Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent
Abstract
1. Introduction
2. Bioavailability and Pharmacokinetics of Astaxanthin
3. Astaxanthin for Neurological Disorders
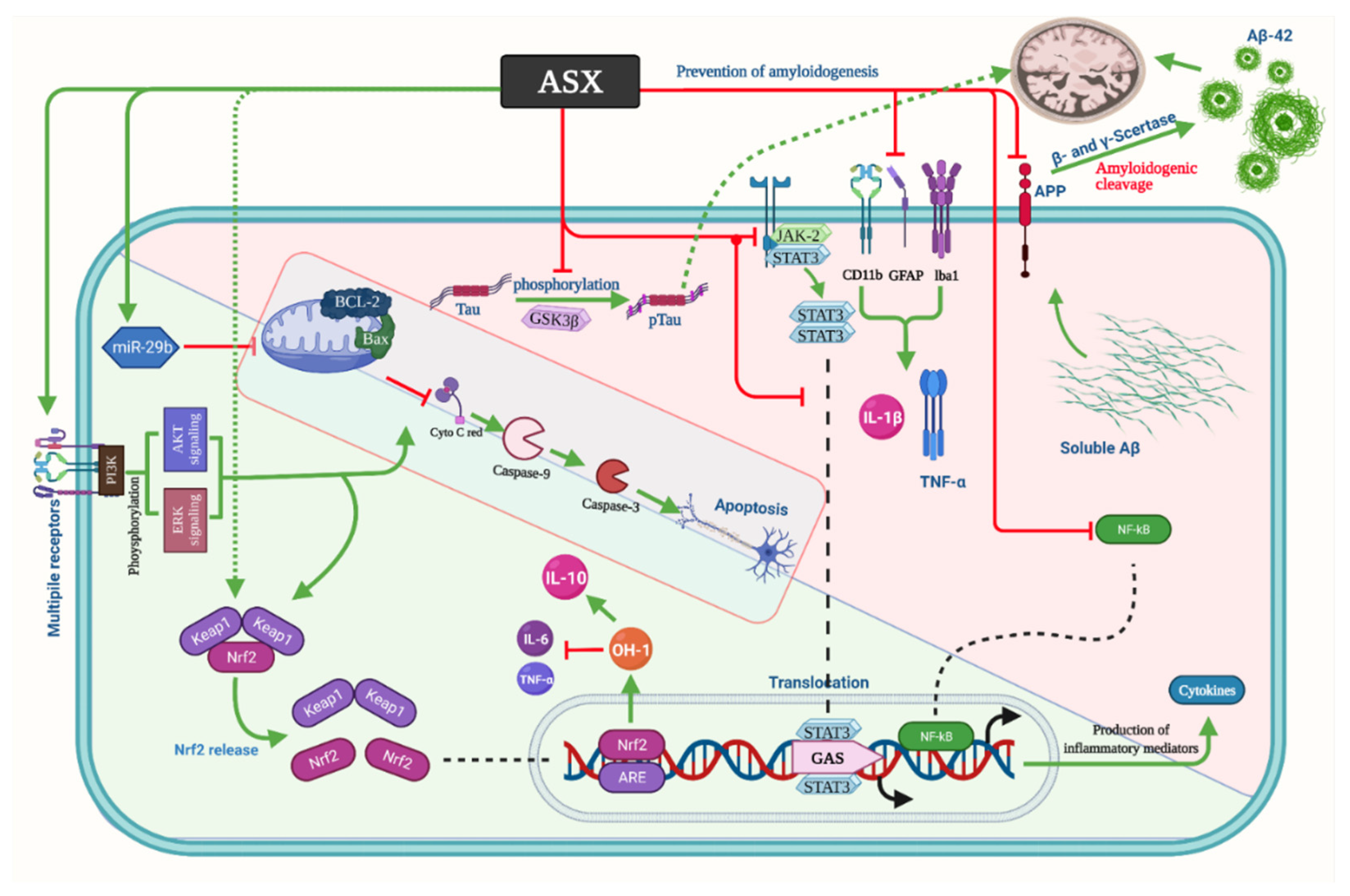
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Neuropathic Pain and Central Nervous System Injuries
3.4. Autism
3.5. Cerebral Ischemia
4. Potential of Astaxanthin in Counteracting Neuroinflammation
5. Safety of Astaxanthin
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010, 17, e1113–e1188. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N.; Tsushima, M.; Komemushi, S.; Mezaki, T.; Iwase, F.; Takahashi, Y.; Sameshima, N.; Mori, M.; Sakagami, Y. Carotenoids in marine invertebrates living along the Kuroshio current coast. Mar. Drugs 2011, 9, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. IJCB 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. A J. Clin. Ther. 2011, 16, 355–364. [Google Scholar]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Too, H.P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Martín, J.F.; Gudiña, E.; Barredo, J.L. Conversion of beta-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb. Cell Factories 2008, 7, 3. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications--a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Wang, M.; Deng, X.; Xie, Y.; Chen, Y. Astaxanthin Attenuates Neuroinflammation in Status Epilepticus Rats by Regulating the ATP-P2X7R Signal. Drug Des. Dev. Ther. 2020, 14, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, J.; Yin, W.; Ding, X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int. J. Clin. Exp. Pathol. 2015, 8, 6083–6094. [Google Scholar]
- Lu, Y.; Wang, X.; Feng, J.; Xie, T.; Si, P.; Wang, W. Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res. Bull. 2019, 148, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Marin, D.P.; Bolin, A.P.; de Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C., Jr.; Guerra, B.A.; Polotow, T.G.; Vardaris, C.; Mattei, R.; et al. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem. Biol. Interact. 2012, 197, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Otton, R.; Marin, D.P.; Bolin, A.P.; De Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C., Jr.; Guerra, B.A.; Leite, J.R.; Barros, M.P.; Mattei, R. Combined fish oil and astaxanthin supplementation modulates rat lymphocyte function. Eur. J. Nutr. 2012, 51, 707–718. [Google Scholar] [CrossRef]
- Page, G.I.; Davies, S.J. Astaxanthin and canthaxanthin do not induce liver or kidney xenobiotic-metabolizing enzymes in rainbow trout (Oncorhynchus mykiss Walbaum). Comp. Biochem. Physiology. Toxicol. Pharmacol. CBP 2002, 133, 443–451. [Google Scholar] [CrossRef]
- Olson, J.A. Absorption, transport and metabolism of carotenoids in humans. Pure Appl. Chem. 1994, 66, 1011–1016. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Kelly, G.S. The interaction of cigarette smoking and antioxidants. Part I: Diet and carotenoids. Altern. Med. Rev. A J. Clin. Ther. 2002, 7, 370–388. [Google Scholar]
- Kvaavik, E.; Totland, T.H.; Bastani, N.; Kjøllesdal, M.K.; Tell, G.S.; Andersen, L.F. Do smoking and fruit and vegetable intake mediate the association between socio-economic status and plasma carotenoids? Eur. J. Public Health 2014, 24, 685–690. [Google Scholar] [CrossRef]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Mercke Odeberg, J.; Lignell, A.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Karlawish, J.; Jack, C.R., Jr.; Rocca, W.A.; Snyder, H.M.; Carrillo, M.C. Alzheimer’s disease: The next frontier-Special Report. Alzheimer’s Dement. 2017, 13, 374–380. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M..T.; Rahman, M..S.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5858. [Google Scholar] [CrossRef]
- Sayed, A.; Bahbah, E.I.; Kamel, S.; Barreto, G.E.; Ashraf, G.M.; Elfil, M. The neutrophil-to-lymphocyte ratio in Alzheimer’s disease: Current understanding and potential applications. J. Neuroimmunol. 2020, 349, 577398. [Google Scholar] [CrossRef] [PubMed]
- Bahbah, E.I.; Fathy, S.; Negida, A. Is Alzheimer’s disease linked to Herpes simplex virus type 1 infection? A mini-review of the molecular correlation and the possible disease connections. Clin. Exp. Neuroimmunol. 2019, 10, 192–196. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mamun, A.A.; Mathew, B.; Aleya, L.; Barreto, G.E.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Revisiting the role of brain and peripheral Aβ in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 2020, 416, 116974. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Elhelaly, A.E.; AlBasher, G.; Alfarraj, S.; Almeer, R.; Bahbah, E.I.; Fouda, M.M.A.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut Res. Int. 2019, 26, 35151–35162. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungau, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Env. Sci. Pollut. Res. Int. 2020, 27, 11554–11564. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Kakouri, E.; Lambrou, G.I.; Bethanis, K.; Tarantilis, P.A. Antioxidant Properties of Crocus Sativus L. and Its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s and Parkinson’s Disease. Curr. Neuropharmacol. 2019, 17, 377–402. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxidative Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef] [PubMed]
- Taksima, T.; Chonpathompikunlert, P.; Sroyraya, M.; Hutamekalin, P.; Limpawattana, M.; Klaypradit, W. Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta(1-42) rat model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Kuedo, Z.; Sangsuriyawong, A.; Klaypradit, W.; Tipmanee, V.; Chonpathompikunlert, P. Effects of Astaxanthin from Litopenaeus Vannamei on Carrageenan-Induced Edema and Pain Behavior in Mice. Molecules 2016, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Fan, Y.C.; Wang, M.; Wang, D.; Li, X.H. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β1-42-induced rat model of Alzheimer’s disease. Clin. Interv. Aging 2013, 8, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Komaki, A.; Ebrahim-Habibi, A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet- fed rats. Metab. Brain Dis. 2017, 32, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 110, 47–58. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef]
- Kim, H.A.; Miller, A.A.; Drummond, G.R.; Thrift, A.G.; Arumugam, T.V.; Phan, T.G.; Srikanth, V.K.; Sobey, C.G. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 953–959. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Lefter, R.; Serban, I.L.; Stefanescu, C.; Chirita, R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013, 25, 401–409. [Google Scholar]
- Hongo, N.; Takamura, Y.; Nishimaru, H.; Matsumoto, J.; Tobe, K.; Saito, T.; Saido, T.C.; Nishijo, H. Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer’s Disease-Related Pathological Progression in the Hippocampus of App(NL-G-F/NL-G-F) Mice. Front. Pharmacol. 2020, 11, 307. [Google Scholar] [CrossRef]
- Solomonov, Y.; Hadad, N.; Levy, R. The Combined Anti-Inflammatory Effect of Astaxanthin, Lyc-O-Mato and Carnosic Acid In Vitro and In Vivo in a Mouse Model of Peritonitis. J. Nutr. Food Sci. 2018, 8. [Google Scholar] [CrossRef]
- Kim, Y.H.; Koh Hk Fau-Kim, D.-S.; Kim, D.S. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, X.B.; Xu, Y.X.; Zhao, H.; Zhu, Q.Y.; Zhu, C.Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Huang, A.; Hu, J.; Zhong, Z.; Liu, Y.; Li, Z.; Pan, X.; Liu, Z. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3β pathway. Neuroscience 2015, 303, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Hao, C.; Mao, X.; Zhang, L. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J. Funct. Foods 2015, 16, 137–151. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, W.; Lee, J.H.; Jeon, S.J.; Choi, Y.H.; Kim, B.W.; Chang, H.I.; Nam, S.W. Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J. Microbiol. Biotechnol. 2009, 19, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumoto, J.; Takamura, Y.; Ishii, Y.; Sasahara, M.; Ono, T.; Nishijo, H. Relationships among parvalbumin-immunoreactive neuron density, phase-locked gamma oscillations, and autistic/schizophrenic symptoms in PDGFR-β knock-out and control mice. PLoS ONE 2015, 10, e0119258. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, S.; Masuch, A.; Nestel, S.; Katzmarski, N.; Meyer-Luehmann, M.; Biber, K. Forebrain microglia from wild-type but not adult 5xFAD mice prevent amyloid-β plaque formation in organotypic hippocampal slice cultures. Sci. Rep. 2015, 5, 14624. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Chen, Q.; Yang, H.; Xie, X. Astaxanthin Promotes Nrf2/ARE Signaling to Alleviate Renal Fibronectin and Collagen IV Accumulation in Diabetic Rats. J. Diabetes Res. 2018, 2018, 6730315. [Google Scholar] [CrossRef]
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014, 5, 3496. [Google Scholar] [CrossRef]
- Hafez, H.A.; Kamel, M.A.; Osman, M.Y.; Osman, H.M.; Elblehi, S.S.; Mahmoud, S.A. Ameliorative effects of astaxanthin on brain tissues of alzheimer’s disease-like model: Cross talk between neuronal-specific microRNA-124 and related pathways. Mol. Cell. Biochem. 2021. [Google Scholar] [CrossRef]
- Shalash, A.S.; Hamid, E.; Elrassas, H.; Bahbah, E.I.; Mansour, A.H.; Mohamed, H.; Elbalkimy, M. Non-motor symptoms in essential tremor, akinetic rigid and tremor-dominant subtypes of Parkinson’s disease. PLoS ONE 2021, 16, e0245918. [Google Scholar] [CrossRef]
- Elfil, M.; Bahbah, E.I.; Attia, M.M.; Eldokmak, M.; Koo, B.B. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson’s Disease. Mov. Disord. 2021, 36, 570–580. [Google Scholar] [CrossRef]
- Strickland, D.; Bertoni, J.M. Parkinson’s prevalence estimated by a state registry. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Archibald, N.; Miller, N.; Rochester, L. Neurorehabilitation in Parkinson disease. Handb. Clin. Neurol. 2013, 110, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Shtilbans, A.; Henchcliffe, C. Biomarkers in Parkinson’s disease: An update. Curr. Opin. Neurol. 2012, 25, 460–465. [Google Scholar] [CrossRef]
- Kwan, L.C.; Whitehill, T.L. Perception of Speech by Individuals with Parkinson’s Disease: A Review. Parkinsons Disease 2011, 2011, 389767. [Google Scholar] [CrossRef]
- Ge, H.; Yan, Z.; Zhu, H.; Zhao, H. MiR-410 exerts neuroprotective effects in a cellular model of Parkinson’s disease induced by 6-hydroxydopamine via inhibiting the PTEN/AKT/mTOR signaling pathway. Exp. Mol. Pathol. 2019, 109, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Vivarelli, S.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Marchetti, B.; Iraci, N. microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 2698. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.J.; Murray, T.K.; Bengoa-Vergniory, N.; Cordero-Llana, O.; Cooper, J.; Buckley, A.; Wade-Martins, R.; Uney, J.B.; O’Neill, M.J.; Wong, L.F.; et al. Loss of MicroRNA-7 Regulation Leads to α-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Titze-de-Almeida, R.; Titze-de-Almeida, S.S. miR-7 Replacement Therapy in Parkinson’s Disease. Curr. Gene Ther. 2018, 18, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.F.; Qi, H.P.; Ma, C.; Chang, M.X.; Zhang, W.N.; Song, R.R. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci. Res. 2020. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget 2018, 9, 10388–10401. [Google Scholar] [CrossRef]
- Wang, C.C.; Shi, H.H.; Xu, J.; Yanagita, T.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Docosahexaenoic acid-acylated astaxanthin ester exhibits superior performance over non-esterified astaxanthin in preventing behavioral deficits coupled with apoptosis in MPTP-induced mice with Parkinson’s disease. Food Funct. 2020, 11, 8038–8050. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Chen, W.; Fu, X.T.; Ma, J.K.; Wang, M.H.; Hou, Y.J.; Tian, D.C.; Fu, X.Y.; Fan, C.D. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, X.; Huang, B.; Zhu, Y.; Chen, X. Astaxanthin suppresses MPP(+)-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar. Drugs 2013, 11, 1019–1034. [Google Scholar] [CrossRef]
- Ye, Q.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Astaxanthin protects against MPP(+)-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012, 13, 156. [Google Scholar] [CrossRef]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.L.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.; Liu, L.J.; Ramer, M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2017, 95, 1295–1306. [Google Scholar] [CrossRef]
- Lampert, A.; Hains, B.C.; Waxman, S.G. Upregulation of persistent and ramp sodium current in dorsal horn neurons after spinal cord injury. Exp. Brain Res. 2006, 174, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Saghaei, E.; Abbaszadeh, F.; Afhami, M.; Haeri, A.; Rahimi, F.; Jorjani, M. Role of microglia and astrocyte in central pain syndrome following electrolytic lesion at the spinothalamic tract in rats. J. Mol. Neurosci. Mn 2013, 49, 470–479. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, R.; Morreale, A.; Donadio, V.; Boriani, S.; Maraldi, N.; Plazzi, G.; Liguori, R. Neuropathic pain following spinal cord injury: What we know about mechanisms, assessment and management. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3257–3261. [Google Scholar]
- Finnerup, N.B.; Otto, M.; McQuay, H.J.; Jensen, T.S.; Sindrup, S.H. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain 2005, 118, 289–305. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, Q.; Liu, F.; Jing, C.; Ding, L.; Zhang, L.; Pang, C. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci. Lett. 2018, 662, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur. J. Pain 2019, 23, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res. Bull. 2018, 143, 217–224. [Google Scholar] [CrossRef]
- Marvizón, J.C.; McRoberts, J.A.; Ennes, H.S.; Song, B.; Wang, X.; Jinton, L.; Corneliussen, B.; Mayer, E.A. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J. Comp. Neurol. 2002, 446, 325–341. [Google Scholar] [CrossRef]
- Gorman, A.L.; Yu, C.G.; Ruenes, G.R.; Daniels, L.; Yezierski, R.P. Conditions affecting the onset, severity, and progression of a spontaneous pain-like behavior after excitotoxic spinal cord injury. J. Pain 2001, 2, 229–240. [Google Scholar] [CrossRef]
- Yu, C.G.; Fairbanks, C.A.; Wilcox, G.L.; Yezierski, R.P. Effects of agmatine, interleukin-10, and cyclosporin on spontaneous pain behavior after excitotoxic spinal cord injury in rats. J. Pain 2003, 4, 129–140. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog. Brain Res. 2007, 161, 195–203. [Google Scholar] [CrossRef]
- Ji, R.R.; Woolf, C.J. Neuronal plasticity and signal transduction in nociceptive neurons: Implications for the initiation and maintenance of pathological pain. Neurobiol. Dis. 2001, 8, 1–10. [Google Scholar] [CrossRef]
- Lerch, J.K.; Puga, D.A.; Bloom, O.; Popovich, P.G. Glucocorticoids and macrophage migration inhibitory factor (MIF) are neuroendocrine modulators of inflammation and neuropathic pain after spinal cord injury. Semin. Immunol. 2014, 26, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Baastrup, C.; Finnerup, N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 2008, 22, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, R.; Aihara, M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol. Vis. 2014, 20, 1796–1805. [Google Scholar]
- Ikonomidou, C.; Bosch, F.; Miksa, M.; Bittigau, P.; Vöckler, J.; Dikranian, K.; Tenkova, T.I.; Stefovska, V.; Turski, L.; Olney, J.W. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999, 283, 70–74. [Google Scholar] [CrossRef]
- Alexander, J.K.; Cox, G.M.; Tian, J.B.; Zha, A.M.; Wei, P.; Kigerl, K.A.; Reddy, M.K.; Dagia, N.M.; Sielecki, T.; Zhu, M.X.; et al. Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp. Neurol. 2012, 236, 351–362. [Google Scholar] [CrossRef]
- Islam, M.S.; Kanak, F.; Iqbal, M.A.; Islam, K.F.; Al-Mamun, A.; Uddin, M.S. Analyzing the Status of the Autism Spectrum Disorder Amid Children with Intellectual Disabilities in Bangladesh. Biomed. Pharmacol. J. 2018, 11, 689–701. [Google Scholar] [CrossRef]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Geier, M.R. Evidence of neurodegeneration in autism spectrum disorder. Transl. Neurodegener. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Kemper, T.L.; Bauman, M. Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Lee, M.; Martin-Ruiz, C.; Graham, A.; Court, J.; Jaros, E.; Perry, R.; Iversen, P.; Bauman, M.; Perry, E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain A J. Neurol. 2002, 125, 1483–1495. [Google Scholar] [CrossRef]
- Courchesne, E.; Pierce, K.; Schumann, C.M.; Redcay, E.; Buckwalter, J.A.; Kennedy, D.P.; Morgan, J. Mapping early brain development in autism. Neuron 2007, 56, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Sabit, H.; Tombuloglu, H.; Rehman, S.; Almandil, N.B.; Cevik, E.; Abdel-Ghany, S.; Rashwan, S.; Abasiyanik, M.F.; Yee Waye, M.M. Gut microbiota metabolites in autistic children: An epigenetic perspective. Heliyon 2021, 7, e06105. [Google Scholar] [CrossRef]
- Granot, E.; Kohen, R. Oxidative stress in childhood--in health and disease states. Clin. Nutr. 2004, 23, 3–11. [Google Scholar] [CrossRef]
- Evans, T.A.; Siedlak, S.L.; Lu, L.; Fu, X.; Wang, Z.; McGinnis, W.R.; Fakhoury, E.; Castellani, R.J.; Hazen, S.L.; Walsh, W.J. The autistic phenotype exhibits a remarkably localized modification of brain protein by products of free radical-induced lipid oxidation. Am. J. Biochem. Biotechnol. 2008, 4, 61–72. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.; Lipinski, B.; Windom, H.; Audhya, T.; McGinnis, W. Oxidative stress in autism: Elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008, 4, 73–84. [Google Scholar]
- Sajdel-Sulkowska, E.M.; Xu, M.; McGinnis, W.; Koibuchi, N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum 2011, 10, 43–48. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Valachovicova, M.; Mislanova, C.; Hudecova, Z.; Sustrova, M.; Ostatnikova, D. Plasma concentrations of selected antioxidants in autistic children and adolescents. Bratisl Lek Listy 2009, 110, 247–250. [Google Scholar] [PubMed]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Prevention or Amelioration of Autism-Like Symptoms in Animal Models: Will it Bring Us Closer to Treating Human ASD? Int. J. Mol. Sci. 2019, 20, 1074. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp. Biol. Med. (Maywood) 2018, 243, 613–620. [Google Scholar] [CrossRef]
- Fernández, M.J.F.; Valero-Cases, E.; Rincon-Frutos, L. Food Components with the Potential to be Used in the Therapeutic Approach of Mental Diseases. Curr. Pharm. Biotechnol. 2019, 20, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Rahman, M.M.; Khan, F.R.; Zaman, F.; Mahmud Reza, H. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav. Brain Res. 2015, 286, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhu, X.L.; Sun, M.H.; Dang, Y.K. Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling pathway in mice with focal cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, C.; Zhang, S.; Xu, Y. Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3β/Nrf2 signalling pathway in vitro. J. Cell. Mol. Med. 2020, 24, 8977–8985. [Google Scholar] [CrossRef] [PubMed]
- Nai, Y.; Liu, H.; Bi, X.; Gao, H.; Ren, C. Protective effect of astaxanthin on acute cerebral infarction in rats. Hum. Exp. Toxicol. 2018, 37, 929–936. [Google Scholar] [CrossRef]
- Cakir, E.; Cakir, U.; Tayman, C.; Turkmenoglu, T.T.; Gonel, A.; Turan, I.O. Favorable Effects of Astaxanthin on Brain Damage due to Ischemia- Reperfusion Injury. Comb. Chem. High Throughput Screen. 2020, 23, 214–224. [Google Scholar] [CrossRef]
- Carelli, S.; Giallongo, T.; Gombalova, Z.; Rey, F.; Gorio, M.C.F.; Mazza, M.; Di Giulio, A.M. Counteracting neuroinflammation in experimental Parkinson’s disease favors recovery of function: Effects of Er-NPCs administration. J. Neuroinflamm. 2018, 15, 333. [Google Scholar] [CrossRef]
- Stewart, J.S.; Lignell, A.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety assessment of astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 3030–3036. [Google Scholar] [CrossRef]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, N. Allergies. Scientific Opinion on the safety of astaxanthin-rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. EFSA J. 2014, 12, 3757. [Google Scholar]
- Additives, E.P.O.; Feed, P.O.S.U.I.A. Scientific Opinion on the safety and efficacy of synthetic astaxanthin as feed additive for salmon and trout, other fish, ornamental fish, crustaceans and ornamental birds. EFSA J. 2014, 12, 3724. [Google Scholar]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Kajita, M.; Kato, T.; Yoshimoto, T.; Masuda, K. Study on the safety of high-dose administration of astaxanthin. Folia Jpn. Ophthalmol. Clin. 2010, 3, 365–370. [Google Scholar]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine Int. J. Phytother. Phytopharm. 2008, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, T.; Ishibashi, T.; Kyle, D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicol. Rep. 2014, 1, 582–588. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, M.S.; Simal-Gandara, J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs 2021, 19, 201. https://doi.org/10.3390/md19040201
Bahbah EI, Ghozy S, Attia MS, Negida A, Emran TB, Mitra S, Albadrani GM, Abdel-Daim MM, Uddin MS, Simal-Gandara J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Marine Drugs. 2021; 19(4):201. https://doi.org/10.3390/md19040201
Chicago/Turabian StyleBahbah, Eshak I., Sherief Ghozy, Mohamed S. Attia, Ahmed Negida, Talha Bin Emran, Saikat Mitra, Ghadeer M. Albadrani, Mohamed M. Abdel-Daim, Md. Sahab Uddin, and Jesus Simal-Gandara. 2021. "Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent" Marine Drugs 19, no. 4: 201. https://doi.org/10.3390/md19040201
APA StyleBahbah, E. I., Ghozy, S., Attia, M. S., Negida, A., Emran, T. B., Mitra, S., Albadrani, G. M., Abdel-Daim, M. M., Uddin, M. S., & Simal-Gandara, J. (2021). Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Marine Drugs, 19(4), 201. https://doi.org/10.3390/md19040201









