Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of Fucoidan Extracts
2.2. Endotoxicity
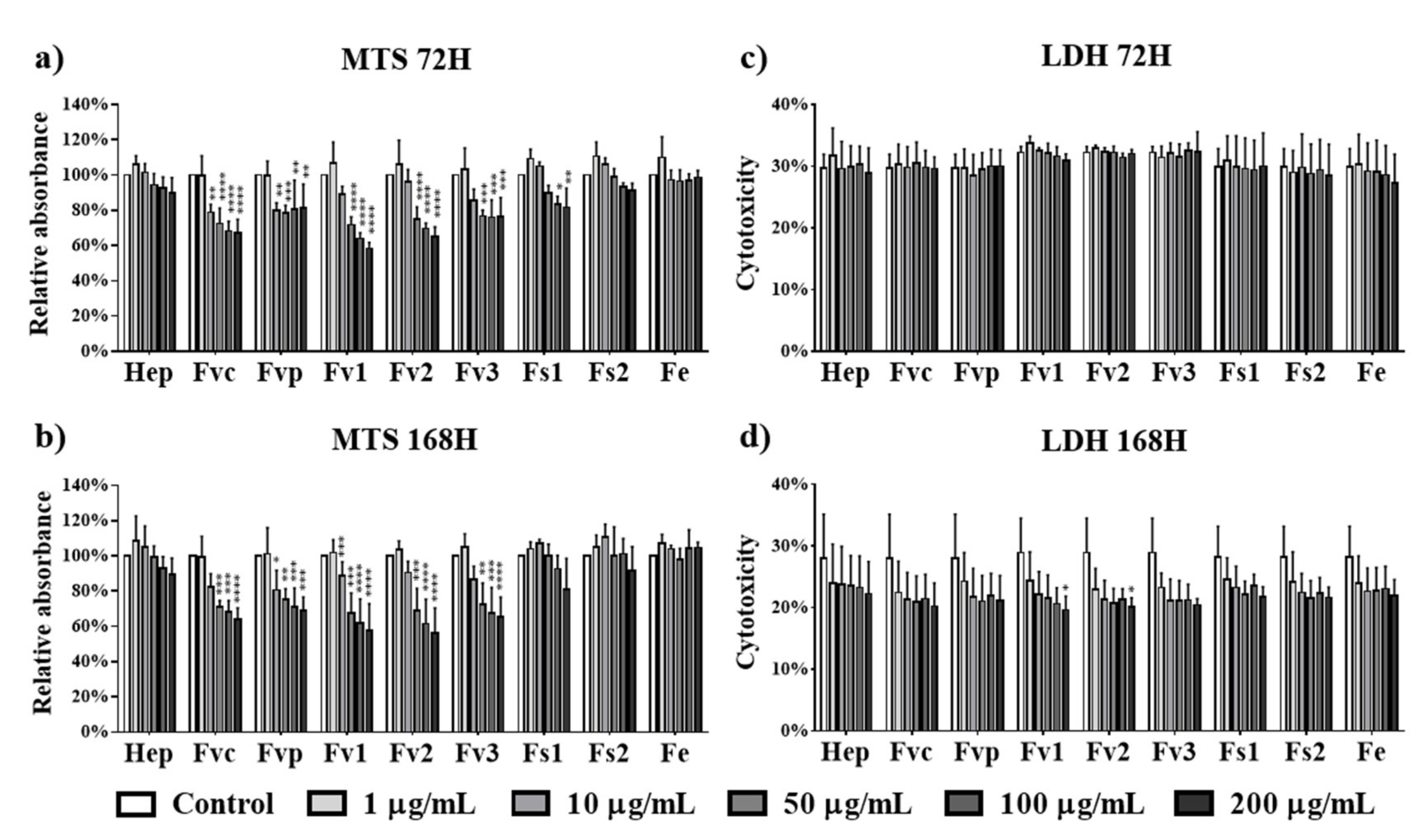
2.3. Effect of Fucus Extracts on Metabolic Activity and Cytotoxicity in Endothelial Cells
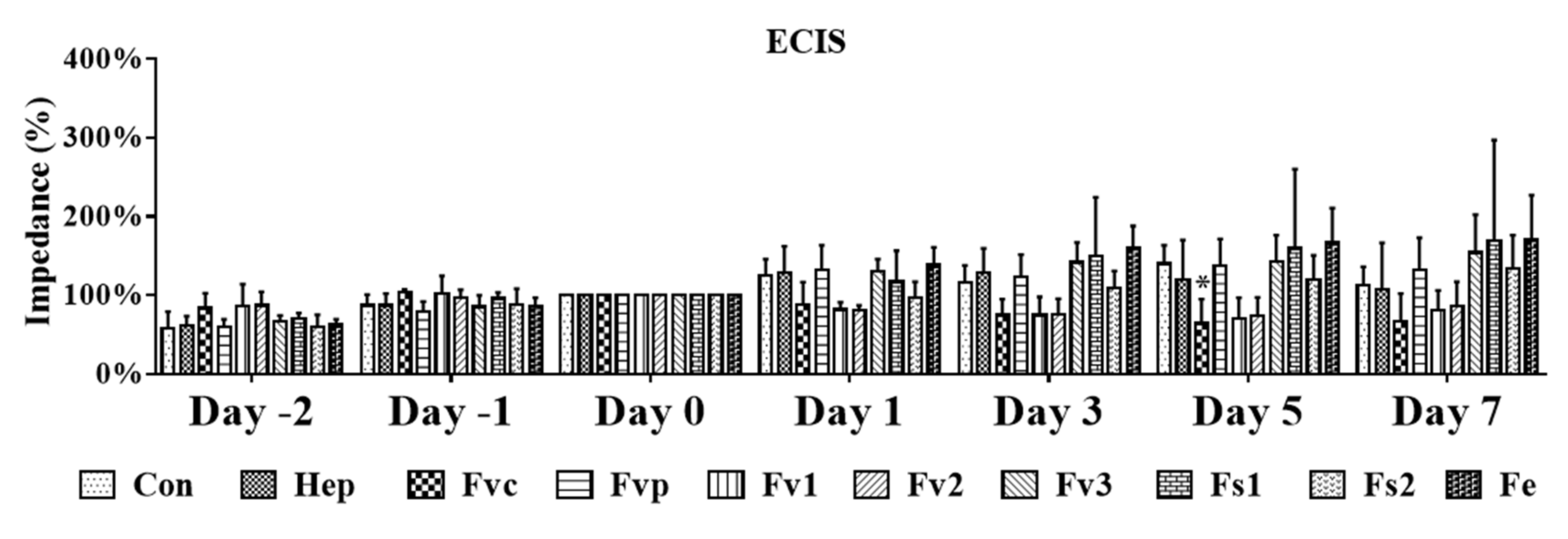
2.4. Impact of Fucus Extracts on the Endothelial Barrier
2.5. Effects of Fucus Extracts on Endothelial Activation and Inflammation
2.6. Effect of Fucus Extracts on Metabolic Activity and Cytotoxicity in Human Mesenchymal Stem Cells
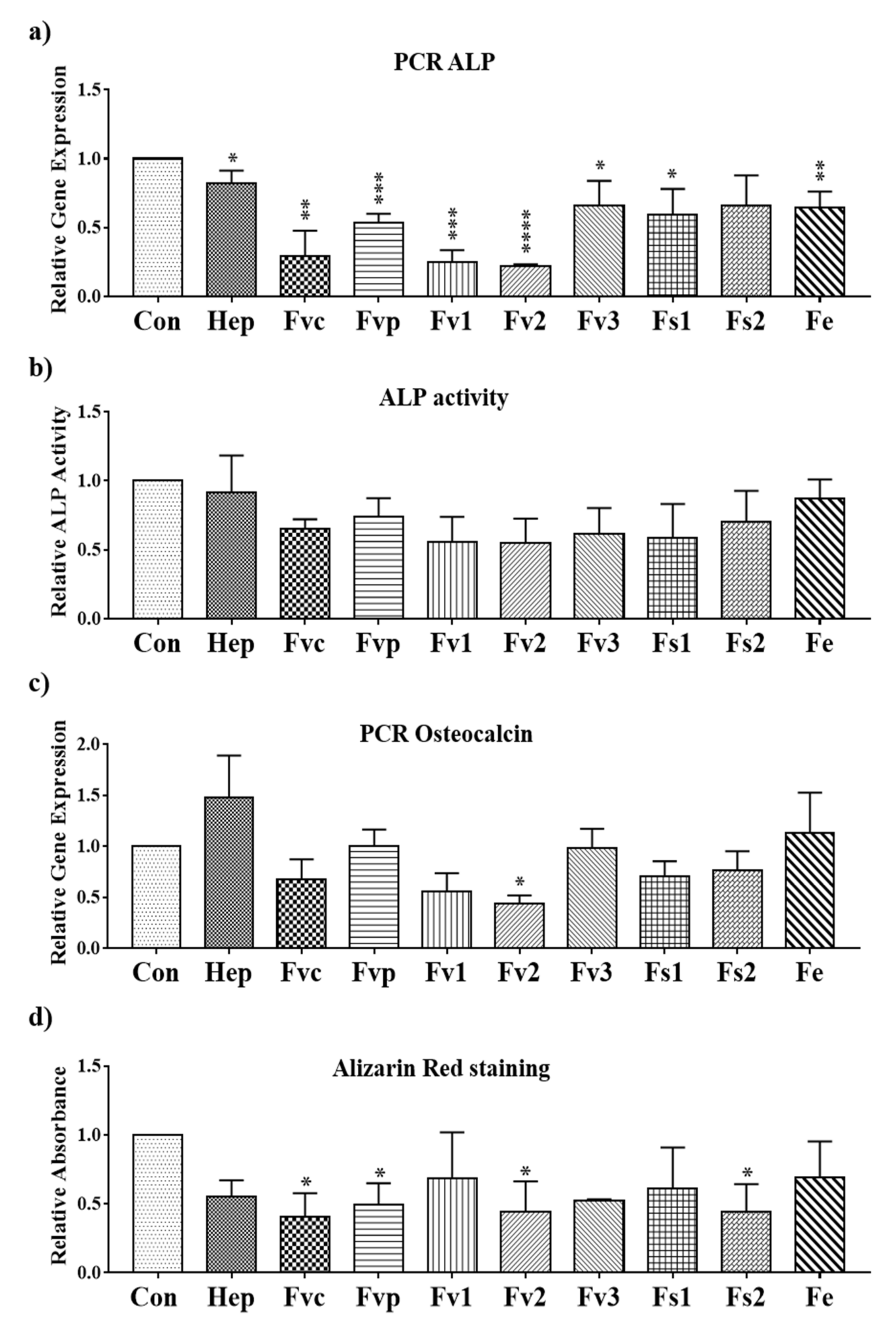
2.7. Impact of Fucus Extracts on the Osteogenic Activity in Human Mesenchymal Stem Cells
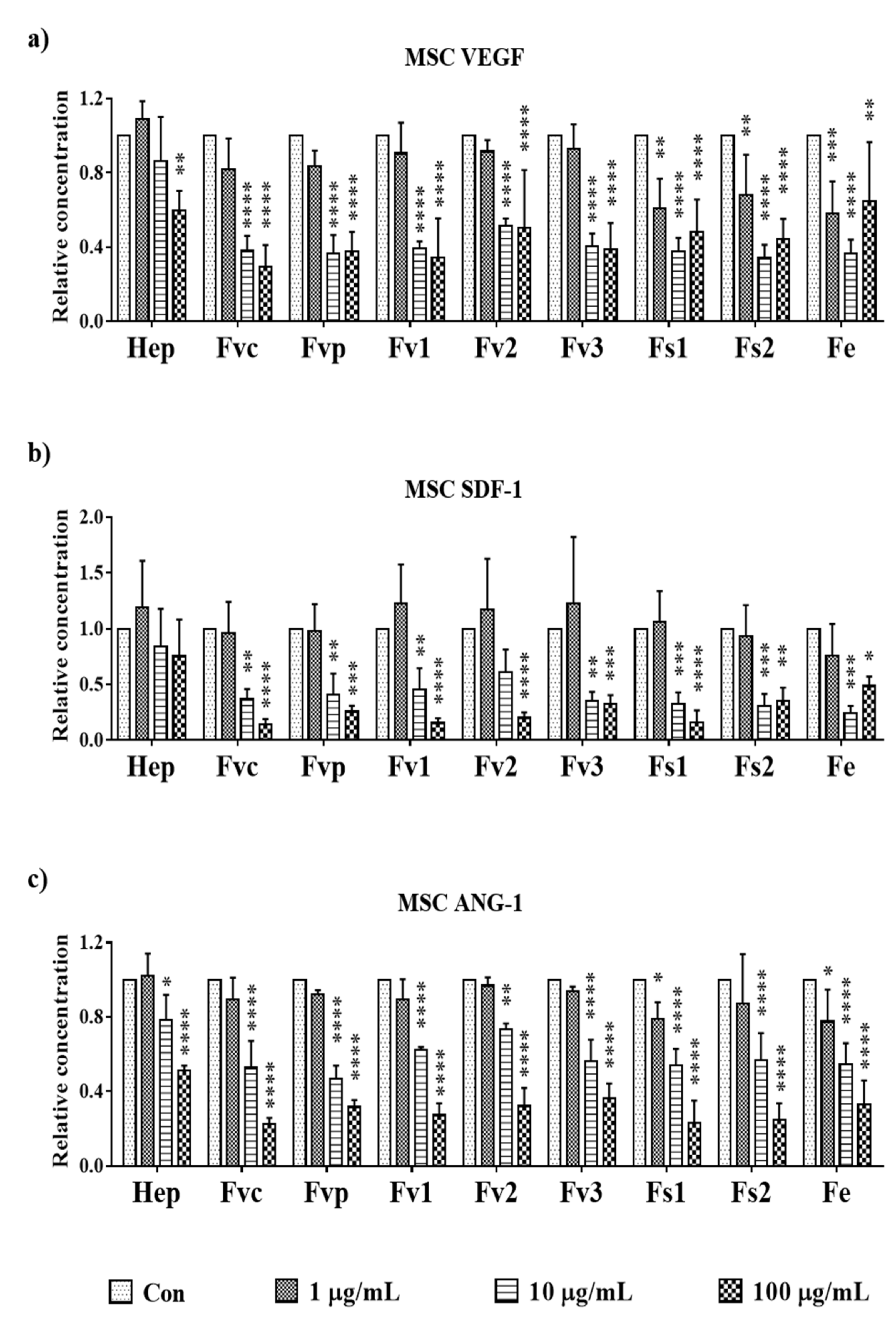
2.8. Effects of Fucus Extracts on Regulatory Molecules for Angiogenesis in MSC
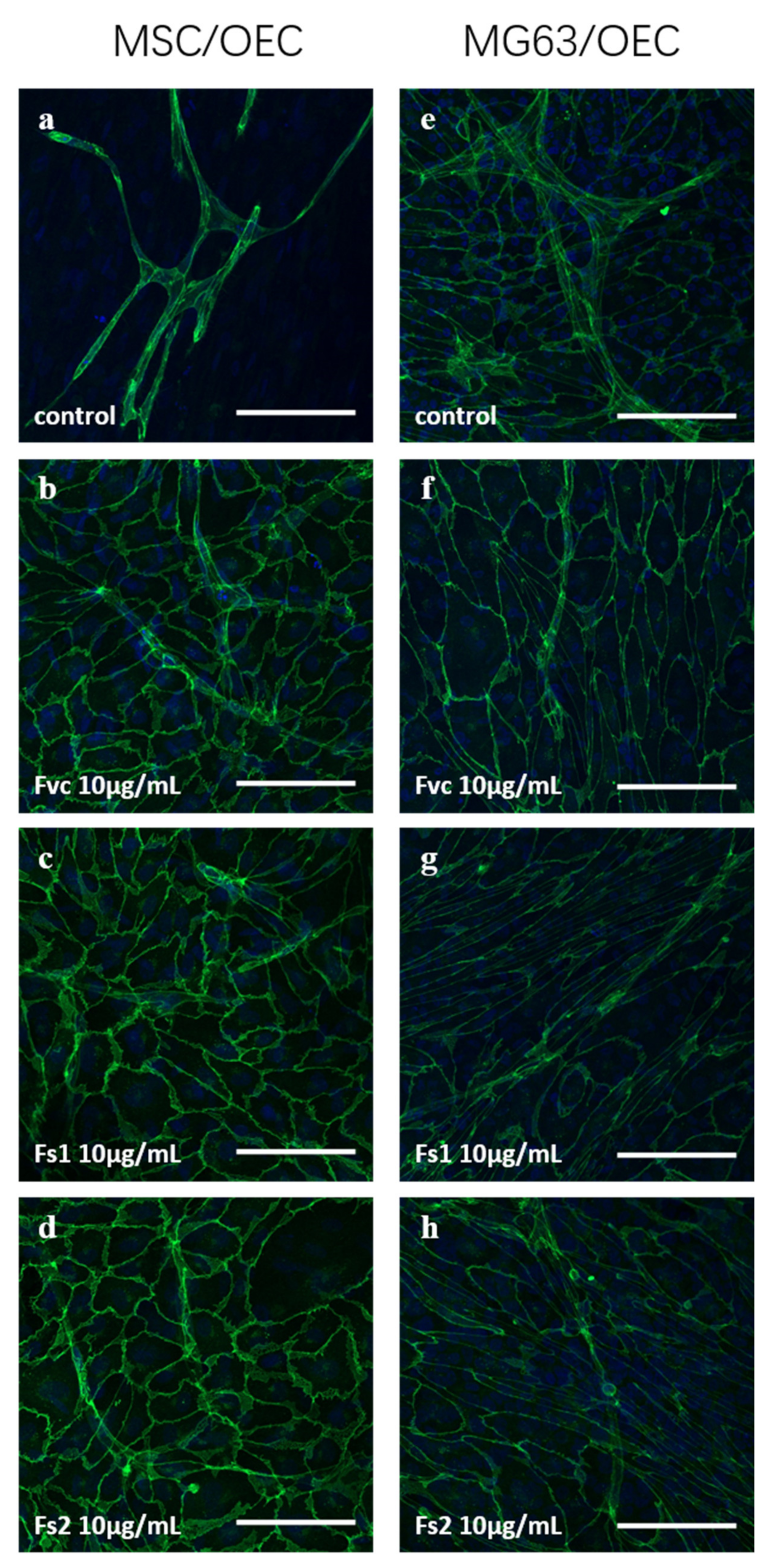
2.9. Influence of Selected Fucus Extracts on the Formation of Vascular Structures in MSC–OEC Co-Culture Models
3. Discussion
4. Materials and Methods
4.1. Fucoidan Extraction
4.2. Chemical Characterization
4.2.1. Elemental Analysis
4.2.2. Monosaccharide Composition by GLC
4.2.3. SEC-MALS-VIS Analysis
4.3. EndoLISA® Endotoxicity Assay
4.4. Ethical Approval for the Use of Human Cells
4.5. Isolation and Culture of Human Mesenchymal Stem Cells (MSCs)
4.6. Isolation and Culture of Human Outgrowth Endothelial Cells (OECs)
4.7. Cell Seeding and Fucoidan Treatment of Individual Cell Types
4.8. MTS Cell Metabolic Activity Assay
4.9. LDH Cytotoxicity Assay
4.10. Immunofluorescence Staining and Visualization of OECs
4.11. Electrical Cell-Substrate Impedance Sensing (ECIS) for MSC and OEC
4.12. Quantification of DNA Content
4.13. Gene Expression Analysis
4.14. Enzyme Linked Immunosorbent Assay (ELISA)
4.15. Quantitative Analysis of Osteogenesis
4.16. Alkaline Phosphatase (ALP) Activity Assay
4.17. Co-Cultures
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, P.; Cui, N.; Song, S.; Liang, H.; Ji, A. Stichopus japonicus Polysaccharide, Fucoidan, or Heparin Enhanced the SDF-1α/CXCR4 Axis and Promoted NSC Migration via Activation of the PI3K/Akt/FOXO3a Signaling Pathway. Cell Mol. Neurobiol. 2016, 36, 1311–1329. [Google Scholar] [CrossRef]
- Brandi, M.L.; Collin-Osdoby, P. Vascular biology and the skeleton. J. Bone Miner. Res. 2006, 21, 183–192. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process Biochem. 2019, 81, 182–187. [Google Scholar] [CrossRef]
- Ohmes, J.; Xiao, Y.; Wang, F.; Mikkelsen, M.D.; Nguyen, T.T.; Schmidt, H.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Mar. Drugs 2020, 18, 481. [Google Scholar] [CrossRef] [PubMed]
- Lahrsen, E.; Liewert, I.; Alban, S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydr. Polym. 2018, 192, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Bittkau, K.S.; Alban, S. Size distribution and chain conformation of six different fucoidans using size-exclusion chromatography with multiple detection. J. Chromatogr. A 2020, 1612, 460658. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Piconi, L.; Quagliaro, L.; Da Ros, R.; Assaloni, R.; Giugliano, D.; Esposito, K.; Szabo, C.; Ceriello, A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: The role of poly(ADP-ribose) polymerase. J. Thromb. Haemost 2004, 2, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Bayer, O.; Brunkhorst, F.; Meisner, M. Markers of endothelial damage in organ dysfunction and sepsis. Crit. Care Med. 2002, 30 (Suppl. 5), S302–S312. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Nakamura, T.; Kanetake, H.; Kanda, S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J. Cell Sci. 2002, 115, 175–183. [Google Scholar] [PubMed]
- Yamaguchi, J.; Kusano, K.F.; Masuo, O.; Kawamoto, A.; Silver, M.; Murasawa, S.; Bosch-Marce, M.; Masuda, H.; Losordo, D.W.; Isner, J.M.; et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003, 107, 1322–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liekens, S.; Schols, D.; Hatse, S. CXCL12-CXCR4 Axis in Angiogenesis, Metastasis and Stem Cell Mobilization. Curr. Pharm.. Des. 2010, 16, 3903–3920. [Google Scholar] [CrossRef]
- Kolbe, M.; Xiang, Z.; Dohle, E.; Tonak, M.; Kirkpatrick, C.J.; Fuchs, S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng. Part A 2011, 17, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kramer, G.; Schröder, A.; Kirkpatrick, C.J.; Seekamp, A.; Schmidt, H.; Fuchs, S. Early endothelial progenitor cells as a source of myeloid cells to improve the pre-vascularisation of bone constructs. Eur. Cell Mater. 2014, 27, 64–79. [Google Scholar] [CrossRef]
- Leal, D.; Mansilla, A.; Matsuhiro, B.; Moncada-Basualto, M.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; De Borggraeve, W.M. Chemical structure and biological properties of sulfated fucan from the sequential extraction of subAntarctic Lessonia sp (Phaeophyceae). Carbohydr. Polym. 2018, 199, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Lahrsen, E.; Schoenfeld, A.K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lahrsen, E.; Schoenfeld, A.K.; Alban, S. Degradation of Eight Sulfated Polysaccharides Extracted from Red and Brown Algae and Its Impact on Structure and Pharmacological Activities. ACS Biomater. Sci. Eng. 2019, 5, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2012, 24, 715–723. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. [Google Scholar] [CrossRef]
- Reijers, J.A.A.; Malone, K.E.; Bajramovic, J.J.; Verbeek, R.; Burggraaf, J.; Moerland, M. Adverse immunostimulation caused by impurities: The dark side of biopharmaceuticals. Br. J. Clin. Pharmacol. 2019, 85, 1418–1426. [Google Scholar] [CrossRef]
- Burrell, R. Human responses to bacterial endotoxin. Circ. Shock 1994, 43, 137–153. [Google Scholar] [PubMed]
- Janga, H.; Cassidy, L.; Wang, F.; Spengler, D.; Oestern-Fitschen, S.; Krause, M.F.; Seekamp, A.; Tholey, A.; Fuchs, S. Site-specific and endothelial-mediated dysfunction of the alveolar-capillary barrier in response to lipopolysaccharides. J. Cell Mol. Med. 2018, 22, 982–998. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Alvira, C.M.; Cornfield, D.N. Developmental differences in focal adhesion kinase expression modulate pulmonary endothelial barrier function in response to inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L66–L77. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Midy, V.; Plouet, J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem. Biophys. Res. Commun. 1994, 199, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Lowik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef]
- Boisson-Vidal, C.; Zemani, F.; Caligiuri, G.; Galy-Fauroux, I.; Colliec-Jouault, S.; Helley, D.; Fischer, A.M. Neoangiogenesis induced by progenitor endothelial cells: Effect of fucoidan from marine algae. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Dithmer, M.; Fuchs, S.; Shi, Y.; Schmidt, H.; Richert, E.; Roider, J.; Klettner, A. Fucoidan reduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PLoS ONE 2014, 9, e89150. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Hakanpaa, L.; Sipila, T.; Leppanen, V.M.; Gautam, P.; Nurmi, H.; Jacquemet, G.; Eklund, L.; Ivaska, J.; Alitalo, K.; Saharinen, P. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat. Commun. 2015, 6, 5962. [Google Scholar] [CrossRef]
- Tait, C.R.; Jones, P.F. Angiopoietins in tumours: The angiogenic switch. J. Pathol. 2004, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eman, R.M.; Öner, F.C.; Kruyt, M.C.; Dhert, W.J.A.; Alblas, J. Stromal Cell-Derived Factor-1 Stimulates Cell Recruitment, Vascularization and Osteogenic Differentiation. Tissue Eng. Part A 2013, 20, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, R.; Oppenheim, J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003, 10, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lv, F.; Zhang, J.; Li, H. SDF-1 Expression is Associated with Poor Prognosis in Osteosarcoma. Ann. Clin. Lab. Sci. 2016, 46, 508–514. [Google Scholar]
- Benslimane-Ahmim, Z.; Pereira, J.; Lokajczyk, A.; Dizier, B.; Galy-Fauroux, I.; Fischer, A.M.; Heymann, D.; Boisson-Vidal, C. Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR4 axis and promotes tumour development by increasing neovascularization. Cancer Lett. 2017, 395, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Hofmann, A.; Kirkpatrick, C. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007, 13, 2577–2588. [Google Scholar] [CrossRef]
- Gupta, D.; Silva, M.; Radziun, K.; Martinez, D.C.; Hill, C.J.; Marshall, J.; Hearnden, V.; Puertas-Mejia, M.A.; Reilly, G.C. Fucoidan Inhibition of Osteosarcoma Cells Is Species and Molecular Weight Dependent. Mar. Drugs 2020, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Ptak, S.H.; Christensen, K.V.; Meichßner, R.; Fretté, X. Improving fucoidan yield from fucus brown algae by microwave extraction. Chem. Eng. 2019, 74, 109–114. [Google Scholar]
- Albersheim, P.; Nevins, D.J.; English, P.D.; Karr, A. A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Fuchs, S.; Hermanns, M.I.; Kirkpatrick, C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006, 326, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, M.; Dohle, E.; Katerla, D.; Kirkpatrick, C.J.; Fuchs, S. Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng. Part C Methods 2010, 16, 877–886. [Google Scholar] [CrossRef] [Green Version]




| Monosaccharide Composition (mol%) a | Degree of Sulfation b DS | Protein Content c (%) | Mw (kDa) | Rms Radius (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Xyl | Gal | Glc | |||||
| Fv1 | 50.2 | 9.5 | 7.9 | 27.7 | 0.28 | 1.88 | 449 | 35 |
| Fv2 | 57.7 | 18.5 | 13.2 | 2.9 | 0.26 | 1.08 | 730 | 33 |
| Fv3 | 81.4 | 8.5 | 6.3 | 0.7 | 0.35 | 0.33 | 173 | 24 |
| Fe | 76.7 | 9.8 | 5.7 | 0.0 | 0.41 | 2.18 | 84 | 21 |
| Fs1 | 76.2 | 6.5 | 3.3 | 11.2 | 0.61 | 0.52 | 272 | 41 |
| Fs2 | 56.1 | 4.1 | 5.0 | 32.6 | 0.32 | 0.51 | 172 | 36 |
| Fucoidan Extracts/Heparin | Shortage | Endotoxicity (EU/mL) |
|---|---|---|
| Heparin (for physico-chemical analysis, Y0001282) | Hep. | 0.0778 |
| Fucoidan from Fucus vesiculosus crude (Fvc, Sigma, F5631-1G) | Fvc | 0.0746 |
| Fucoidan from Fucus vesiculosus pure (Fvp,Sigma, F8190) | Fvp | 0.0750 |
| Fv_KF_7-7-2017_SDU_24H_M1-0.1-HCL-22C_frac1 | Fv1 | 0.0059 |
| Fv_KF_10-10-17_SDU_24H_M1-22C_frac3 | Fv2 | 0.0206 |
| Fv_KF_170707_SDU_180405_M313D0.2 | Fv3 | 0.0743 |
| Fs_KF_171010_SDU_180501_M342D0.2 | Fs1 | 0.0743 |
| Fs_KF_171010_SDU_180405_M331D0.2 | Fs2 | 0.0743 |
| Fe_KF_170707_SDU_180405_M313D0.2 | Fe | 0.0732 |
| Ex. | Species | Harvest | Extracted | ||
|---|---|---|---|---|---|
| in | at | for | |||
| Fv1 | F. vesiculosus | July | 100 mM hydrochloric acid | RT | 24 h |
| Fv2 | F. vesiculosus | October | 100 mM hydrochloric acid × 3 | RT | 24 h |
| Fv3 | F. vesiculosus | October | demineralized water * | 120 °C | 30 min |
| Fe | F. evanescens | July | demineralized water * | 120 °C | 30 min |
| Fs1 | F. serratus | October | 10 mM sulfuric acid * | 100 °C | 30 min |
| Fs2 | F. serratus | October | 100 mM hydrochloric acid * | 80 °C | 30 min |
| Gene Name | Primer Assay | Catalogue No. |
|---|---|---|
| ALP | Hs_ALPL_1_SG QuantiTect Primer Assay | QT00012957 |
| Angiopoietin 1 | Hs_ANGPT1_1_SG QuantiTect Primer Assay | QT00046865 |
| Angiopoietin 2 | Hs_ANGPT2_1_SG QuantiTect Primer Assay | QT00100947 |
| Osteocalcin | Hs_BGLAP_1_SG QuantiTect Primer Assay | QT00232771 |
| ICAM | Hs_ICAM1_1_SG QuantiTect Primer Assay | QT00074900 |
| IL-6 | Hs_IL6_1_SG QuantiTect Primer Assay | QT00083720 |
| SDF-1 | Hs_CXCL12_1_SG QuantiTect Primer Assay | QT00087591 |
| VCAM-1 | Hs_VCAM1_1_SG QuantiTect Primer Assay | QT00018347 |
| VEGF | Hs_VEGFA_2_SG QuantiTech Primer Assay | QT01036861 |
| RPL13A | Hs_RPL13A_1_SG QuantiTect Primer Assay | QT00089915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Xiao, Y.; Neupane, S.; Ptak, S.H.; Römer, R.; Xiong, J.; Ohmes, J.; Seekamp, A.; Fretté, X.; Alban, S.; et al. Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization. Mar. Drugs 2021, 19, 194. https://doi.org/10.3390/md19040194
Wang F, Xiao Y, Neupane S, Ptak SH, Römer R, Xiong J, Ohmes J, Seekamp A, Fretté X, Alban S, et al. Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization. Marine Drugs. 2021; 19(4):194. https://doi.org/10.3390/md19040194
Chicago/Turabian StyleWang, Fanlu, Yuejun Xiao, Sandesh Neupane, Signe Helle Ptak, Ramona Römer, Junyu Xiong, Julia Ohmes, Andreas Seekamp, Xavier Fretté, Susanne Alban, and et al. 2021. "Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization" Marine Drugs 19, no. 4: 194. https://doi.org/10.3390/md19040194
APA StyleWang, F., Xiao, Y., Neupane, S., Ptak, S. H., Römer, R., Xiong, J., Ohmes, J., Seekamp, A., Fretté, X., Alban, S., & Fuchs, S. (2021). Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization. Marine Drugs, 19(4), 194. https://doi.org/10.3390/md19040194






