri, B.J.; Chang, J.S.T.; Neidlinger, T.R. Βcryptoxanthin- and α-Carotene-Rich Foods Have Greater Apparent Bioavailability than Βcarotene-Rich Foods in Western Diets. Br. J. Nutr. 2011, 105, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of Lutein and Zeaxanthin in Visual and Cognitive Function throughout the Lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids Bioavailability from Foods: From Plant Pigments to Efficient Biological Activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Hempel, J.; Schädle, C.N.; Sprenger, J.; Heller, A.; Carle, R.; Schweiggert, R.M. Ultrastructural Deposition Forms and Bioaccessibility of Carotenoids and Carotenoid Esters from Goji Berries (Lycium Barbarum L.). Food Chem. 2017, 218, 525–533. [Google Scholar] [CrossRef]
- Gille, A.; Neumann, U.; Louis, S.; Bischoff, S.C.; Briviba, K. Microalgae as a Potential Source of Carotenoids: Comparative Results of an in Vitro Digestion Method and a Feeding Experiment with C57BL/6J Mice. J. Funct. Foods 2018, 49, 285–294. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Chitchumroonchokchai, C.; Mariutti, L.R.B.; Mercadante, A.Z.; Failla, M.L. Comparison of Two Static in Vitro Digestion Methods for Screening the Bioaccessibility of Carotenoids in Fruits, Vegetables, and Animal Products. J. Agric. Food Chem. 2017, 65, 11220–11228. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids Modulate the Hallmarks of Cancer Cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Marco, G.J. A Rapid Method for Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress-Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and Cancer: Advances and New Agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.H.; Qian, Z.J.; Choi, I.W.; Jung, W.K. Anti-Inflammatory Effect of Fucoxanthin Derivatives Isolated from Sargassum Siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-Inflammatory Effect of Apo-9′-Fucoxanthinone via Inhibition of MAPKs and NF-KB Signaling Pathway in LPS-Stimulated RAW 264.7 Macrophages and Zebrafish Model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.H.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of Fucoxanthin on Lipopolysaccharide-Induced Inflammation in Vitro and in Vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ahn, G.; Heo, S.; Kang, S.; Kang, M.; Yang, H.; Kim, D.; Woon, S.; Kim, S.; Jeon, B.; et al. Inhibition of Tumor Growth in Vitro and in Vivo by Fucoxanthin against Melanoma B16F10 Cells. Environ. Toxicol. Pharmacol. 2012, 35, 39–46. [Google Scholar] [CrossRef]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-Inducing Effect of Fucoxanthin on Human Leukemia Cell Line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef]
- Farruggia, C.; Kim, M.B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.K.; Lee, J.Y. Astaxanthin Exerts Anti-Inflammatory and Antioxidant Effects in Macrophages in NRF2-Dependent and Independent Manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin Alleviated Acute Lung Injury by Inhibiting Oxidative/Nitrative Stress and the Inflammatory Response in Mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-Inhibitory Effects of the Astaxanthin-Rich Alga Haematococcus Pluvialis in Human Colon Cancer Cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary Astaxanthin Inhibits Colitis and Colitis-Associated Colon Carcinogenesis in Mice via Modulation of the Inflammatory Cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zou, Z.Y.; Xiao, X.; Huang, Y.M.; Wang, X.; Lin, X.M. Effects of Lutein Supplement on Serum Inflammatory Cytokines, ApoE and Lipid Profiles in Early Atherosclerosis Population. J. Atheroscler. Thromb. 2013, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; MacKey, A.D.; Dimmit, R.A.; Hartmann, E.E.; et al. Effect of Carotenoid Supplementation on Plasma Carotenoids, Inflammation and Visual Development in Preterm Infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef]
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Ito, M.; Aizawa, R.; Murakoshi, M.; Uemura, S.; Khachik, F.; Nishino, H. Inhibitory Effects of Natural Carotenoids, α-Carotene, β-Carotene, Lycopene and Lutein, on Colonic Aberrant Crypt Foci Formation in Rats. Cancer Lett. 1996, 107, 137–142. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C.; Molina, M.G.D.; Ugas, R.; Midas, P.; Méndez, V.E. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: Programs and Collection Procedures. Vital Health Stat. 1. 1994, 7, 1–407. [Google Scholar]
- Min, K.B.; Min, J.Y. Serum Carotenoid Levels and Risk of Lung Cancer Death in US Adults. Cancer Sci. 2014, 105, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dang, F.; Deng, C. β-Cryptoxanthin Induced Anti-Proliferation and Apoptosis by G0/G1 Arrest and AMPK Signal Inactivation in Gastric Cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.Q.; Choi, S.W.; Ausman, L.M.; Wang, X.D. β-Cryptoxanthin Restores Nicotine-Reduced Lung SIRT1 to Normal Levels and Inhibits Nicotine-Promoted Lung Tumorigenesis and Emphysema in A/J Mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef]
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.-D. β-Cryptoxanthin Supplementation Prevents Cigarette Smoke-Induced Lung Inflammation, Oxidative Damage, and Squamous Metaplasia in Ferrets. Cancer Prev. Res. 2011, 4, 1255–1266. [Google Scholar] [CrossRef]
- Palozza, P.; Sestito, R.; Picci, N.; Lanza, P.; Monego, G.; Ranelletti, F.O. The Sensitivity to β-Carotene Growth-Inhibitory and Proapoptotic Effects Is Regulated by Caveolin-1 Expression in Human Colon and Prostate Cancer Cells. Carcinogenesis 2008, 29, 2153–2161. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. An Int. Rev. J. 2017, 8, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Boyd, K.; Matanoski, G.; Tao, X.; Chen, L.; Lam, T.K.; Shiels, M.; Hammond, E.; Robinson, K.A.; Caulfield, L.E.; et al. Carotenoids and the Risk of Developing Lung Cancer: A Systematic Review. Am. J. Clin. Nutr. 2008, 88, 372–383. [Google Scholar] [CrossRef] [PubMed]
- The ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: Design, Methods, Participant Characteristics, and Compliance. Ann. Epidemiol. 1994, 4, 1–10. [Google Scholar] [CrossRef]
- Lai, G.Y.; Weinstein, S.J.; Taylor, P.R.; McGlynn, K.A.; Virtamo, J.; Gail, M.H.; Albanes, D.; Freedman, N.D. Effects of α-Tocopherol and β-Carotene Supplementation on Liver Cancer Incidence and Chronic Liver Disease Mortality in the ATBC Study. Br. J. Cancer 2014, 111, 2220–2223. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality: A Prospective Cohort Study. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, J.G.; Meisner, C.; Bode, J.C.; Bode, C. Lycopene, β-Carotene, and Colorectal Adenomas. Am. J. Clin. Nutr. 2003, 78, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.G.; Zhang, Q.L.; Zheng, J.L.; Li, H.L.; Zhang, W.; Tang, W.G.; Xiang, Y.B. Dietary, Circulating Beta-Carotene and Risk of All-Cause Mortality: A Meta-Analysis from Prospective Studies. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS One 2015, 10, 1–20. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial Potential of Carotenoid Pigments from Microalgae: Current Trends and Future Prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as Versatile Cellular Factories for Valued Products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus Pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.; Barredo, J.L. Carotenoids Production: A Healthy and Profitable Industry. Methods Mol. Biol. 2018, 1852, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliam. In Official Journal L; Eur-lex: Luxembourg, 2015; Volume 327, pp. 1–22.
- Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. In Official Journal L.; Eur-lex: Luxembourg, 2004; Volume 139, pp. 1–54.
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; Eur-lex: Luxembourg, 2006; Volume 18, pp. 244–259.

| Mol. | Algae | Extraction | Concentration | Applications | Ref. |
|---|---|---|---|---|---|
| FU | Fucus vesiculosus | Enzyme-assisted extraction | 0.66 mg/g DW | Development of value-added nutraceutical products from seaweed | [42] |
| Fucus serratus | Supercritical fluid extraction | 2.18 mg/g DW | Obtaining high-purity fucoxanthin | [43] | |
| Laminaria japonica | Microwave-assisted extraction | 0.04 mg/g DW | Obtaining high-purity fucoxanthin | [44] | |
| Laminaria japonica | Maceration | 0.10 mg/g DW | Drug against chronic kidney disease | [45] | |
| Undaria pinnatifida | Microwave-assisted extraction | 0.90 mg/g DW | Obtention of high-purity fucoxanthin | [44] | |
| Undaria pinnatifida | Maceration | 3.09 mg/g DW | Scones | [46] | |
| Undaria pinnatifida | Supercritical fluid extraction | 0.99 mg/g DW | Carotenoid isolation | [3] | |
| Undaria pinnatifida | Maceration | 2.67 mg/g DW | Drug development | [47] | |
| Padina tetrastromatica | Ultrasonic-assisted extraction | 0.75 mg/g DW | Nutraceuticals and biomedical applications | [48] | |
| Cystoseira hakodatensis | Maceration | 3.47 mg/g DW | Optimization of the environmental conditions | [49] | |
| Himanthalia elongata | Maceration | 18.60 mg/g DW | Commercial fucoxanthin production | [50] | |
| Tisochrysis lutea | Ultrasonic-assisted extraction | 0.25 mg/g DW | Nutraceutical, cosmetic and pharmaceutical applications, such as for the treatment of metastatic melanoma | [51] | |
| Pavlova lutheri | Ultrasonic-assisted extraction | 0.03 mg/g DW | Yogurt | [52] | |
| Phaeodactylum tricornutum | Maceration | 0.1 mg/g DW | Milk | [53] | |
| AS | Haematococcus pluvialis | Conventional extraction | 900 kg/2 ha/year | Antioxidant, anti-tumor, anti-inflammatory, ocular protective effect, antidiabetic, coloring agent | [54] |
| Haematococcus pluvialis | Two-stage system | 3.8% dw | [55] | ||
| Haematococcus pluvialis | Enzyme | 3.6% dw | [56] | ||
| Haematococcus pluvialis | Conventional extraction | 2–3% dw | [57] | ||
| Haematococcus pluvialis | Pressurized extraction | 99% of total AS | [58] | ||
| LU | Chlorella protothecoides | Maceration | 83.8 mg/L | Antioxidant, light-filtering, eye protection, colorant, potential therapeutic use against several chronic diseases, lower risk of cancer, anti-inflammatory benefits | [59] |
| Chlorella protothecoides | Mechanical | 83.8 mg/L | [60] | ||
| Chlorella protothecoideswas | Mechanical | 4.92 mg/g | [61] | ||
| Chlorella vulgaris | Heptane–ethanol–water extraction | 30 mg/g | [62] | ||
| Scenedesmus almeriensis | - | 0.54% wt | [63] | ||
| Dunaliella salina | Conventional extraction | 15.4 mg m−2 d−1 | [64] | ||
| ZEA | Nannochloropsis oculata | Supercritical fluids extraction | 13.17 mg/g | Antioxidant, anti-inflammatory, eyes and UV light protection, prevention of coronary syndromes, anti-tumoral, anti-cardiovascular diseases, and structural actions in neural tissue | [65] |
| Chlorella ellipsoidea | Pressurized liquid extraction | 4.26 mg/g | [66] | ||
| Synechocystis sp | Pulse electric field | 1.64 mg/g | [1] | ||
| Himanthalia elongata | Pulse electric field | 0.13 mg/g | [1] | ||
| Heterochlorella luteoviridis | Moderate electric field | 244 µg/g | [9] | ||
| CRY | Spirulina platensis | Supercritical fluid extraction | 7.5 mg/100 g | Antioxidant, anti-inflammatory, anticancer (lung, oral, pharyngeal), improves respiratory function, stimulation of bone formation and protection, modulation response to phytosterols in post-menopausal women, decreases risk of degenerative diseases | [34,67] |
| Palisada perforata | Conventional extraction | 14.2% total carotenoids | [68] | ||
| Gracilaria gracilis | Conventional extraction | 10.2% total carotenoids | [68] | ||
| Pandorina morum | Maceration | 2.38 µg/g DW | [69] | ||
| Nanochlorum eucaryotum | Enzyme extraction | - | [70] | ||
| SIP | Codium fragile | Maceration | 16 mg/kg fresh algae | Anti-angiogenic, antioxidant, cancer-preventing action; inhibit adipogenesis | [71] |
| Caulerpa lentillifera | Maceration | 0.1% DW | [72] | ||
| Umbraulva japonica | Maceration | 0.1% DW | [35] | ||
| DIAD | Phaeodactylum tricornutum | MeOH extraction | 19% of total pigments | Antioxidant | [73] |
| Phaeodactylum tricornutum | MeOH extraction | - | [74] | ||
| Odontella aurita | EtOH extraction | 10% total carotenoids | [75] | ||
| Phaeodactylum tricornutum | Whole | 14 µg/L | [76] | ||
| DIAT | Phaeodactylum tricornutum | MeOH extraction | 17% of total pigments | Antioxidant | [73] |
| Mol. | Delivery System | Assay | Benefits | Results | Use | Ref. |
|---|---|---|---|---|---|---|
| FU | Palm stearin solid lipid core | In vitro | Increase stability during storage | Release of FU of 22.92% during 2 h in SGF and 56.55% during 6 h SIF | Oral supplements | [149] |
| Nanoparticles of zein | ABTS DPPH | Increase antioxidant activity | More antioxidant than free FU | Foods and beverages | [150] | |
| Nanoemulsion | In vitro | Increase stability during storage; antiobesity | 95% of FU remains in the emulsion after 4 weeks | Food, beverages, nutraceutics | [151] | |
| Nanoemulsion (LCT) | In vitro digestion and bioability assays in rats | Increase stability | Increase FU level in serum blood (LCT > MCT) | Functional foods and nutraceutics | [152] | |
| Chitosan–glycolipid nanogels | In vitro | Significant increase in bioavailability | Lpx levels (nmol MDA/mL) higher in control (30.9) than in emulsions (17.0–12.15) | Foods and nutraceutics | [153] | |
| AS | Fish oil | In vitro | Useful for supplementation | Better antioxidant effect | Oral supplements | [154] |
| Encapsulation | TBARS Peroxide enzymes | Increase stability | Better antioxidant effect | Foods | [155] | |
| Pectin–chitosan multilayer | Stability Assays | Increase stability | Better stability than monolayer | Nutraceuticals, functional, medical foods | [156] | |
| l-lacic acid | Release and stability test | Increase stability | Enhance stability | Functional foods and nutraceutics | [157] | |
| Ascobyl palmitate emulsion | Stability assay | Sublingual delivery | Enhance sports performance, skin protection, cardioprotective | Dietetic supplementation in sports | [158] | |
| LU | β-CD | In vitro | Increase stability | More stable against oxidating agents | Foods | [159] |
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [159] | |
| ZEA | Sea Buckthorn oil and water emulsion | Stability and digestive assays | Increase bioaccesibility | Increase 64.55% | Functional foods and nutraceutics | [160] |
| High-pressure treatment | Stability and digestive assays | Improve Nannochloropsis sp. ZEA disponibility | Foods | [161] | ||
| Glycyrrhizic acid, arabinogalactan | In vitro | Solubility enhancement | Prevention of H-aggregates formation, increase of photostability | Foods | [159] |
| Study | Model | Dose | Experimental Design | Observations | Ref. |
|---|---|---|---|---|---|
| Fucoxanthin | |||||
| Anti-inflammatory | In vitro. RAW 264.7 macrophages with LPS-induced inflammation | 15–60 μM | Expression of inflammatory mediators | D-d reduction of expression of IL6-IL-1, NO, and TNF-α | [212] |
| In vitro (Apo-9′). RAW 264.7 macrophages and zebrafish model | 25–100 μg/mL | Reduction of LPS-induced inflammation | D-d reduction of NO, ROS, TNF-α, and COX production | [213] | |
| In vitro and in vivo. RAW 264.7 and aqueous humor of rats | 10 mg/kg | Reduction of LPS-induced inflammation | D-d reduction of PGE2, NO, TNF-α by inhibiting iNOS and COX-2 | [214] | |
| Anti-cancer | Ex vivo. B16F10 cell culture implanted in mice | 200 μM | Growth inhibition of melanoma | D-d growth inhibition by inducing G0/G1 cell cycle arrest and apoptosis; inhibition production of retinoblastoma protein | [215] |
| In vitro. Human leukemic HL-60 cells | 15.2 μM | Inhibited the proliferation | DNA fragmentation | [216] | |
| Astaxanthin | |||||
| Anti-inflammatory | In vitro. RAW 264.7, splenocytes, and bone-narrow macrophages | 25 μM | Expression of inflammatory mediators in LPS-induced inflammation | D-d significant reduction of IL-6, IL-1β, and ROS production | [217] |
| In vivo. Mice with induced acute lung injury | 60 mg/kg/day for 14 days | Analysis of inflammation markers, tissue damage | Significant reduction of mortality, histological damage, inflammatory infiltration, and iNOS and NF-κβ levels | [218] | |
| Anti-cancer | In vitro. Human colon cancer lines HCT-116, SW480, WiDr, HT-29 and LS-174 | 5–25 µg/mL | Growth inhibition of with H. pluvialis astaxanthin-rich extract | D-d cell cycle arrest and apoptosis induction by lowering expression of Bcl-2, AKT and induced expression of apoptotic MAPK | [219] |
| In vivo. Chemically induced colitis and colon carcinogenesis mice | 200 ppm | Analysis of inflammatory biomarkers | D-d inhibition of NF-κβ, TNF-α, IL-1β, IL-6, and COX-2 expression; lower iNOS expression at high dosage | [220] | |
| Lutein | |||||
| Anti-inflammatory | Observational study. Early atherosclerosis patients (n = 65) | 20 mg/day for 3 months | Differences in serum cytokines, and metabolic biomarkers | Significant reduction in serum IL-6 MCP-1 and LDL-cholesterol after 3 months of supplementation | [221] |
| Observational study. Preterm infants (n = 203) | 30 mL/ kg/ day until 40 weeks post-menstrual age | Differences in inflammation biomarkers | Enhanced retinal development and reduced C-reactive protein levels | [222] | |
| Anti-cancer | In vivo. Rats | 3–30 g/L | Inhibition of N-methylnitrosourea-induced colon crypt foci formation | Significantly lowered formation of aberrant crypt foci | [223] |
| β-cryptoxanthin | |||||
| Anti-cancer | Prospective cohort study. Smokers and non-smokers from NHANES III (n = 10,382) | Dietary contribution | 20-year cohort | Higher serum levels of β-CRY were associated with lower death risk, but not for non-smokers | [224,225] |
| Ex vivo. Human gastric cell lines AGS and SGC-7901 implanted in mice | 0–40μM | Growth and proliferation inhibition | D-d growth and proliferation inhibitory activity by reducing cyclins, endothelial growth factor, PKA and increasing cleaved caspases expression | [226] | |
| In vivo. Mice | 10 mg/kg diet | Induced emphysema and lung tumorigenesis | D-d tumor mass reduction, decreased levels of IL-6 and AKT and restoration of silenced tumor-suppressor genes | [227] | |
| In vivo. Cigarette smoke-exposed ferrets | 7.5–37.5 μg/kg/day | Inflammation biomarkers and tissue damage analysis | D-d inhibition of NF-κβ, TNF-α, AP-1 expression as well as lung tissue squamous metaplasia and inflammation | [228] | |
| Siphonaxanthin | |||||
| Anti-cancer | In vitro. Human leukemia (HL-60) cells | 5–20 μM | Analysis on cell viability and apoptosis | D-d reduction of cell viability and induction of apoptosis by increasing levels of DR5, lower expression of Bcl-2 and increase in caspase-3 | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. https://doi.org/10.3390/md19040188
Pereira AG, Otero P, Echave J, Carreira-Casais A, Chamorro F, Collazo N, Jaboui A, Lourenço-Lopes C, Simal-Gandara J, Prieto MA. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Marine Drugs. 2021; 19(4):188. https://doi.org/10.3390/md19040188
Chicago/Turabian StylePereira, Antia G., Paz Otero, Javier Echave, Anxo Carreira-Casais, Franklin Chamorro, Nicolas Collazo, Amira Jaboui, Catarina Lourenço-Lopes, Jesus Simal-Gandara, and Miguel A. Prieto. 2021. "Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids" Marine Drugs 19, no. 4: 188. https://doi.org/10.3390/md19040188
APA StylePereira, A. G., Otero, P., Echave, J., Carreira-Casais, A., Chamorro, F., Collazo, N., Jaboui, A., Lourenço-Lopes, C., Simal-Gandara, J., & Prieto, M. A. (2021). Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Marine Drugs, 19(4), 188. https://doi.org/10.3390/md19040188