Abstract
Indonesia is one of the most biodiverse countries in the world and a promising resource for novel natural compound producers. Actinomycetes produce about two thirds of all clinically used antibiotics. Thus, exploiting Indonesia’s microbial diversity for actinomycetes may lead to the discovery of novel antibiotics. A total of 422 actinomycete strains were isolated from three different unique areas in Indonesia and tested for their antimicrobial activity. Nine potent bioactive strains were prioritized for further drug screening approaches. The nine strains were cultivated in different solid and liquid media, and a combination of genome mining analysis and mass spectrometry (MS)-based molecular networking was employed to identify potential novel compounds. By correlating secondary metabolite gene cluster data with MS-based molecular networking results, we identified several gene cluster-encoded biosynthetic products from the nine strains, including naphthyridinomycin, amicetin, echinomycin, tirandamycin, antimycin, and desferrioxamine B. Moreover, 16 putative ion clusters and numerous gene clusters were detected that could not be associated with any known compound, indicating that the strains can produce novel secondary metabolites. Our results demonstrate that sampling of actinomycetes from unique and biodiversity-rich habitats, such as Indonesia, along with a combination of gene cluster networking and molecular networking approaches, accelerates natural product identification.
1. Introduction
It is now 80 years ago that Selman Waksman and Boyd Woodruff discovered actinomycin from Actinomyces (Streptomyces) antibioticus, which was the first antibiotic that was isolated from an actinomycete [1]. Since then, actinomycetes have been widely used as sources for drug discovery and development [2]. Most antibiotics and other useful natural products applied in human medicine, veterinary, and agriculture are derived from these filamentous bacteria [3,4]. Within the family of Actinomycetales, Streptomyces is the most prominent genus in respect to the production of bioactive secondary metabolites since it is the origin of more than 50% of all clinically useful antibiotics [5]. Successfully, the intensive screening campaigns of soil-derived streptomycetes yielded many currently recognized drugs, such as the antibacterial substance streptomycin, the antifungal metabolite nystatin, and the anticancer compound doxorubicin during the golden era of antibiotics [6,7]. However, in the last few decades, discovering and developing new drugs from these soil microorganisms has declined immensely, while the need for new drugs to overcome multidrug resistance has become greater than ever [8]. Nowadays, one of the major problems in antibiotic screening programs, in particular with streptomycetes, is the high rediscovery rate of already-known antibacterial compounds through the classical bioactivity-guided paradigms [3].
Sampling actinomycetes from conventional environments such as soils often leads to the rediscovery of known species producing already-known antibiotics [9]. Thus, gaining access to unusual unique habitats with the pursuit to isolate new strains as sources of novel bioactive compounds represents a current barrier in drug discovery research [9]. In recent years, the bioprospection of underexplored niches such as extreme or marine environments has become an efficient approach to find novel Streptomyces species that might produce novel compounds [10,11]. S. asenjonii strain KNN 42.f, isolated from a desert soil sample, is one example of a novel Streptomyces species from an extreme habitat, which produces the three new bioactive compounds asenjonamides A–C [12]. Another example displays the marine S. zhaozhouensis CA-185989 that produces three new bioactive polycyclic tetramic acid macrolactams [13]. Micromonospora sp. as turbinimicin producer represents a further example of prolific marine bacteria that can deliver new antifungal compounds [14]. These are only a few examples demonstrating that unusual or aquatic territories can be promising avenues as new natural products reservoirs.
Indonesia is the world’s largest archipelagic country, spanning into three time zones, covering more than 17,000 islands, with 88,495,000 hectares of tropical forest, 86,700 square kilometers of coral reefs, and 24,300 square kilometers of mangrove areas [15,16]. It has the second-highest level of terrestrial biodiversity globally after Brazil [17], while being ranked as first if marine diversity is taken into account [16,17]. With the given species-rich flora and fauna besides endemic and ecologically adapted species, mega biodiversity of microbial species is gratifyingly represented across various unique habitats [18,19,20], such as acidic hot springs [21], peatland forests [22], the Thousand Islands reef complex [23], Enggano Island [24], fish species [25], and leaves of traditional medicinal plants [26]. Thus, since unique Indonesian niches are expected to deliver untapped potential actinomycetal strains that may produce novel bioactive secondary metabolites, different locations were targeted for the sampling of actinomycetes in this study.
The latest analyses of genome sequence data from actinomycetes revealed a remarkable discrepancy between the genetic potential of the secondary metabolism, known to be encoded by biosynthetic gene clusters (BGCs), and the actual natural compound production capacity of such isolates, upon their growth under standard laboratory conditions. This is attributed to the fact that numerous BGCs are not expressed under conventional lab parameters and occur as so-called “silent” or “cryptic” BGCs [27]. The activation of these silent clusters allows one to unlock the chemical diversity of the tested organisms and enables the discovery of new molecules for medical and biotechnological purposes [28]. Thus, several efforts, e.g., involving genetic and cultivation methods, are employed to activate the expression of silent gene clusters [29]. One cultivation-based approach to exploit the metabolic capacity of the natural compound producers is the “one strain many compounds (OSMAC)” strategy [3,28,30]. Such a strategy simply relies on the variation of media compositions as a basis to test for different natural compound production profiles since global changes in the specialized metabolic pathways can occur under variable cultivation conditions [31]. The OSMAC concept represents a well-established model that was suggested nearly two decades ago; however, it still leads to the discovery of new chemotypes, such as the novel aromatic polyketide lugdunomycin from Streptomyces sp. QL37 [32] or an eudesmane sesquiterpenoid and a new homolog of the Virginiae Butanolides (VB-E) from from Lentzea violacea strain AS 08 [33]. Along the lines of the OSMAC concept, an elicitor screening approach has recently been suggested, which intends to mimic natural trigger molecules that can induce the biosynthesis of formerly unknown metabolites. This format has been conducted in a high-throughput approach and was coupled with MALDI-MS analysis. In the case of S. ghanaensis, this strategy led to the discovery of the antibiotically active depsipeptide cinnapetide [34].
Besides the variable trials to elicit the BGCs via pleiotropic approaches, a mass spectrometry dereplication step is frequently included in the current screening programs to address the formerly stated challenge of the high rediscovery rate prior to the tedious screening, isolation, and purification processes [35,36,37]. The utility of such a platform is to pinpoint known compounds in the initial phase of the discovery pipeline and leverage the process of finding new drugs. Integrated genomic and metabolomic mining methods have proven as an efficient dereplication strategy for compound identification in recent years [38,39,40,41]. While genome mining involves the identification of putative BGCs based on the genome sequences of the natural compound producers [42,43] using in silico bioinformatics tools such as antiSMASH [44], metabolome mining encompasses sorting out the chemical compounds in extracts of natural compound producers via their mass fragmentation patterns. Counting on the fact that metabolites with a similar chemical architecture tend to generate similar mass fragmentation patterns in mass spectrometry (MS) analysis, the implementation of the computational platform Global Natural Product Social (GNPS) to group the structurally related entities, often derive from a common biosynthetic origin, as a connected set of a molecular family cluster is an overgrowing necessity [45]. Such a platform iteratively proves its effectiveness to arrange seamlessly large numbers of samples enabling dereplication and tentative structural identification and/or classification [46]. The combinatorial employment of both computational tools side by side empowers the rapid identification of new substances, which can be highlighted by discovering the antibacterial substance thiomarinol from Pseudoalteromonas luteoviolacea [38] and microviridin 1777, a chymotrypsin inhibitor from M. aeruginosa EAWAG 127a [47].
Taken all together with the promises that highly biodiverse habitats can offer in synergy with an effective and practical mining technique, this study aimed to characterize the secondary metabolomes of selected actinomycetes isolated from three different locations within Indonesia. A collection of 422 actinomycetes from Lombok, Bali, and Enggano Islands were sampled and preliminary filtered with different bioactivity tests, where nine actinomycetes with the most bioactive potential were nominated for a hybrid genome mining and molecular networking approach in order to assess their biosynthetic capacity for the production of novel natural compounds.
2. Results and Discussion
2.1. Isolation and Characterization of Indonesian Actinomycetes
To isolate actinomycetes, soil samples were collected from two specific habitats (terrestrial and marine) in three different geographic areas of Indonesia using standard isolation protocols [48,49,50,51,52]. Enggano Island was chosen as a sampling location for terrestrial habitats since it is a pristine island with many endemic species and high biodiversity [53,54], whereas Bali and Lombok Island were selected as sampling sites for marine habitats resulting in 422 strains in total (Table 1). Among all sampling locations, the Enggano Island soil samples contributed to the highest number of actinomycetes isolates (56.2%), followed by sediment samples from Lombok (37.2%) and Bali island (6.6%).

Table 1.
Indonesian strains, isolation method, source of isolation (compare Figure 1), and most closely related species (%) based on 16S rRNA gene sequence phylogenetic analysis with EzTaxon.
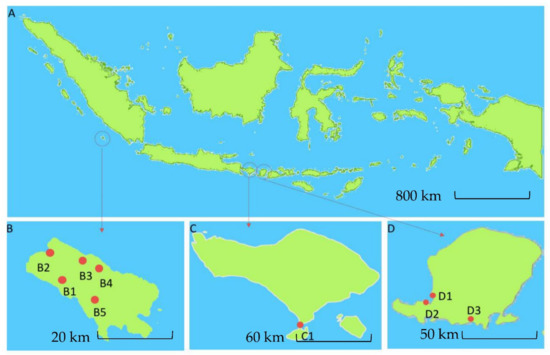
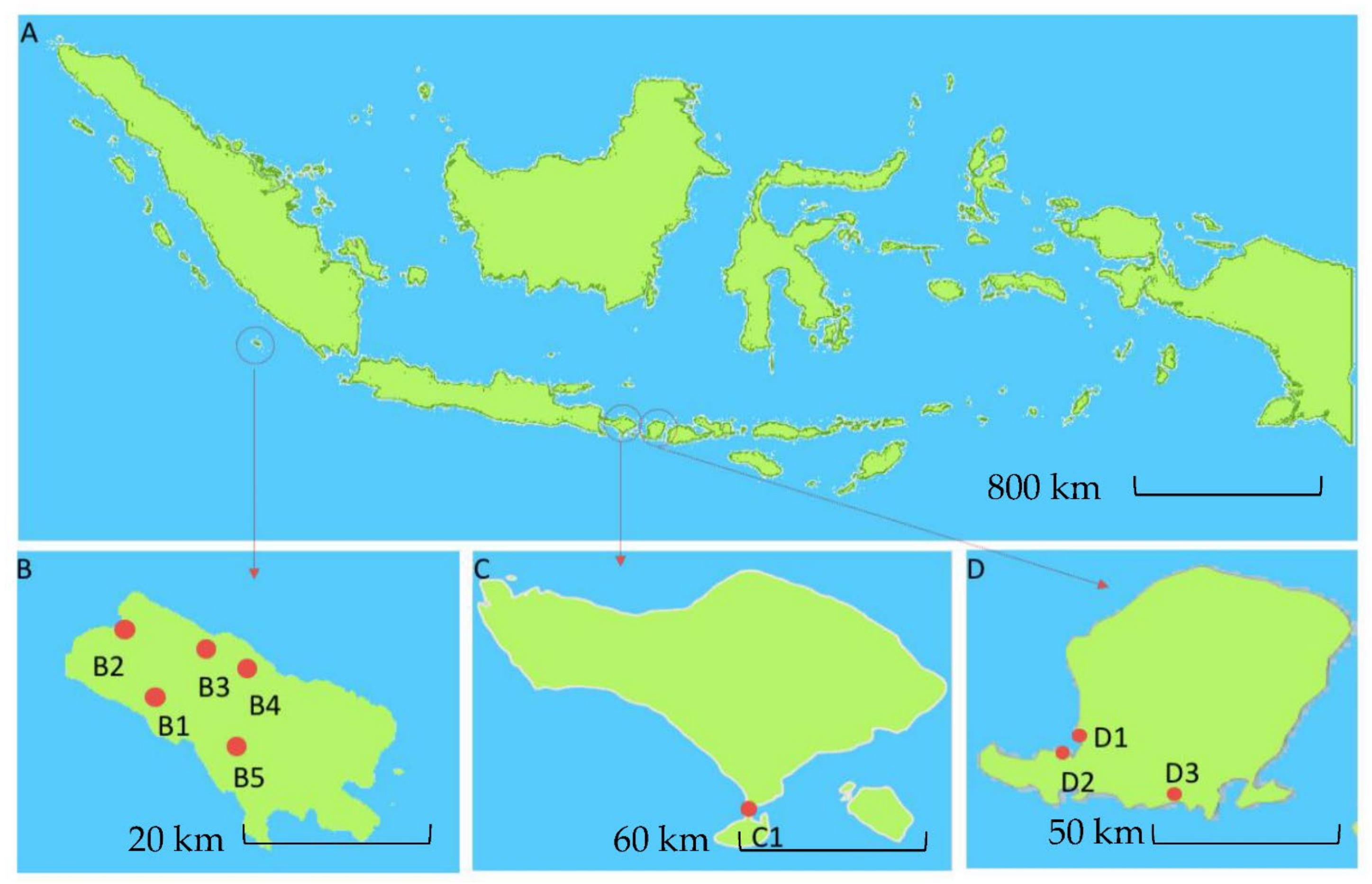
Figure 1.
Map of Indonesia showing three geographical regions (A). Sampling site location in Enggano Island (B), Bali Island (C), and Lombok Island (D). Red dot shows the sampling locations at Enggano Island, B1: Desa Meok; B2: Desa Banjar Sari; B3: Bak Blau Waterspring, Desa Meok; B4: Desa Boboyo; B5: Desa Malakoni; at Bali Island C1 for Kuta; and Lombok Island D1: Pantai Cemara, Lembar; D2: Pantai Tanjung Kelor, Sekotong; D3: Pantai Koeta.
Table 1.
Indonesian strains, isolation method, source of isolation (compare Figure 1), and most closely related species (%) based on 16S rRNA gene sequence phylogenetic analysis with EzTaxon.
| Strain (Streptomyces sp.) | Isolation Method | Source of Isolation | Most Closely Related Species Based on 16S rDNA Analysis |
|---|---|---|---|
| SHP 22-7 | phenol | Soil under a ketapang tree (Terminalia catappa) from Desa Meok (B1), Enggano Island | Streptomyces rochei NBRC 12908T (99.59%) |
| SHP 20-4 | phenol | Soil under a kina tree (Cinchona sp.), Desa Banjarsari (B2), Enggano Island | Streptomyces hydrogenans NBRC 12908T (99.68%) |
| SHP 2-1 | phenol | Soil under a hiyeb tree (Artocarpus elastica) near Bak Blau water spring, Desa Meok (B3), Enggano Island | Streptomyces griseoluteus NBRC 13375T (98.96%) |
| DHE 17-7 | dry heat | Soil under a ficus tree (Ficus sp.), Desa Boboyo (B4), Enggano Island | Streptomyces lannensis TA4-8T (99.78%) |
| DHE 12-3 | dry heat | Soil under a cempedak tree (Artocarpus integer), Desa Boboyo (B4), Enggano Island | Streptomyces coerulescens ISP 51446T (98.87%) |
| DHE 7-1 | dry heat | Soil under a terok tree (Artocarpus elastica), desa Boboyo (B4), Enggano Island | Streptomyces adustus WH-9T (99.59%) |
| DHE 6-7 | dry heat | Soil under forest snake fruit tree (Salacca sp.), Desa Malakoni (B5), Enggano Island | Streptomyces parvulus NBRC 13193T (98.55%) |
| DHE 5-1 | dry heat | Soil under a banana tree (Musa sp.), Desa Banjar sari (B2), Enggano Island | Streptomyces parvulus NBRC 13193T (99.79%) |
| BSE 7-9 | NBRC medium 802 | Mangrove sediment near plant rhizosphere, Kuta (C1), Bali Island | Streptomyces bellus ISP 5185T (99.06%) |
| BSE 7F | NBRC medium 802 | Mangrove sediment near plant rhizosphere, Kuta (C1), Bali Island | Streptomyces matensis NBRC 12889T (99.72%) |
| I3 | humic acid-vitamin + chlorine 1% | Mangrove sediment from Pantai Tanjung Kelor, Sekotong (D2), West Lombok Island | Streptomyces longispororuber NBRC 13488T (99.23%) |
| I4 | humic acid-vitamin + chlorine 1% | Mangrove sediment from Pantai Tanjung Kelor, Sekotong (D2), West Lombok Island | Streptomyces griseoincarnatus LMG 19316T (99.89%) |
| I5 | humic acid-vitamin + chlorine 1% | Mangrove sediment from Pantai Tanjung Kelor, Sekotong (D2), West Lombok Island | Streptomyces viridodiasticus NBRC13106T (99.31%) |
| I6 | humic acid-vitamin + chlorine 1% | Mangrove sediment from Pantai Tanjung Kelor, Sekotong (D2), West Lombok Island | Streptomyces spongiicola HNM0071T (99.78%) |
| I8 | humic acid-vitamin | Sea sands from Pantai Koeta (D3), Lombok Island | Streptomyces smyrnaeus SM3501T (98.44%) |
| I9 | humic acid-vitamin | Sea sands from Pantai Koeta (D3), Lombok Island | Streptomyces gancidicus NBRC 15412T (98.82%) |
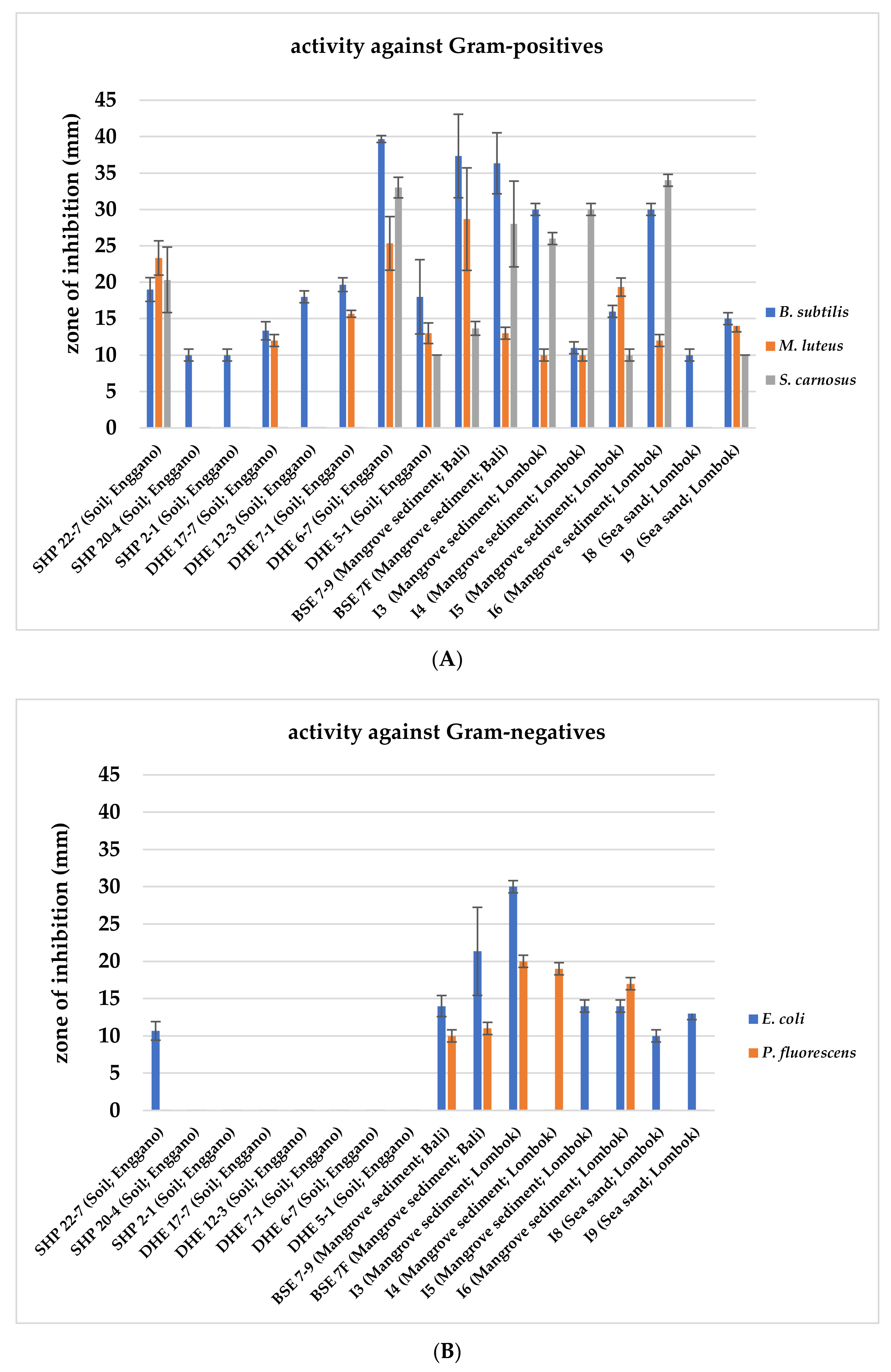
Within the frame of a preliminary bioactivity screening, all 422 isolates were evaluated for their antimicrobial activities in agar plug diffusion bioassays against selected Gram-positive (Bacillus subtilis, Micrococcus luteus, and Staphylococcus carnosus) and Gram-negative bacteria (Escherichia coli and Pseudomonas fluorescens). The 16 most potent isolates were selected based on their antimicrobial activity against the tested organisms, indicated by the largest inhibition zones around the agar plug. All 16 isolates showed bioactivity against the Gram-positive test organism B. subtilis (Figure 2A), and nine exerted further activity against Gram-negative test strains (Figure 2B), while only four strains (BSE 7–9, BSE 7F, I3, and I6) displayed potency against both (Figure 2).
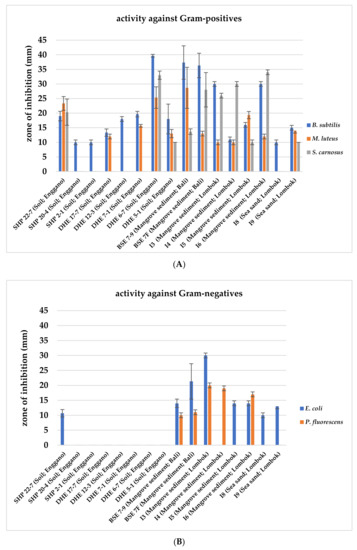
Figure 2.
Antimicrobial bioassays with 16 Indonesian actinomycetes strain samples against Gram-positive (A) and Gram-negative test strains (B). Inhibition zone diameters of agar plug test assays are given in mm. Agar plugs were used after ten days of growth of the respective actinomycetes strains. Data shown are as the result of three independent biological replicates.
To investigate the phylogenetic relationship of the 16 bioactive actinomycetal isolates, 16S rRNA gene sequence analyses were performed. For this purpose, the genomic DNA was isolated from each and was used as a template in a PCR approach with 16S rRNA gene-specific primers. The resulting 16S rRNA gene amplifications were sequenced, and the 16S rRNA gene sequences were compared using the EzTaxon database (www.ezbiocloud.net/, accessed on 28 May 2018) to determine the phylotype of the strains [55]. EzTaxon analysis revealed that all isolates belong to the genus Streptomyces with similarity values amongst the various predicted related species ranging from 98.44–99.89% (Table 1).
Subsequently, nine strains were prioritized based on their bioactivity profile and taxonomic position. Strains SHP 22-7, BSE 7-9, BSE 7F, I3, I4, I5, and I6 were selected since they showed antibacterial activity against Gram-positive and Gram-negative bacteria (Figure 2A,B). DHE 17-7 and DHE 7-1 were selected as they exerted bioactivity against at least two different Gram-positive test strains. DHE 6-7 and DHE 5-1, which showed bioactivity against all Gram-positive test strains, were not chosen for further analysis because both strains showed a close phylogenetic relationship to Streptomyces parvulus (Table 1), which is a known producer of the polypeptide antibiotic actinomycin D [56]. In an initial attempt with HPLC-MS analysis of the methanolic extracts of culture samples from DHE 6-7 and DHE 5-1, actinomycin D was detected as a product (Figure S1), ruling out both strains from further investigations.
2.2. Phylogenomic Analysis of Nine Prioritized Indonesian Streptomyces Strains
To obtain a better understanding of the phylogenetic relationship about the prioritized nine Streptomyces strains, a phylogenetic analysis based on their full-length genomes sequences was performed. For this purpose and the genome mining studies mentioned below, the genomic DNA was isolated from each sample and sequenced by using the Pacific Biosciences RS II (PacBioRSII) platform [57,58,59]. The resultant genome sequences ranged in sizes between 7.05 Mbp (Streptomyces sp. I6) and 8.36 Mbp (DHE 17-7) and GC contents between 72.08% (DHE 7-1) and 72.47% (Streptomyces sp. I6) (Table S1), which share comparable values reported for Streptomyces species (genome sizes of 6-12 Mb [60] and GC contents of 72–73% [61,62]).
In order to run a whole-genome phylogenetic analysis, the genome sequences were submitted to the Type (Strain) Genome Server (TYGS) (https://tygs.dsmz.de, accessed on 13 December 2019) [63], which allows a phylogenetic analysis based on full-length genome sequences and compares genomic data with the database genomes. The resulting phylogenetic information is more authentic than those obtained from 16S rDNA- or multi-locus sequence analysis (MLSA)-based classifications, which only use small sequence fragments as a basis for sequence comparisons [63]. The TYGS analysis provides information on the similarity of a strain to its nearest related type strain, derived from the digital DNA-DNA hybridization (dDDH) values calculated by the genome-to-genome distance calculator (GGDC) 2.1 (http://ggdc.dsmz.de, accessed on 13 December 2019) [64]. TYGS phylogenomic analysis revealed that all nine isolates belong to the genus Streptomyces. The dDDH values between the nine Indonesian strains and their closest relatives ranged between 31.4% (Streptomyces sp. I4) and 51.5% (Streptomyces sp. I6) (using GGDC distance formula d4) (Table 2), which is below the threshold of 70% used for species delineation [65,66], proposing a novel collection of Streptomyces species.

Table 2.
Data from pairwise comparisons between genome sequences from nine Indonesian strains and their closest related strains based on dDDH analysis. “Query strain” refers to analyzed strain, and “subject strain” refers to most closely related Indonesian strain sample. Degree of relatedness is given as dDDH distance formula d4 as previously described by Meyer-Kolthoff et al. [66].
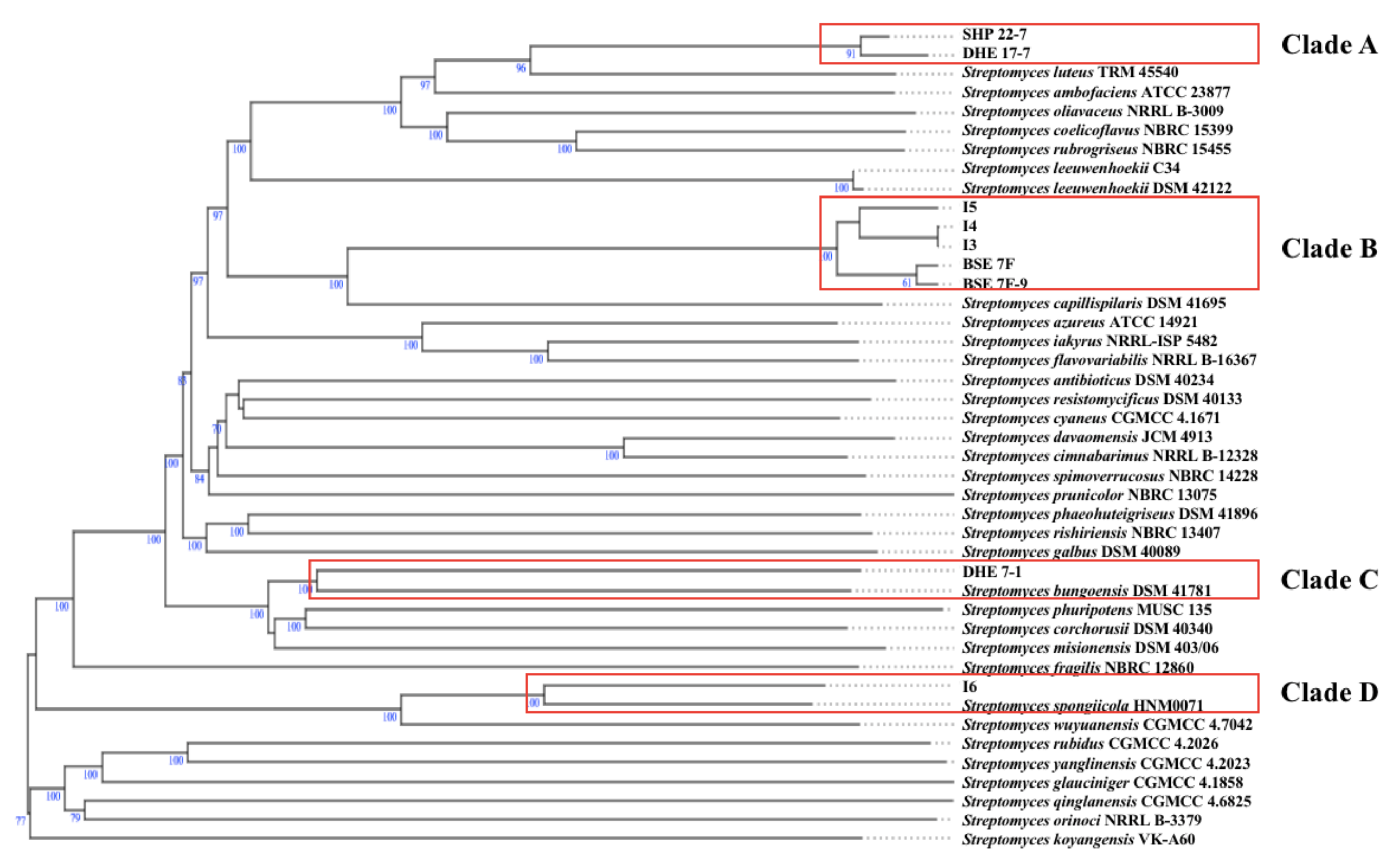
According to the TYGS phylogenomic tree, the terrestrial Enggano Island strains SHP 22-7 and DHE 17-7 belong to the same clade (clade A) (Figure 3) and most likely resemble the same type of species with a dDDH value of 86.7% (Table 2). Both bacteria are found to be closely related to S. luteus TRM 45540, isolated from a soil sample from China [67]. All mangrove isolates originating from sediments of Lombok Island (Streptomyces sp. I3, I4, and I5) and Bali Island (BSE 7F, and BSE 7-9) were allied in clade B, suggesting a correlative connection (Figure 3). Additionally, the dDDH analysis showed that BSE 7F is closely related to BSE 7-9 with a value of 95.7% and thus most likely represent the same subspecies (Table 2), while Streptomyces sp. I3 and I4 probably represent the same species having a dDDH score of almost 100% (Table 2). The nearest related type strain of all five mangrove strains is S. capillispiralis DSM 41695 isolated from a Sweden soil sample [68].
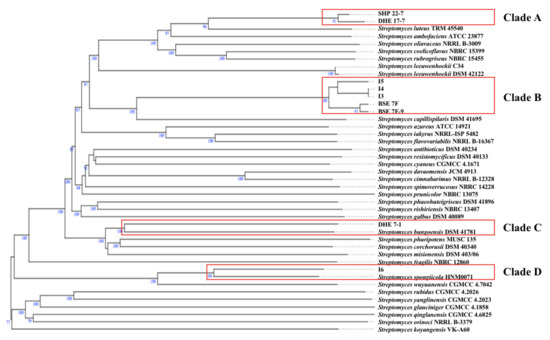
Figure 3.
Whole-genome sequence tree generated with the TYGS web server for nine Indonesian Streptomyces isolates (highlighted by red boxes) and closely related type strains. Tree inferred with FastME from GBDP distances was determined from genome sequences. The branch lengths are scaled in terms of GBDP distance formula d5. The numbers above branches indicate GBDP pseudobootstrap support values > 60% from 100 replications, with an average branch support of 84.4%. The tree was rooted at the midpoint.
By contrast, the soil sample DHE 7-1 and mangrove Streptomyces sp. I6 were found to group separately in distinct clades (clade C and D, respectively) (Figure 3). The soil S. bungoensis DSM 41781 collected in Japan [69] shares a dDDH value of 32.3% as the closest related strain to DHE 7-1 (Table 2), while the nearest related neighbor of Streptomyces sp. I6 is S. spongiicola HNM0071, isolated from a marine sponge collected from China [70] with a dDDH value of 51.5% (Table 2). Additional information on the specific polyphasic characteristics of the representative type strains from each clade can be found in the Supplementary Material. Altogether, 16S rRNA gene-based phylogenetic and phylogenomic studies revealed that all nine prioritized isolates belong to the genus Streptomyces and, based on dDDH analysis, represent novel species (Figure 3).
2.3. Genetic Potential for Secondary Metabolite Biosynthesis of Nine Indonesian Streptomyces Strains
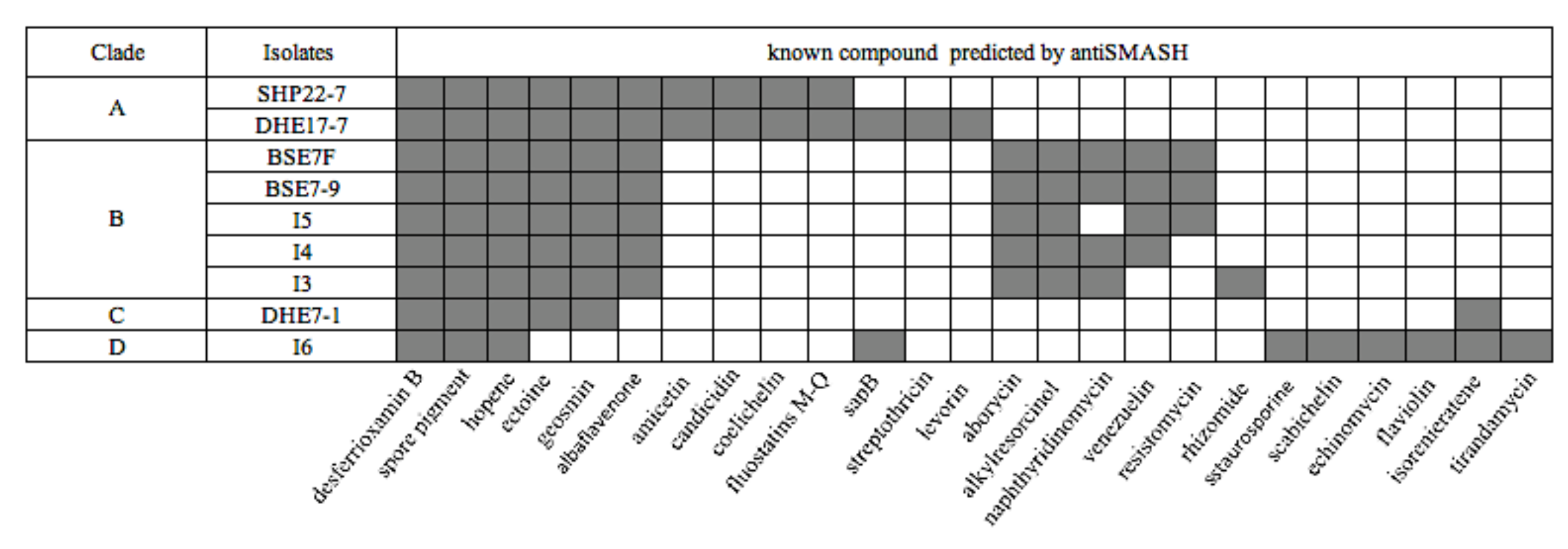
To infer the genetic potential of the strains for the biosynthesis of secondary metabolites, the genomes were analyzed bioinformatically using the web tool antiSMASH version 5.0 (https://antismash.secondarymetabolites.org, accessed on 13 November 2019) [44]. The antiSMASH analysis yielded a sum of 206 potential BGCs for the nine isolates (Table 3) with the lowest BGC count of 17 for strain Streptomyces sp. I3 and the highest number of 30 BGCs for strain DHE 17-7 (Table 3). On average, this makes 23 BGCs per strain, which is lower than the average value of 40 BGCs reported for Streptomyces genomes [71]. However, the lower BGC count is most likely a result of the underlying PacBio genome sequences, which generally yield less contigs than other sequencing technologies, resulting in less interrupted BGCs and thus less BGC counts in antiSMASH analyses. The genome of DHE 17-7 exhibited a slight correlation between genome size (8.4 Mbp) and the observable number of BGCs (30 BGCs) (Table 3 and Table S1). Several of the identified BGCs from the nine Indonesian isolates showed a high similarity (>60%) to already-known BGCs (Figure 4), e.g., all strains harbored BGCs encoding compounds that are commonly produced by streptomycetes, such as desferrioxamine, which is a vital siderophore for the growth and development [72], hopene, as a substance of the cytoplasmic membrane modulating membrane fluidity and stability [73], and a spore pigment for protection against UV radiation [74]. This result is consistent with previous observations, where these BGCs have been reported for most analyzed Streptomyces genomes [71]. Ectoine and geosmin BGCs were found in all Indonesian isolates except for Streptomyces sp. I6 (Figure 4). Moreover, albaflavenone BGCs were uncovered in all strains, excluding Streptomyces sp. I6 and DHE 7-1. Interestingly, in the genomes of the mangrove-derived isolates BSE 7F, BSE 7-9, I3, I4, and I5, two ectoine BGC were identified, suggesting that the additional ectoine BGCs may play a role in the adaptation of these organisms to the osmotic stress of such high-salinity environments.

Table 3.
List of Indonesian actinomycetes strains with number and type of BGCs as predicted by antiSMASH analysis.
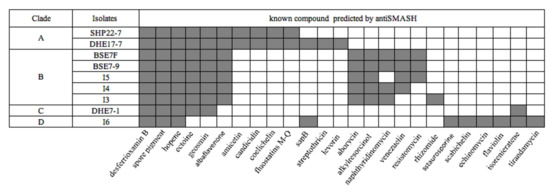
Figure 4.
Presence (grey color) and absence (white color) of BGCs in nine Indonesian strains as predicted by antiSMASH analysis with similarity above 60%.
Aborycin and alkylresorcinol gene clusters were discovered in the five mangrove Streptomyces strains, whereas amicetin, candicidin, coelichelin, and fluostatin M-Q BGCs were only detected for the soil-based isolates SHP 22-7 and DHE 17-7. Candicidin, as an example of a fungizide [75], is most likely produced by terrestrial streptomycetes in order to defend themselves against local fungal competitors. Coelichelin is a further spotted siderophore which might be necessary for the soil-living streptomycetes to sequester poorly soluble environmental Fe3+ [76], which is quite scarce and highly contested by other microorganisms in soils. The discovery of the same BGC composition in strains derived from the same habitat, such as soil or mangroves, is probably attributed to the fact that each biosynthetic product has its specific biochemical relevance in the respective environment. Of the nine strains, only Streptomyces sp. I6 harbored a staurosporine, scabichelin, echinomycin, flaviolin, and tirandamycin BGC. Likewise, DHE 7-1 together with Streptomyces sp. I6 were the only representatives comprising an isorenieratene BGC among the nine strains (Figure 4). Both strains, I6 and DHE 7-1, were found to be phylogenetically distant from the other strains (Figure 3), outlining that phylogenetically related isolates tend to have similar biosynthetic elements known as BGCs shaped by the environmental conditions. A similar finding has already been made by Meij et al., who reported that ecological conditions play an important role in controlling the formation of secondary metabolites in actinomycetes [77].
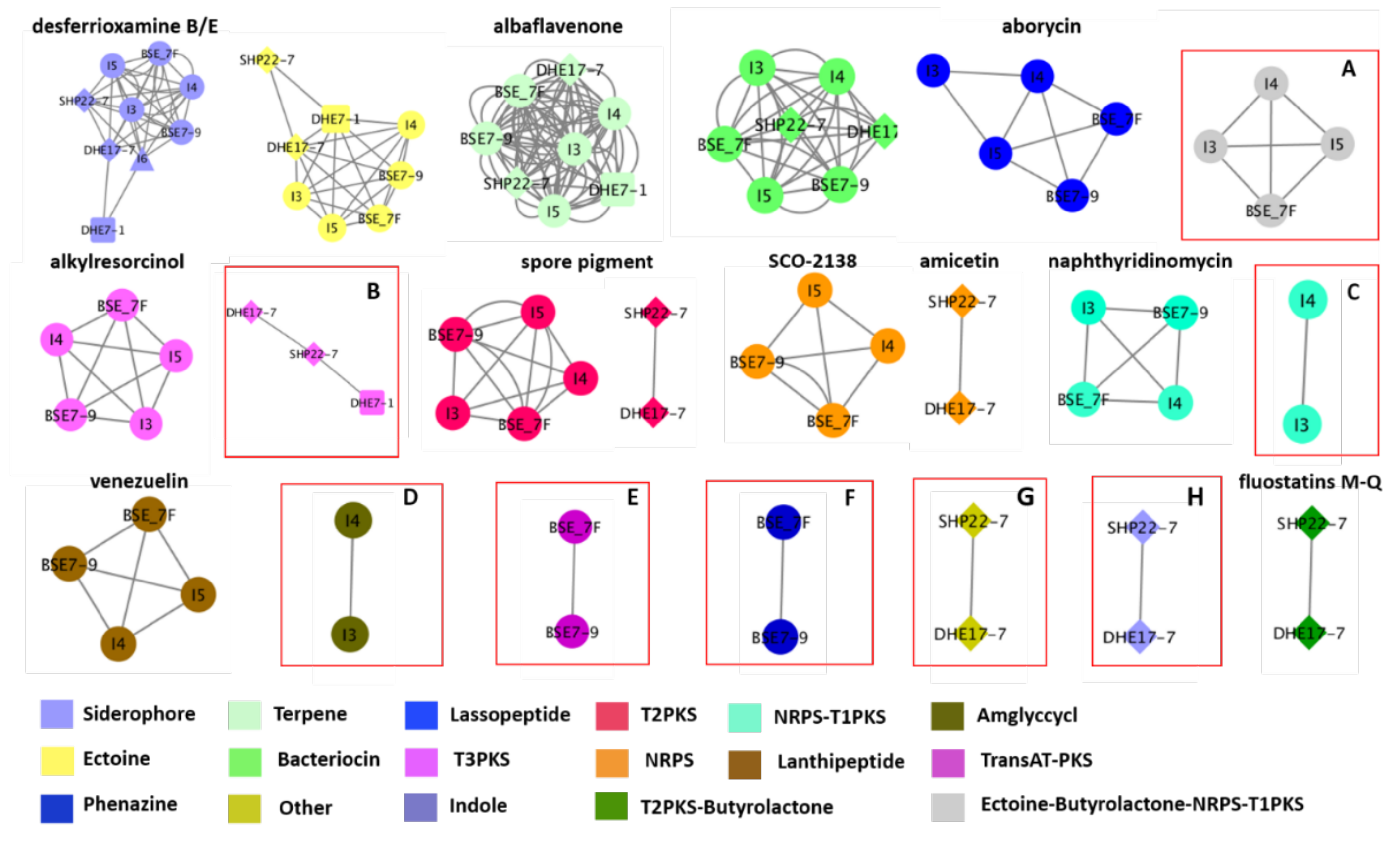
To glean a more detailed picture about the BGC distribution amongst the strains, the genome sequences from the nine strains have been analyzed using the BiG-SCAPE software (https://bigscape-corason.secondarymetabolites.org/, accessed on 13 November 2019) [78]. BiG-SCAPE allows fast computation and visual exploration of BGC similarities by grouping BGCs into gene cluster families (GCF) based on their sequences and Pfam protein families similarities [79]. Comparing all shared BGCs within the nine Indonesian strains with BiG-SCAPE allows visualization of the more common BGCs (large nodes) and the less frequent ones (doubletons, (singletons are not shown) (Figure 5). With this approach, we visualized the occurrence of eight GCFs with a similarity of less than 60% similarity to known BGCs as predicted by antiSMASH. Ectoine-butyrolactone-NRPS-T1PKS GCF, which has similarities with polyoxypeptin (48%) or aurantimycin A (51%), was distributed among strains I3, I4, I5 and BSE 7F (Figure 5, Tables S6, S7, S9 and S10). A type III polyketide (T3PKS) GCF was shared amongst the strains DHE 17-7, SHP 22-7, and DHE 7-1, which showed 7–8% BGC similarity to the herboxidiene BGC (Figure 5, Tables S4, S5 and S11). In the strains Streptomyces sp. I3 and I4 of clade B, we found the others-type I polyketide (otherks-T1PKS), which showed 48–55% BGC similarity to the nataxazole BGC, and an aminoglycoside/aminocyclitol (amglyccycl) BGC type, which led to 2% similarity to the BGC of cetoniacytone A (Figure 5, Tables S6 and S7). We identified two unique GCFs in the strains BSE 7F and BSE 7-9 of clade B, namely a transAT-PKS GCF, which showed 54–58% similarity to the weishanmycin and phenazine BGC types, and did not show any similarity to any BGC in the antiSMASH database (Figure 5, Tables S9 and S10). Moreover, we detected two GCFs of an indole, which showed 23–33% BGC similarity to the 5-isoprenylindole-3-carboxylate β-D-glycosyl ester BGC and other BGC type, which do not belong to any BGCs in the antiSMASH database for the phylogenetically related species of SHP 22-7 and DHE 17-7 of clade A (Figure 5, Tables S4 and S5). Altogether, the Big-SCAPE analysis revealed eight unique GCFs, which could not be associated with known BGCs and may have the potential to encode for new substances. Furthermore, the obtained data disclosed that phylogenetically related strains derived from a similar environmental habitat tend to share similar BGC composition profiles. Inferred from this observation, one can conclude that it is worth it to make an effort to sample actinomycetes from unique environmental habitats, since this may lead to the isolation of phylogenetically unique species, which have a higher potential of producing novel natural compounds, as also previously described by Hug et al. [9].
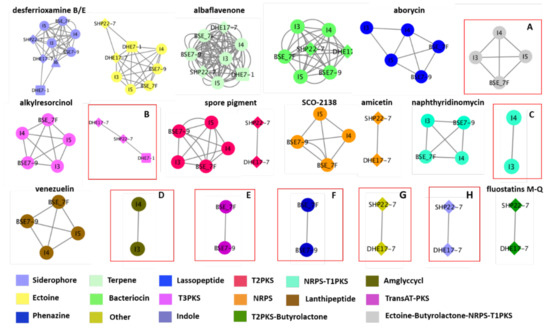
Figure 5.
Similarity network of the predicted biosynthetic gene clusters (BGCs) of the nine Indonesian Streptomyces strains. Shared similar BGCs are indicated by a connected line. Each node represents a specific BGC type (labeled with different colors). The shape node represents the same species, i.e., clade A (SHP 22-7 and DHE 17-7) indicated with diamond, clade B (I4, I5, BSE 7F, and BSE 7-9) shown with ellipse, clade C (DHE 7-1) with a cube, and clade D (I6) indicated with a triangle. BGCs with similarities less than 60% are highlighted by red boxes: (A) Ectoine-butyrolactone-NRPS-T1PKS; (B) T3PKS; (C) Otherks-T1PKS, (D) Amglyccyc; (E) TransAT-PKS; (F) Phenazine; (G) Other; and (H) Indole.
2.4. Optimal Cultivation Conditions for Compound Production of Nine Indonesian Streptomyces Strains
In order to infer the biosynthetic capacity of the prioritized nine isolates in a bioactivity context, various media following the OSMAC strategy were screened to define the optimal production conditions [30,31]. For this purpose, SHP 22-7, DHE 17-7, DHE 7-1, BSE 7-9, BSE 7F, I3, I4, I5, and I6 were each grown in twelve different liquid cultivation media (SGG, YM, OM, R5, MS, TSG, NL19, NL300, NL330, NL500, NL550, and NL800 (Table S2)), and culture samples were harvested at different time points (48, 72, 96, and 168 h). Cell cultures were extracted with ethyl acetate, concentrated in vacuo, and then re-dissolved in methanol. Methanolic extracts were tested in bioassays against a selected panel of pathogenic strains B. subtilis, M. luteus, S. carnosus, E. coli, and P. fluorescens. Samples with the largest inhibition zones in bioassay tests were defined as the ones grown under optimal cultivation conditions. For each Indonesian Streptomyces strain, the optimal production conditions have been defined for cultivation in liquid media (Table S3). In addition, it is hypothesized that filamentous actinomycetes as soil organisms grow and develop better on solid nutrient substrates and that a well-grown healthy culture produces more diverse secondary metabolites [80]. Thus, to extend the probability of finding new substances by exploring the biosynthetic potential of the nine strains for secondary metabolite production, we recruited an antibiotic extraction also from solid media. For this purpose, each isolate was spread on agar plates consisting of the respective abovementioned media and incubated for 7–10 days at 28 °C until spores formed. Grown agar samples were squeezed out and concentrated. The aqueous phase of the solid medium extract was used for bioassays and further chemical analysis.
For Streptomyces sp. I3 and I4, the same cultivation parameters were found to be optimal. Both strains showed a promising potency upon their growth in liquid NL550 medium for 72 h on solid MS medium (Table S3). Such similar production behavior might be ascribed to their most possible likelihood to represent the same species as suggested above. Furthermore, we found that most of the nine isolates (Streptomyces sp. I3, I4, I5, and I6) produced best on solid MS medium (Table S3). In general, MS is a suitable medium for streptomycetes regarding spore isolation [81]. This would support the hypothesis that strains produce better, when they show healthy growth and development.
2.5. Identification of Natural Compounds from Nine Indonesian Actinomycetes
To putatively identify the specialized bioactive substances which are produced by the nine isolates under the various conditions, the culture extract samples were submitted to high-resolution mass spectrometry (HRMS) coupled with the GNPS platform. For this purpose, the obtained extracts from the optimal medium in liquid and solid were firstly fractionated by solid-phase extraction (SPE) and then qualitatively profiled against their main crudes and media controls using HPLC. Subsequently, the prioritized profiles and/or SPE fractions that mainly cover the whole metabolomes with fewer media components were chosen for further metabolomics mass identification through HRMS/MS. The acquired tandem-MS mass spectra from the positive mode were recruited to build a feature-based molecular network, while the negative ionization was consulted, if needed, during the annotation step to further validate the feature identities [45,82]. The dereplication of the known compounds, chemical analogues, and potential novel chemical structures was carried out either by matching their MS/MS spectra against the literature if available, GNPS spectral libraries [45] and/or assisted by manual in silico annotation via Sirius+CSI: FingerID 4.0.1 integrated with Antibase and Pubchem databases [83,84] (see Material and Methods).
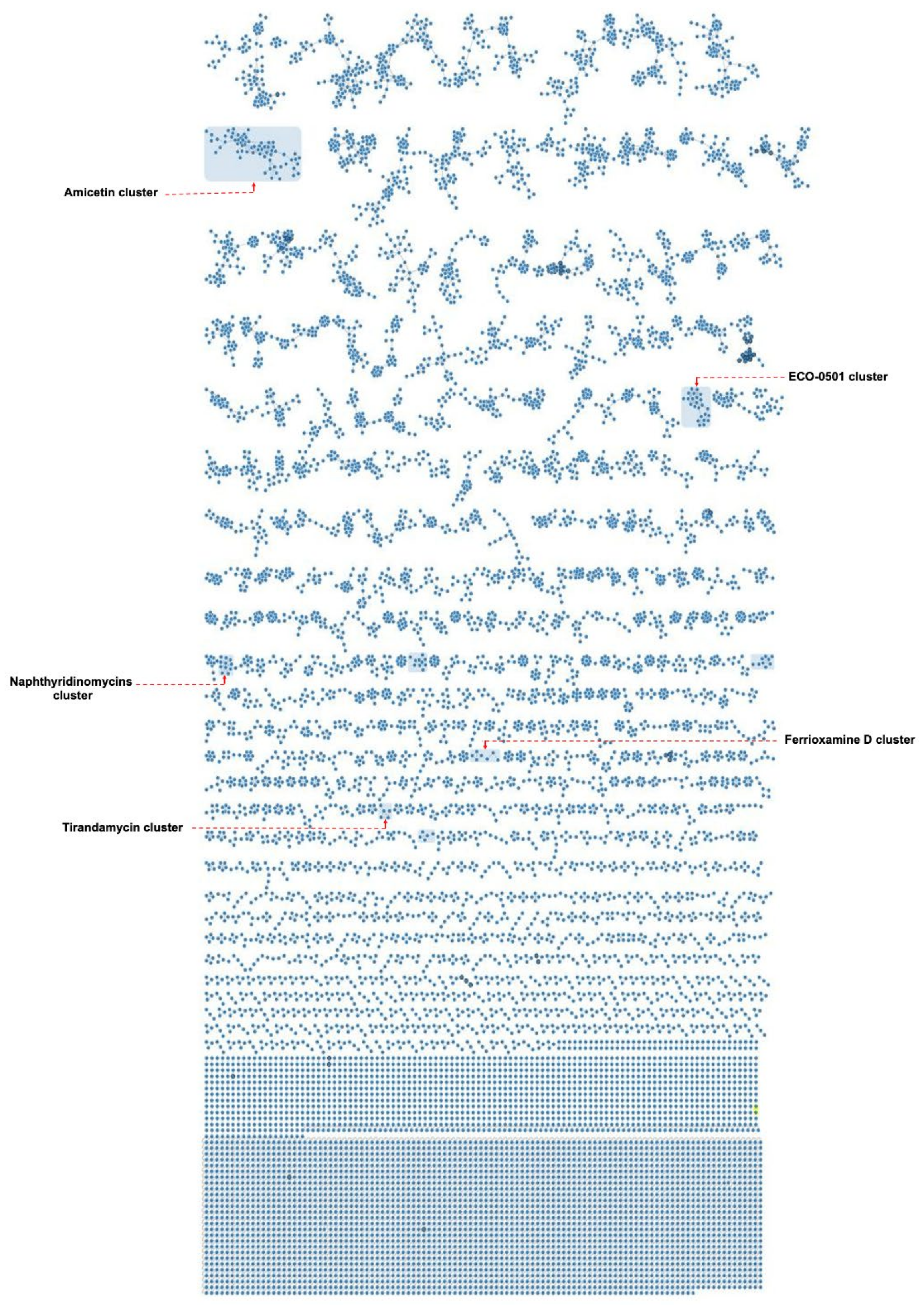
Among the numerous identified secondary metabolites from the nine isolates, antimycins cluster were swiftly retrieved through the identical similarity of their MS/MS spectra to the publicly shared ones of GNPS libraries (Figures S2–S6). Tracking down such features in liquid BSE 7F fractions, particularly the one eluted with 100% MeOH in negative mode, expanded this set with further known members (Figures S5 and S6). In alignment with the formerly described positional and stereogenic isomers of the antimycin family entities, the extracted ion chromatograms (EICs) unambiguously displayed such an isomeric behavior under both modes (Figures S2, S4 and S5) [85,86,87]. In a similar fashion to antimycins, a different cluster comprising ferrioxamines was deciphered with the aid of GNPS spectral libraries. Ferroxamine D1, 656.2830 Da in size as C27H48N6O9 [88,89], was displayed as the primary ion linked with an additional unknown analogue, 627.3303 Da as C26H51FeN8O6 (Figures S7–S9). Despite the fact of observing these two features under only solid cultivation parameters across different isolates (Streptomyces sp. I3, I4, I6, BSE 7F, DHE 17-7, and SHP 22-7) with variable concentrations, two extra unknown amphiphilic trihydroxamate-containing siderophores were also grouped (Figures S7 and S10). Interestingly, BSE 7-9 was the sole producer of such amphiphilic entities under exclusive liquid conditions. Moreover, two additional unknown ferrioxamines were retrieved as unique features singly produced by the DHE 17-7 isolate (Figure S11).
Analogously, staurosporine, with two further congeners, was dereplicated from the I6 sample assisted by shared spectral repositories (Figure S12). Manual annotation of a pair of singletons, 1137.45 as [M+H]+ and 560.22 as [M + 2H]2+ from the I6 extract, uniquely grown under solid conditions, could decipher echinoserine and depsiechinoserine, respectively (Figures S13–S15) [90,91]. Although the two features were supposed to group together considering their skeletons, the MS/MS spectra of their triggered singly and doubly pseudomolecular ions were different enough not to serve such a purpose resulting in scattered self-looped nodes (Figures S13 and S14). Furthermore, traces of the structurally related echinomycin [92] were also observed within Streptomyces sp. I6 extracts, expanding in this way the molecular compound family (Figure S15). Likewise, a tirandamycins cluster was disclosed in Streptomyces sp. I6 extracts upon liquid cultivation depicting the known tirandamycin A in connectivity with further related chemotypes (Figure S16). In parallel, the observed UV absorbance of the annotated mass ion at m/z 418.18 as tirandamycin A was in alignment with its reported characteristic value [93,94], additionally confirming the identity of the dereplicated feature (Figure S17). Notably, the anticipated molecular formula of the grouped ions of the tirandamycin cluster, besides their degrees of unsaturation, was also reflected by their observed UV absorbances, which differed from the characteristic known one (Figure S17).
An additional constellation of ions mainly derived from isolates BSE 7-9 and I5 was uncovered through manual annotation as naphthyridinomycins cluster (Figure S18). The in silico annotation considering the molecular formula prediction and their MS2 spectra deconvoluted naphthyridinomycin-A, aclidinomycin A, and bioxalomycin-β2 besides several unknown related products (Figures S19–S22) [95,96,97]. Similarly, the manual interrogation of an exclusive group of ions derived from DHE 17-7 led to the putative dereplication of ECO-501, a PKS product so far only reported from Amycolatopsis orientalis ATCC 43491 [98] (Figures S23–S26). Interestingly, the putative annotation of such a feature was in complete alignment regarding the observed UV absorbance and the formerly reported MS/MS spectra (Figures S27–S29). Moreover, amicetin and cytosaminomycins as structurally related entities were uncovered from SHP 22-7 samples as a big group of ions (Figures S27–S29), encompassing a wide scope of structural modifications as expected according to previous reports in addition to a putatively new set of congeners (Figure S30) [99,100,101].
The compound naphthyridinomycin was detected in several culture extract samples from strains of mangrove origin, such as Streptomyces sp. I3, I4, I5, BSE 7F, and BSE 7-9 (Figure 6, Table 4), while amicetin was detected as a biosynthetic product from the isolates SHP 22-7 and DHE 17-7 obtained from soil samples of Enggano Island (Figure 6, Table 4). Furthermore, we observed that Streptomyces sp. I6 produces echinomycin (Figure 6, Table 4), a substance also reported as the biosynthetic product from the closely related type strain Streptomyces spongiicola HNM0071, which was originally derived from a marine sponge [102]. These results underline our assumption that phylogenetically related strains are likely to produce similar compounds as a response to their natural-habitat environmental conditions. Specifically, the isolates Streptomyces sp. I3 and I4 have been found to most likely represent the same species derived from a similar habitat as indicated by the dDDH value of almost 100% and the high overall similarities of BGC composition and secondary metabolite production profile of both strains (see above). In this context, it should be mentioned that current antibiotic research often addresses the problem of dereplication of known compounds during drug-screening approaches [103,104,105]. However, what should also be taken into account is the fact that there is also an issue of dereplication of producer strains as observed in the current study. Thus, it is worth it to put effort into phylogenetic profiling at the beginning of the screening strategy in order to sort out known producer strains.
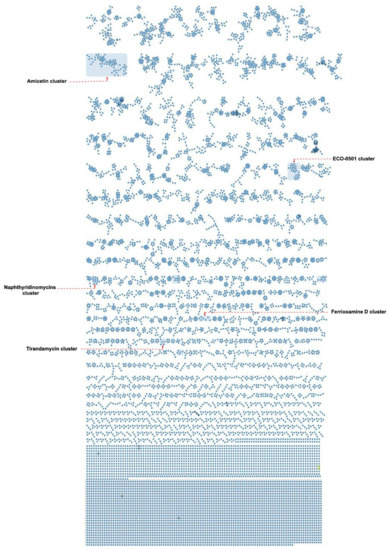
Figure 6.
Molecular networking of extract and fraction samples from nine Indonesian Streptomyces strains. Molecular families containing a known substance are highlighted by blue boxes.

Table 4.
Correlation between known compounds and BGC distribution in the nine Indonesian strains. A checkmark (√) indicates identified BGC in the studied strain, a question mark (?) indicates that BGC is not identified in the studied strain, and a minus sign (-) indicates the compound is not present in the medium.
Interestingly, the ferrioxamine molecular family was only detected for samples of strains grown on solid media (Table 4 and Table 5). In addition to the abovementioned metabolites, the solid media uniquely delivered a putative new molecular family consisting of likely three peptides with m/z 598.2834 [M + 2H]2+, 662.8048 [M + 2H]2+, and 727.3259 [M + 2H]2+, for which no known substance could be associated. These compounds were detected in samples of strains I3, I5, and BSE 7F (Figure S31, Table 5), highlighting that cultivation conditions have a substantial effect on the chemical profiles. A further example of rendering the impact of the adopted cultivation method was represented with an additional cluster of unknown features from SHP 22-7 isolate, designated compound group I, which were exclusively produced under nonliquid fermentation (Figure S32).

Table 5.
Overview of analogs and putative new compounds identified for the nine Indonesian Streptomyces strains. A minus sign (-) indicates that the compound is not present in the medium.
Within the same context, strain DHE 17-7 also offered several putative new compounds (compound group II) which were detected when grown in a liquid medium and presented themselves only as a set of doubly charged entities (Figure S33) (Table 5). Thus, in regard to drug-discovery efforts, strain DHE17-7 is the most promising strain to be investigated further. The potent biosynthetic capacity is also reflected by the genetically encoded biosynthetic potential since DHE17-7 has a total of 30 BGCs, which is the largest BGC set compared to the other Indonesian strains (Table 3). In summary, 16 potential novel compounds (Table 5) have been identified as biosynthetic products from the Indonesian strains, which could not be associated with any known compound and thus demonstrate the value of new strains for drug-discovery research.
Furthermore, we observed a correlation between growth conditions and compound production. It is known that sources of complex nitrogen such as soybean meal and corn steep liquor can increase ferrioxamine production in streptomycetes [106,107]. Interestingly, ferrioxamine B/D and its analogs has been mainly identified for strains grown on solid media, such as MS agar (Streptomyces sp. I3, I4, I6) and NL300 agar (SHP 22-7) (Table 4 and Table S3), which contain soy flour and cotton seed powder, respectively (Table S2). We could detect ferrioxamines only in samples obtained from strains grown on solid medium. This might be because in liquid media iron (Fe3+) is more evenly distributed compared to solid media. Thus, cells grown on solid media might be faced with local iron depletion conditions, which lead to induction of ferrioxamine biosynthesis [76]. In addition to ferrioxamine and its analogs, several known and unknown compounds were only discovered in samples from strains grown on solid medium, i.e., the three known compound echinomycin, staurosporine, and tirandamycin for Streptomyces sp. I6, and three putative new peptides for Streptomyces sp. I3; I5; BSE 7F, as well as the putative new compound group I for S. sp. SHP 22-7 (Table 4 and Table 5). Apart from that, we also found some unknown and known compounds in strains grown in liquid media only, such as amicetin (SHP 22-7 and DHE 17-7), antimycin and its analogs (BSE 7F), ECO-0501 (DHE 17-7), or the putative new compound group II for strains Streptomyces sp. DHE 17-7 (Table 4 and Table 5). This indicates that cultivation conditions significantly affect the formation of substances. Therefore, both the liquid and solid cultivation approach are feasible for increasing the probability of discovering new compounds.
2.6. Identification of Potential BGCs Responsible for Compound Production in the Nine Indonesian Streptomyces Strains
To identify the BGCs responsible for compound production in the nine Indonesian Streptomyces strains, we aimed to link the compound production profile and BGC composition by correlating the BGCs data with the MS-based molecular networking results. As described above, strains SHP 22-7, I3, I4, and I6 produce desferrioxamine B/D when grown on solid media (Table 4). We observed that the corresponding BGCs associated with desferrioxamine B/D biosynthesis were present in all of the four strains. Furthermore, we were able to assign the BGC responsible for the biosynthesis of naphthyridinomycin in the strains BSE 7F, BSE 7-9, I3, I4, and I5 (Table 4). Additional BGCs could be assigned to the compound formations of amicetin in SHP 22-7 and DHE 17-7, antimycin in BSE 7F, echinomycin, staurosporine, and tirandamycin A in I6 (Table 4). Furthermore, we could not identify the BGC encoding the biosynthesis of ECO-0501 in strain DHE 17-7 based on the antiSMASH output. A potential candidate gene cluster could be cluster region 24, which is a predicted type I PKS BGC that shows some similarity (<55%) to BGCs encoding structurally related macrolactam natural products, such as vicenistatin, sceliphrolactam, and streptovaricin (Figure S34).
In addition to the metabolites mentioned earlier, we also discovered a group of new peptides, which were detected in samples of strains Streptomyces sp. I3, I5, and BSE 7F grown on solid media (Table 5). Notably, for all three strains a bacteriocin BGC could be detected (Tables S6, S8 and S9), which showed 42–57% similarity to the informatipeptin BGC. Alternatively, all three strains also share a combined NRPS/ectoine/butyrolactone/other/T1PKS gene cluster (Tables S6, S8 and S9), and it is also conceivable that the peptide group might be encoded from this region. A similar cluster was found on regions 21 and 22 for the phylogenetically related strain BSE 7-9, for which, however, no respective compound was detected (Table S10). Moreover, we found the putative new compound group IIwith masses ranging from 435–474 Da, produced by strain DHE 17-7 when grown in liquid medium (Table 5). Five BGCs are present in DHE 17-7 (region 10, 16, 17, 22, and 28), which do not show any similarity to known BGCs in the antiSMASH database and nine BGCs (region 3, 4, 6, 11, 13, 19, 24, 25, and 26) have similarities of less than 50%. Thus, the so far unknown metabolites might be encoded by some of the unique BGCs from DHE 17-7 (Table S4). The same applies for the putative new compound group I detected in strain SHP 22-7. Its genome comprises 13 BGCs with similarities of less than 50% and therefore represents all putative candidates. Similar observations have been made in comparable studies, where it has been shown that BGCs encoding ectoine, desferrioxamine, spore pigment, and bacteriocin production are very abundant in actinobacterial natural compound producers; however, each strain still possesses numerous BGCs that code for potential yet unknown substances [108,109,110]. That Indonesian habitats can serve as a promising reservoir for antibiotic active substances has already been highlighted in several previous screening studies [111,112,113,114,115]. Especially Indonesian actinomycetes have been reported as producer strains of new secondary metabolites, as for example shown for the Indonesian Streptomyces sp. strains ICBB8230, ICBB8309, and ICBB8415, which produced new angucyclinones [116,117], Streptomyces sp. ICBB8198, producing new phenazine derivatives [118], and Streptomyces sp. ICBB9297, which produced new elaiophylin macrolides [119]. Furthermore, Indonesian non-Streptomyces strains, as for example Micrococcus sp. ICBB8177 and Amycolatopsis sp. ICBB8242, have also been reported to produce novel compounds, as for example the limazepines or succinylated apoptolidins, respectively [120,121]. Thus, Indonesian habitats can indeed be considered a promising source for new bioactive natural products.
Altogether, the combined GNPS and cluster networking approach disclosed several potentially novel compounds from the Indonesian strains Streptomyces sp. I3, I4, I5, I6, BSE 7F, BSE 7-9, and DHE 17-7—some of which could be assigned to potential encoding BGCs, and some are expected to be encoded by unique BGCs. The new Indonesian isolates thus represent a valuable resource for further drug research and development approaches. We conclude that the combined phylogenomic, GNPS, and cluster-networking approach is an efficient strategy to prioritize phylogenetically unique producer strains and focus on potentially novel compounds encoded by special BGCs.
3. Materials and Methods
3.1. Sample Collection and Treatment
Soil samples were collected from Enggano Island (5°22′57.0792″ S, 102°13′28.2792″ E), Indonesia, in December 2015 (Figure 1B). Marine samples were collected from marine sediments from Bali Island (8°43’5.5″ S, 115°10′7.8″ E), Indonesia, in May 2014 (Figure 1C), and Lombok Island West Nusa Tenggara (8°24′17.133″ S, 116°15′57.228″ E), Indonesia, in May 2017 (Figure 1D). Soil and sediment samples were taken aseptically from 10 cm depth of soil samples and the center of sediment in mangrove and tidal area. Soil and sediment samples were transferred into sterile 50 mL conical tubes and placed on ice and then stored at 4 °C until further treatment.
3.2. Isolation of Actinomycetes
Isolation and enumeration of actinomycetes were done using a serial dilution of Humic Acid-Vitamin (HV) medium [48] and/or NBRC No. 802 Medium [49] by using the direct method [50], the dry heat method [51], and the phenol method [51]. In the direct method, an air-dried soil sample or marine sediment was ground in a mortar and heated in a hot-air oven at 110 °C for 30 min. One gram of the heated samples was transferred to 10 mL of sterile water and mixed for 2 min, then diluted with sterile water to 10−1, 10−2, and 10−3 times. In total, 200 µL of each dilution was inoculated on isolation medium agar of HV [48] or NBRC No. 802 Medium [49] with or without the addition of 1% NaCl. The inoculated plates were incubated for 2–4 weeks at 28 °C. The colonies showing the Streptomyces morphological characteristics were selected and streaked on fresh plates of the modified Streptomyces International Project 2 (ISP2 ≙ YM) agar [52]. The cultures were resuspended in sterile 0.9% (w/v) saline supplemented with 15% (v/v) glycerol and stored at −80 °C. This dry-heat method [51] was used to isolate heat-tolerant actinomycetes spores. In the dry-heat method, the soil or sediment samples were incubated at 100 °C for 40 min and then cooled to 28 °C in a desiccator. The samples were distributed on HV medium agar plates with a spatula tip and incubated at 28 °C for 2–3 weeks. The phenol method was used to select for spores, which survive in the presence of phenol. In total, 1 mL of 10−1 dilution of one gram of oven-dried soil or marine sample was transferred to 9 mL of sterile 5 mM-phosphate buffer (pH 7.0) containing phenol at a final concentration of 1.5%. The sample was then heated and diluted in serial dilution (10−1, 10−2, 10−3). Next, 100 or 200 µL of each dilution was spread over the surface of HV medium agar plates and incubated for 2–4 weeks at 28 °C.
3.3. Antimicrobial Bioassays
The preliminary screening of actinomycetal strains for antimicrobial activity was performed using the agar plug diffusion method (see Supplementary for test plate preparation). Gram-positive (B. subtilis ATCC6051, M. luteus, and S. carnosus TM300) and Gram-negative bacteria (E. coli K12 W3110 and P. fluorescens) were chosen as test organisms. The isolates were spread evenly over the agar plate surface of soya flour mannitol medium (MS) (mannitol 20 g, soy flour (full fat) 20 g, agar 16 g in 1 L of distilled water) [80] and incubated for 10 days at 28 °C. Agar discs of the 10 days inoculum were cut aseptically with a cork borer (9 mm diameter) and placed on the bioassay test plate. Bioassays to determine optimal cultivation conditions in the liquid culture were examined using a disc diffusion assay against the test Gram-positive (B. subtilis ATCC6051, M. luteus, and S. carnosus TM300) and Gram-negative bacteria (E. coli K12 W3110 and P. fluorescens). In total, 10 µL methanolic extract obtained from liquid cultures of the actinomycetal strains was pipetted on a filter disc (6 mm) and then placed on the respective test plates. In addition, 5 µL kanamycin (50 μg/mL) was used as positive control and 10 µl methanol as a negative control.
The bioassay plates were incubated overnight at 37 °C for B. subtilis, E. coli, and S. carnosus and at 28 °C for M. luteus and P. fluorescens to allow for the test organisms’ growth. The antimicrobial activity of the isolates was assessed by measuring the diameter of the inhibition zone (mm) around the agar plug or the discs. All bioassay tests were carried out as three independent biological replicates.
3.4. Isolation of Genomic DNA and 16S rDNA Phylogenetic Analysis
For isolation of genomic DNA, the producer strains were grown for two days in 50 mL of R5 medium at 30 °C [81]. The genomic DNA was extracted and purified with the Nucleospin® Tissue kit from Macherey-Nagel (catalog number 740952.50) following the standard protocol from the manufacturer. The DNA was applied as a PCR template for 16S rRNA gene amplification using polymerase chain reaction (PCR). Primers used for PCR were 27Fbac (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492Runi (5′-TACGGTTACCTTACGACTT-3′). The PCR amplicons were subcloned into the cloning vector pDrive (Qiagen) using basic DNA manipulation procedures as previously described by Sambrook et al. [122]. The respective 16S rDNA fragments were sequenced at MWG Eurofins (Ebersberg, Germany) with primers 27Fbac. The 16S rDNA sequence data were analyzed using the EzTaxon database (https://www.ezbiocloud.net, accessed on 28 May 2018).
3.5. Phylogenomic and Genome Mining Analysis
For phylogenomic and genome mining studies, full-genome sequence data have been obtained as reported previously [57,58,59]. Genomic DNA was isolated to construct a 10–20 kb paired-end library for sequencing by Macrogen (Seoul, South Korea) with the Pacific Biosciences RS II technology (Pacbio). The genome was assembled using Hierarchical Genome Assembly (HGAP) V.3. and annotated with Prokka version 1.12b and the NCBI Prokaryotic Genome Annotation Pipeline (PGAP). The phylogenomic analysis of the nine selected strains was carried out with the Type (Strain) Genome Server (TYGS), a free bioinformatics tool (https://tygs.dsmz.de/, accessed on 13 December 2019) for whole-genome-based taxonomic analysis [63]. The identification of potential biosynthesis gene clusters (BGCs) was accomplished by analyzing the genome sequences with antiSMASH version 5.0 [44]. The antiSMASH results were further analyzed using the BiG-SCAPE platform [78] to cluster the predicted BGCs into gene cluster families (GCFs) based on their sequences and Pfam protein family similarities [79]. BiG-SCAPE was conducted on global mode with default parameters [78], with the exception of the raw distance cutoff and the “--mix”parameter. Raw distance cutoff was set to 0.4 to ensure that even clusters with a pairwise distance higher than 0.3 (the default) were included in the output. The resulting network of BiG-SCAPE was visualized with Cytoscape version 3.7.2 [82].
3.6. Cultivation Conditions for Optimal Compound Production of Nine Indonesian Strains
To determine optimal cultivation conditions in liquid culture, the nine Indonesian actinomycetes strains, SHP 22-7, DHE 17-7, DHE 7-1, BSE 7-9, BSE 7F, I3, I4, I5, and I6, were each cultivated in 50 mL inoculum medium (NL410) in 500-mL Erlenmeyer flasks (with steel springs) in an orbital shaker (180 rpm) at 28 °C. After 48 h, 10 mL of preculture was inoculated into 100 mL of twelve different production medium (SGG, YM, OM, R5, MS, TSG, NL19, NL300, NL330, NL500, NL550, and NL800 (Table S2) and cultivated for 48–168 h. Cell culture samples were harvested at different time points (48, 72, 96, and 168 h). In addition, 5 mL of each cell culture sample was extracted with the same volume of ethyl acetate (EtOAc) for 30 min at room temperature. The EtOAc was dried in a rotary evaporator and suspended in a total volume of 0.75 mL methanol. The methanolic extracts were used for bioassay experiments. The culture extract samples, which yielded the largest zone of inhibition in the bioassays against the test organisms, were used for further compound identification analysis. To determine optimal cultivation conditions on solid culture, the nine Indonesian strains were each spread on 100 mL agar plates consisting of the respective abovementioned cultivation media and then incubated for 7–10 days at 28 °C until spore formation was visible on agar plates. The overgrown agar was then used for bioassay experiments and further compound identification analysis.
3.7. Sample Preparation for Chemical Identification
For chemical identification in the liquid sample, the nine Indonesian isolates were each cultivated in 50 mL of NL410 medium. After 48 h, 10 mL of the preculture was inoculated into 100 mL of optimal production medium. The 100 mL whole broth of each cell culture was extracted as described above. Then, the extracts were used for further experiment. For chemical identification from samples grown on solid medium, overgrown agar was cut into pieces and transferred to 50 mL Falcon tubes. The Falcon tubes were centrifuged at 13,000 rpm for 30 min at room temperature. The aqueous phase was concentrated to 1/5 of the original volume in the Genevac Centrifugal Evaporator EZ-2 Elite (SP Scientific). The concentrated aqueous phase was used for further chemical profiling.
The culture extract samples obtained from liquid medium extraction and the aqueous phase of the solid medium extraction were separated by solid-phase extraction (SPE) columns. The columns were washed twice with 2 mL methanol and 2 mL distilled water for activating the columns. The samples were prepared by adding 100% methanol to the culture extract samples and the aqueous phase until the samples were dissolved completely. The methanolic samples were applied onto the activated columns with a flow rate of 2 mL/min. The column was washed twice with distilled water. The column was eluted consecutively with 2 mL of 100% methanol, 50% methanol, and distilled water. Samples from the elution column were defined as fractions. The column was eluted with 100% methanol as the 100% fraction, with 50% methanol as the 50% fraction, and distilled water as the distilled water fraction. The fractions were dried in the Genevac EZ-2 Elite (SP Scientific) and then dissolved with 0.5 mL methanol. The crude extracts and all fractions were analyzed with HPLC and high-resolution mass spectrometry (HRMS).
3.8. HPLC-HRMS/MS Analysis
The HRMS analysis was carried out on MaXis 4G instrument (Bruker Daltonics, Bremen, Germany) coupled to an Ultimate 3000 HPLC (Thermo Fisher Scientific, Bremen, Germany). HPLC-method was applied as follows: the spectrometer using a gradient (solvent A: 0.1% formic acid (FA) in H2O, and solvent B: 0.06% formic acid in acetonitrile), a gradient of 10–100% B in 45 min, 100% B for an additional 10 min, using a flow rate of 0.3 mL/min; 5 μL injection volume and UV detector (UV/VIS) wavelength monitoring at 210, 254, 280, and 360 nm. The separation was carried out on a Nucleoshell 2.7 μm 150 × 2 mm column (Macherey-Nagel, Düren, Germany), and the range for MS acquisition was m/z 100–1800. A capillary voltage of 4500 V, nebulizer gas pressure (nitrogen) of 2 (1.6) bar, ion source temperature of 200 °C, the dry gas flow of 9 (7) l/min source temperature, and spectral rates of 3 Hz for MS1 and 10 Hz for MS2 were used. For acquiring MS/MS fragmentation, the ten most intense ions per MS1 were selected for subsequent collision-induced dissociation (CID) with stepped CID energy applied. The employed parameters for tandem MS were applied as previously detailed by Garg et al. in 2015 [123]. Sodium formate was used as an internal calibrant and Hexakis (2,2-difluoroethoxy) phosphazene (Apollo Scientific Ltd., Stockport, UK) as the lock mass. Data processing was performed using Bruker Daltonics Data Analysis 4.1(Bremen, Germany).
3.9. MS/MS Molecular Networking
Mass-spectral data were analyzed using Compass Data Analysis 4.4 (Bruker Daltonik, Bremen, Germany), whereas MetaboScape 3.0 (Bruker Daltonik, Bremen, Germany) was consulted for molecular features selection. Raw data files were imported into MetaboScape 3.0 for the entire data treatment and preprocessing in which T-ReX 3D (time-aligned region complete extraction) algorithm is integrated for retention time alignment with an automatic detection to decompose fragments, isotopes, and adducts intrinsic to the same compound into one single feature. All the harvested ions were categorized as a bucket table with their corresponding retention times, measured m/z, molecular weights, detected ions, and their intensity within the sample. The Bucket table was prepared with an intensity threshold (1e3) for the positive measurements with a minimum peak length 3, possessing a mass range of 150–1800 Da. For detailed parameters employed for the MetaboScape analysis, see Table S13. The features list of the preprocessed retention time range was exported from MetaboScape as a single MGF file, which was in turn uploaded to the GNPS online platform where a feature-based molecular network (FBMN) was created. The precursor ion mass tolerance was set to 0.03 Da and a MS/MS fragment ion tolerance of 0.03 Da. A network was then created where edges were filtered to have a cosine score above 0.70 and more than 5 matched peaks. Further, edges between two nodes were kept in the network if and only if each of the nodes appeared in each other’s respective top 10 most similar nodes. Finally, the maximum size of a molecular family was set to 100, and the lowest-scoring edges were removed from molecular families until the molecular family size was below this threshold. Cytoscape 3.5.1 was used for molecular network visualization.
4. Conclusions
In this study, we report on the isolation of 422 actinomycetes strains from three different unique areas in Indonesia. A combined genomics and metabolomics approach was applied to nine of the most potent antibiotic producer strains, which allowed us to uncover 16 so far unknown compounds. When cultivating the strains in various liquid and solid media, we found that culture conditions significantly affected the ability to produce specific compounds. Thus, the combination of both cultivation methods, solid and liquid cultivation, is a suitable approach to tap the full biosynthetic potential of actinomycetes. By phylogeny-associated genome mining studies, we found that phylogenetically related species tend to have a similar BGC composition. Additional metabolomics data suggested that the ability of the strains to produce certain compounds may be influenced by the environmental conditions, where the producer strains have been derived from.
Overall, the described methodology represents an efficient strategy for drug discovery and the reported unknown compounds may serve as a basis for further drug development.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19060316/s1, Figure S1. Actinomycin D production in DHE 6-7 (a) and DHE 5-1 (b). Peaks in HPLC representing actinomycin are marked with red colour. Mass spectra of actinomycin D detected in DHE 6-7 (c); Figure S2. Positive extracted ion chromatograms (EICs), ions cluster and predicted molecular formula of antimycins; Figure S3. GNPS spectral libraries hits of antimycins; Figure S4. Positive MS2 spectra of the detected antimycins from isolate BSE7F; Figure S5. Negative EICs and predicted molecular formulae of antimycins; Figure S6. Negative MS2 spectra of the detected antimycins from isolate BSE7F; Figure S7. Ions cluster of ferrioxamines and GNPS spectral libraries hit of ferrioxamine D1; Figure S8. Positive EICs and molecular formula prediction of ferrioxamine D1; Figure S9. Positive EICs, molecular formula prediction and MS2 of an unknown ferrioxamine; Figure S10. Positive EICs and MS1 of unknown amphiphilic ferrioxamines; Figure S11. Positive EICs and MS2 of DHE 17-7 ferrioxamines; Figure S12. Ions cluster of staurosporines and GNPS spectral libraries hit of staurosporine; Figure S13. Ions clusters of echinoserine; Figure S14. Ion singleton of depsiechinoserine, positive EICs and MS2 of depsiechinoserine; Figure S15. Positive EICs and MS1 of echinomycin; Figure S16. Ions cluster, Positive EICs and MS1 of tirandamycin A in addition to its congeners; Figure S17. UV absorbance, and MS2 of tirandamycin A in addition to its congeners; Figure S18. Ion cluster of naphthyridinomycins and their predicted molecular formula (MF); Figure S19. Positive EICs and MS2 of naphthyridinomycin and their related entities from isolate BSE7-9; Figure S20. Positive EICs and MS1 of naphthyridinomycin from isolates BSE7-9 and I5; Figure S21. Positive EICs and MS1 of aclidinomycin A from isolates BSE7-9 and I5; Figure S22. Positive EICs and MS1 of bioxalomycin-β2 from isolates BSE7-9 and I5; Figure S23. Ions cluster of ECO-0501 and its related congeners from isolate DHE 17-7; Figure S24. UV absorbance, MS2 of ECO-0501 and its related congeners from isolate DHE 17-7; Figure S25. Comparative positive MS2 of ECO-0501 from isolate DHE 17-7 and its reported version from Amycolatopsis orientalis; Figure S26. Negative MS2 of ECO-0501 from isolate DHE 17-7 and its proposed fragmentation scheme; Figure S27. Ions cluster of amicetins and its related congeners from isolate SHP 22-7; Figure 28. Comparative positive MS2 of amicetin and streptocytosin A; Figure S29. Comparative negative MS2 of amicetin and streptocytosin A; Figure S30. Comparative positive MS2 of some unknown members of amicetin molecular family; Figure S31. Comparative positive MS2 of some unknowns of likely peptides; Figure S32. Comparative positive MS2 of some unknowns from isolate SHP 22-7; Figure S33. Comparative positive MS2 of some unknowns from isolate DHE 17-7; Figure S34. Cluster similarity between the DHE 17-7 gene region 24 (query sequence) and the streptovaricin, sceliphrolactam and vicenistatin cluster; Table S1. Genome characteristics from nine Indonesian actinomycetes strain isolates; Table S2. media tested for antibiotic production in agar and liquid culture. All data refer to 1 l H2Odeion. For solid media 16 g/l agar is added, except for R5 medium 18 g/l agar is added; Table S3. List of optimal culture conditions (media, time point) and bioactivity profile of nine Indonesian strain isolates; Table S4. List of predicted BGCs of strain DHE 17-7 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S5. List of predicted BGCs of strain SHP22-7 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S6. List of predicted BGCs of strain I3 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S7. List of predicted BGCs of strain I4 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S8. List of predicted BGCs of strain I5 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S9. List of predicted BGCs of strain BSE 7F derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S10. List of predicted BGCs of strain BSE 7-9 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S11. List of predicted BGCs of strain DHE 7-1 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S12. List of predicted BGCs of strain I6 derived from antiSMASH analysis. The minus sign (-) indicates the BGC did not have any similarity with any BGCs in the antiSMASH database; Table S13. Parameters used in MetaboScape analysis;
Author Contributions
S.R. and S.A. isolated strains and performed preliminary bioassays; I.H. carried out phylogenetic analysis and antibiotic bioassays; I.H. and J.K. performed extraction of culture broths; A.K. and H.S. carried out HPLC-MS analysis, H.S. performed GNPS studies; I.H. and Y.M. performed genome-sequence-based bioinformatic analysis, A.G. performed BiG-SCAPE analysis; Y.M. and H.G. conceived the research. Y.M., W.W., H.G., P.L., W.K., and N.Z. supervised the work. I.H. wrote the original draft of paper, which was revised by Y.M., W.W., H.G., P.L., W.K., and N.Z. and approved by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the funding received from the BMBF German–Indonesian cooperation project NAbaUnAk (16GW0124K) and the German Center for Infection Research (DZIF) (TTU 09.811). I.H. is grateful for the RISET-Pro scholarship program from the Indonesian Ministry for Research and Technology (World Bank Loan No. 8245-ID). A.G. is grateful for the support of the Deutsche Forschungsgemeinschaft (DFG; Project ID # 398967434-TRR 261). S.A. is grateful for his Ph.D. scholarships (grant PKZ 91613866), generously provided by the German Academic Exchange Service (DAAD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete genomes sequence data were deposited at the National Center for Biotechnology (NCBI) information data base, https://www.ncbi.nlm.nih.gov/genome (29 December 2020 for all, except SHP 22-7 (7 September 2018) and BSE 7F (4 May 2018)) with the accession numbers QEQV00000000 for BSE 7F, QXMM00000000 for SHP 22-7, SAMN15691494 for DHE 7-1, SAMN15691533 for I3, SAMN15691540 for I4, SAMN15691656 for I5, SAMN15691724 for BSE 7-9, SAMN15692265 for DHE 17-7, and RHDP00000000 for I6. GNPS job data: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=429506a1cc2c4a679b421cc455c0249b (accessed on 12 March 2021).
Acknowledgments
We thank R. Ort-Winklbauer for technical assistance and Dorothee Wistuba for support in HRMS experiments, and the Ministry of Research and Technology, Republic of Indonesia for the RISET-Pro Scholarship support of I.H. (World Bank Loan No. 8245-ID).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Waksman, S.A.; Woodruff, H.B. The Soil as a Source of Microorganisms Antagonistic to Disease-Producing Bacteria. J. Bacteriol. 1940, 40, 581–600. [Google Scholar] [CrossRef]
- Kresge, N.; Simoni, R.D.; Hill, R.L. Selman Waksman: The Father of Antibiotics. J. Biol. Chem. 2004, 279, e7–e8. [Google Scholar] [CrossRef]
- van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of Actinobacteria: New concepts for natural product discovery. Nat. Rev. Microbiol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- van der Heul, H.U.; Bilyk, B.L.; McDowall, K.J.; Seipke, R.F.; van Wezel, G.P. Regulation of antibiotic production in Actinobacteria: New perspectives from the post-genomic era. Nat. Prod. Rep. 2018, 35, 575–604. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R. Antibiotic discovery from actinomycetes: Will a renaissance follow the decline and fall? SIM News 2005, 55, 186–196. [Google Scholar]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural product drug discovery in the genomic era: Realities, conjectures, misconceptions, and opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Hug, J.J.; Bader, C.D.; Remškar, M.; Cirnski, K.; Müller, R. Concepts and methods to access novel antibiotics from actinomycetes. Antibiotics 2018, 7, 44. [Google Scholar] [CrossRef]
- Sivalingam, P.; Hong, K.; Pote, J.; Prabakar, K. Extreme Environment Streptomyces: Potential Sources for New Antibacterial and Anticancer Drug Leads? Int. J. Microbiol. 2019, 2019, 5283948. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K. Pharmaceutically active secondary metabolites of marine. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Abdelkader, M.S.A.; Philippon, T.; Asenjo, J.A.; Bull, A.T.; Goodfellow, M.; Ebel, R.; Jaspars, M.; Rateb, M.E. Asenjonamides A-C, antibacterial metabolites isolated from Streptomyces asenjonii strain KNN 42.f from an extreme-hyper arid Atacama Desert soil. J. Antibiot. 2018, 71, 425–431. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Díaz, C.; De La Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces Zhaozhouensis. Mar. Drugs 2015, 13, 128–140. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, M.; Braun, D.R.; Ericksen, S.S.; Piotrowski, J.S.; Nelson, J.; Peng, J.; Ananiev, G.E.; Chanana, S.; Barns, K.; et al. A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science 2020, 370, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Pelaez, F. Biodiversity, chemical diversity and drug discovery. Prog. Drug Res. 2008, 65, 141, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Huffard, C.L.; Erdmann, M.V.; Gunawan, T. Geographic Priorities for Marine Biodiversity Conservation in Indonesia; Ministry of Marine Affairs and Fisheries and Marine Protected Areas Governance Program: Jakarta, Indonesia, 2012; ISBN 978-602-98450-6-8. Available online: https://www.coraltriangleinitiative.org/sites/default/files/resources/8_Geographic%20Priorities%20for%20Marine%20Biodiversity%20Conservation%20in%20Indonesia.pdf (accessed on 28 May 2021).
- Convention on Biological Diversity Biodiversity Facts: Status and trends of biodiversity, including benefits from biodiversity and ecosystem services. Available online: https://www.cbd.int/countries/profile/?country=id (accessed on 20 January 2021).
- von Rintelen, K.; Arida, E.; Häuser, C. A review of biodiversity-related issues and challenges in megadiverse Indonesia and other Southeast Asian countries. Res. Ideas Outcomes 2017, 3, e20860. [Google Scholar] [CrossRef]
- De Bruyn, M.; Stelbrink, B.; Morley, R.J.; Hall, R.; Carvalho, G.R.; Cannon, C.H.; Van Den Bergh, G.; Meijaard, E.; Metcalfe, I.; Boitani, L.; et al. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Syst. Biol. 2014, 63, 879–901. [Google Scholar] [CrossRef]
- Lohman, D.J.; de Bruyn, M.; Page, T.; von Rintelen, K.; Hall, R.; Ng, P.K.L.; Shih, H.-T.; Carvalho, G.R.; von Rintelen, T. Biogeography of the Indo-Australian Archipelago. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 205–226. [Google Scholar] [CrossRef]
- Aditiawati, P.; Yohandini, H.; Madayanti, F. Akhmaloka Microbial diversity of acidic hot spring (kawah hujan B) in geothermal field of kamojang area, west java-indonesia. Open Microbiol. J. 2009, 3, 58–66. [Google Scholar] [CrossRef]
- Liu, B.; Talukder, M.J.H.; Terhonen, E.; Lampela, M.; Vasander, H.; Sun, H.; Asiegbu, F. The microbial diversity and structure in peatland forest in Indonesia. Soil Use Manag. 2020, 36, 123–138. [Google Scholar] [CrossRef]
- De Voogd, N.J.; Cleary, D.F.R.; Pol, A.R.M.; Gomes, N.C.M. Bacterial community composition and predicted functional ecology of sponges, sediment and seawater from the thousand islands reef complex, West Java, Indonesia. FEMS Microbiol. Ecol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Kanti, A.; Sumerta, I.N. Diversity of Xylose Assimilating Yeast From the Island of Enggano, Sumatera, Indonesia [Keragaman Khamir Pengguna Xilose Yang Diisolasi Dari Pulau Enggano, Sumatera, Indonesia]. Ber. Biol. 2016, 15. [Google Scholar] [CrossRef]
- Hennersdorf, P.; Kleinertz, S.; Theisen, S.; Abdul-Aziz, M.A.; Mrotzek, G.; Palm, H.W.; Saluz, H.P. Microbial diversity and parasitic load in tropical fish of different environmental conditions. PLoS ONE 2016, 11, e0151594. [Google Scholar] [CrossRef]
- Zam, S.I.; Agustien, A.; Djamaan, A.; Mustafa, I. The Diversity of Endophytic Bacteria from the Traditional Medicinal Plants Leaves that Have Anti-phytopathogens Activity. J. Trop. Life Sci. 2019, 9, 53–63. [Google Scholar] [CrossRef]
- Baltz, R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “one strain many compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Handayani, I.; Blin, K.; Kulik, A.; Mast, Y. Disclosing the Potential of the SARP-Type Regulator PapR2 for the Activation of Antibiotic Gene Clusters in Streptomycetes. Front. Microbiol. 2020, 11, 225. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H.; Aon, J.C. Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review. Front. Microbiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Wu, C.; van der Heul, H.U.; Melnik, A.V.; Lübben, J.; Dorrestein, P.C.; Minnaard, A.J.; Choi, Y.H.; van Wezel, G.P. Lugdunomycin, an Angucycline-Derived Molecule with Unprecedented Chemical Architecture. Angew. Chem. Int. Ed. 2019, 58, 2809–2814. [Google Scholar] [CrossRef]
- Hussain, A.; Rather, M.A.; Dar, M.S.; Aga, M.A.; Ahmad, N.; Manzoor, A.; Qayum, A.; Shah, A.; Mushtaq, S.; Ahmad, Z.; et al. Novel bioactive molecules from Lentzea violacea strain AS 08 using one strain-many compounds (OSMAC) approach. Bioorganic Med. Chem. Lett. 2017, 27, 2579–2582. [Google Scholar] [CrossRef]
- Zhang, C.; Seyedsayamdost, M.R. Discovery of a Cryptic Depsipeptide from Streptomyces ghanaensis via MALDI-MS-Guided High-Throughput Elicitor Screening. Angew. Chem. Int. Ed. 2020, 59, 23005–23009. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Current challenges in the discovery of novel antibacterials from microbial natural products. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2009, 46, 9.11.1–9.11.21. [Google Scholar] [CrossRef]
- Wohlleben, W.; Mast, Y.; Stegmann, E.; Ziemert, N. Antibiotic drug discovery. Microb. Biotechnol. 2016, 9, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Maansson, M.; Vynne, N.G.; Klitgaard, A.; Nybo, J.L.; Melchiorsen, J.; Nguyen, D.D.; Sanchez, L.M.; Ziemert, N.; Dorrestein, P.C.; Andersen, M.R.; et al. An Integrated Metabolomic and Genomic Mining Workflow To Uncover the Biosynthetic Potential of Bacteria. mSystems 2016, 1, e00028-15. [Google Scholar] [CrossRef]
- Ong, J.F.M.; Goh, H.C.; Lim, S.C.; Pang, L.M.; Chin, J.S.F.; Tan, K.S.; Liang, Z.-X.; Yang, L.; Glukhov, E.; Gerwick, W.H.; et al. Integrated Genomic and Metabolomic Approach to the Discovery of Potential Anti-Quorum Sensing Natural Products from Microbes Associated with Marine Samples from Singapore. Mar. Drugs 2019, 17, 72. [Google Scholar] [CrossRef]
- Tiam, S.K.; Gugger, M.; Demay, J.; Le Manach, S.; Duval, C.; Bernard, C.; Marie, B. Insights into the diversity of secondary metabolites of Planktothrix using a biphasic approach combining global genomics and metabolomics. Toxins 2019, 11, 498. [Google Scholar] [CrossRef]
- Amiri Moghaddam, J.; Crüsemann, M.; Alanjary, M.; Harms, H.; Dávila-Céspedes, A.; Blom, J.; Poehlein, A.; Ziemert, N.; König, G.M.; Schäberle, T.F. Analysis of the Genome and Metabolome of Marine Myxobacteria Reveals High Potential for Biosynthesis of Novel Specialized Metabolites. Sci. Rep. 2018, 8, 16600. [Google Scholar] [CrossRef]
- Albarano, L.; Esposito, R.; Ruocco, N.; Costantini, M. Genome mining as new challenge in natural products discovery. Mar. Drugs 2020, 18, 199. [Google Scholar] [CrossRef]
- Ward, A.C.; Allenby, N.E.E. Genome mining for the search and discovery of bioactive compounds: The Streptomyces paradigm. FEMS Microbiol. Lett. 2018, 365, fny240. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acid. Res. 2019, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; De Felicio, R.; Fenner, A.; et al. Molecular Networking as a Dereplication Strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef]
- Sieber, S.; Grendelmeier, S.M.; Harris, L.A.; Mitchell, D.A.; Gademann, K. Microviridin 1777: A Toxic Chymotrypsin Inhibitor Discovered by a Metabologenomic Approach. J. Nat. Prod. 2020, 83, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Nonomura, H. Efficacy of artificial humic acid as a selective nutrient in HV agar used for the isolation of soil actinomycetes. J. Ferment. Technol. 1987, 65, 609–616. [Google Scholar] [CrossRef]
- Hamada, M.; Lino, T.; Tamura, T.; Iwami, T.; Harayama, S.; Suzuki, K.I. Serinibacter salmoneus gen. nov., sp. nov., an actinobacterium isolated from the intestinal tract of a fish, and emended descriptions of the families Beutenbergiaceae and Bogoriellaceae. Int. J. Syst. Evol. Microbiol. 2009, 59, 2809–2814. [Google Scholar] [CrossRef]
- Hamada, M.; Shibata, C.; Nurkanto, A.; Ratnakomala, S.; Lisdiyanti, P.; Tamura, T.; Suzuki, K.I. Serinibacter tropicus sp. nov., an actinobacterium isolated from the rhizosphere of a mangrove, and emended description of the genus Serinibacter. Int. J. Syst. Evol. Microbiol. 2015, 65, 1151–1154. [Google Scholar] [CrossRef]
- Hayakawa, M.; Sadakata, T.; Kajiura, T.; Nonomura, H. New methods for the highly selective isolation of Micromonospora and Microbispora from soil. J. Ferment. Bioeng. 1991, 72, 320–326. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Grismer, L.L.; Riyanto, A.; Iskandar, D.T.; Mcguire, J.A. A new species of Hemiphyllodactylus Bleeker, 1860 (Squamata: Gekkonidae) from Pulau Enggano, southwestern Sumatra, Indonesia. Zootaxa 2014, 3821, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Jakl, S. New cetoniine beetle from Enggano and Simeuleu Islands west of Sumatra (Coleoptera: Scarabaeidae: Cetoniinae). Stud. Rep. Dist. Museum Prague-East Taxon. Ser. 2008, 4, 103–110. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.R.; Buddana, S.K.; Tatipamula, V.B.; Naga, Y.V.V.; Ahmad, J. Production of polypeptide antibiotic from Streptomyces parvulus and its antibacterial activity. Braz. J. Microbiol. 2014, 45, 303–312. [Google Scholar] [CrossRef]
- Handayani, I.; Ratnakomala, S.; Lisdiyanti, P.; Fahrurrozi; Kusharyoto, W.; Alanjary, M.; Ort-Winklbauer, R.; Kulik, A.; Wohlleben, W.; Mast, Y. Complete Genome Sequence of Streptomyces sp. Strain SHP22-7, a New Species Isolated from Mangrove of Enggano Island, Indonesia. Microbiol. Resour. Announc. 2018, 7, e01317-18. [Google Scholar] [CrossRef]
- Handayani, I.; Ratnakomala, S.; Lisdiyanti, P.; Fahrurrozi; Alanjary, M.; Wohlleben, W.; Mast, Y. Complete genome sequence of Streptomyces sp. strain BSE7F, a Bali mangrove sediment actinobacterium with antimicrobial activities. Genome Announc. 2018, 6, e00618-18. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Ratnakomala, S.; Lisdiyanti, P.; Ort-Winklbauer, R.; Wohlleben, W.; Mast, Y. Complete Genome Sequence of the Putative Phosphonate Producer Streptomyces sp. Strain I6, Isolated from Indonesian Mangrove Sediment. Microbiol. Resour. Announc. 2019, 8, e01580-18. [Google Scholar] [CrossRef]
- Tidjani, A.R.; Lorenzi, J.N.; Toussaint, M.; Van Dijk, E.; Naquin, D.; Lespinet, O.; Bontemps, C.; Leblond, P. Massive gene flux drives genome diversity between sympatric Streptomyces conspecifics. MBio 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Thakur, V.; Saxena, R.K.; Vadlamudi, S.; Purohit, S.; Kumar, V.; Rathore, A.; Chitikineni, A.; Varshney, R.K. Complete genome sequence of sixteen plant growth promoting Streptomyces strains. Sci. Rep. 2020, 10, 10294. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the Evolutionary History of an Ancient Phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Meier-kolthoff, J.P.; Klenk, H.; Go, M. Taxonomic use of DNA G + C content and DNA–DNA hybridization in the genomic age. Int. J. Syst. Evol. Microbiol. 2014, 64, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B.J.; Rosselló-Móra, R.; Busse, H.J.; Ludwig, W.; Kämpfer, P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int. J. Syst. Evol. Microbiol. 2010, 60, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Luo, X.X.; Kai, L.; Wang, Y.; Wan, C.X.; Zhang, L.L. Streptomyces luteus sp. nov., an actinomycete isolated from soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Mertz, F.P.; Higgens, C.E. Streptomyces capillispiralis sp. nov. Int. J. Syst. Bacteriol. 1982, 32, 116–124. [Google Scholar] [CrossRef]
- Eguchi, T.; Takada, N.; Nakamura, S.; Tanaka, T.; Makino, T.; Oshima, Y. Streptomyces bungoensis sp. nov. Int. J. Syst. Bacteriol. 1993, 43, 794–798. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, S.; Huang, D.; Chen, J.; Zhu, W. Streptomyces spongiicola sp. Nov., An actinomycete derived from marine sponge. Int. J. Syst. Evol. Microbiol. 2016, 66, 738–743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belknap, K.C.; Park, C.J.; Barth, B.M.; Andam, C.P. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.O.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Poralla, K.; Muth, G.; Härtner, T. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 2000, 189, 93–95. [Google Scholar] [CrossRef]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef]
- Waksman, S.A.; Lechevalier, H.A.; Schaffner, C.P. Candicidin and other polyenic antifungal antibiotics. Bull. World Health Organ. 1965, 33, 219. [Google Scholar] [PubMed]
- Tierrafría, V.H.; Ramos-Aboites, H.E.; Gosset, G.; Barona-Gómez, F. Disruption of the siderophore-binding desE receptor gene in Streptomyces coelicolor A3(2) results in impaired growth in spite of multiple iron-siderophore transport systems. Microb. Biotechnol. 2011, 4, 275–285. [Google Scholar] [CrossRef]
- van der Meij, A.; Worsley, S.F.; Hutchings, M.I.; van Wezel, G.P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework for systematic exploration of biosynthetic diversity from large-scale genomic data. bioRxiv 2018, 445270. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Yagüe, P.; Lopez-Garcia, M.T.; Rioseras, B.; Sanchez, J.; Manteca, A. New insights on the development of Streptomyces and their relationships with secondary metabolite production. Curr. Trends Microbiol. 2012, 8, 65–73. [Google Scholar] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, UK, 2000; ISBN 0708406238. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Böcker, S.; Dührkop, K. Fragmentation trees reloaded. J. Cheminform. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- van Tamelen, E.E.; Dickie, J.P.; Loomans, M.E.; Dewey, R.S.; Strong, F.M. The Chemistry of Antimycin A. X. Structure of the Antimycins1. J. Am. Chem. Soc. 1961, 83, 1639–1646. [Google Scholar] [CrossRef]
- Barrow, C.J.; Oleynek, J.J.; Marinelli, V.; Sun, H.H.; Kaplita, P.; Sedlock, D.M.; Gillum, A.M.; Chadwick, C.C.; Cooper, R. Antimycins, inhibitors of ATP-citrate lyase, from a Streptomyces sp. J. Antibiot. 1997, 50, 729–733. [Google Scholar] [CrossRef]
- Hosotani, N.; Kumagai, K.; Nakagawa, H.; Shimatani, T.; Saji, I. Antimycins A10∼A16, Seven New Antimycin Antibiotics Produced by Streptomyces spp. SPA-10191 and SPA-8893. J. Antibiot. 2005, 58, 460–467. [Google Scholar] [CrossRef]
- Sidebottom, A.M.; Johnson, A.R.; Karty, J.A.; Trader, D.J.; Carlson, E.E. Integrated Metabolomics Approach Facilitates Discovery of an Unpredicted Natural Product Suite from Streptomyces coelicolor M145. ACS Chem. Biol. 2013, 8, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Traxler, M.F.; Watrous, J.D.; Alexandrov, T.; Dorrestein, P.C.; Kolter, R. Interspecies Interactions Stimulate Diversification of the Streptomyces coelicolor Secreted Metabolome. MBio 2013, 4, e00459-13. [Google Scholar] [CrossRef]
- Blum, S.; Fielder, H.P.; Groth, I.; Kempter, C.; Stephan, H.; Nicholson, G.; Metzger, J.W.; Jung, G. Biosynthetic capacities of actinomycetes. 4. Echinoserine, a new member of the quinoxaline group, produced by Streptomyces tendae. J. Antibiot. 1995, 48, 619–625. [Google Scholar] [CrossRef]
- Cox, G.; Sieron, A.; King, A.M.; De Pascale, G.; Pawlowski, A.C.; Koteva, K.; Wright, G.D. A Common Platform for Antibiotic Dereplication and Adjuvant Discovery. Cell Chem. Biol. 2017, 24, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Keller-Schierlein, W.; Mihailovié, M.L.; Prelog, V. Stoffwechselprodukte von Actinomyceten. 15. Mitteilung. über die Konstitution von Echinomycin. Helv. Chim. Acta 1959, 42, 305–322. [Google Scholar] [CrossRef]
- Yu, Z.; Vodanovic-Jankovic, S.; Ledeboer, N.; Huang, S.-X.; Rajski, S.R.; Kron, M.; Shen, B. Tirandamycins from Streptomyces sp. 17944 inhibiting the parasite Brugia malayi asparagine tRNA synthetase. Org. Lett. 2011, 13, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.C.; Li, S.; Burr, D.A.; Sherman, D.H. Isolation and Characterization of Tirandamycins from a Marine-Derived Streptomyces sp. J. Nat. Prod. 2009, 72, 2076–2079. [Google Scholar] [CrossRef]
- Kluepfel, D.; Baker, H.A.; Piattoni, G.; Sehgal, S.N.; Sidorowicz, A.; Singh, K.; Vézina, C. Naphthyridinomycin, a new broad-spectrum antibiotic. J. Antibiot. 1975, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Cang, S.; Ohta, S.; Chiba, H.; Johdo, O.; Nomura, H.; Nagamatsu, Y.; Yoshimoto, A. New naphthyridinomycin-type antibiotics, aclidinomycins A and B, from Streptomyces halstedi. J. Antibiot. 2001, 54, 304–307. [Google Scholar] [CrossRef][Green Version]
- Bernan, V.S.; Montenegro, D.A.; Korshalla, J.D.; Maiese, W.M.; Steinberg, D.A.; Greenstein, M. Bioxalomycins, new antibiotics produced by the marine Streptomyces sp. LL-31F508: Taxonomy and fermentation. J. Antibiot. 1994, 47, 1417–1424. [Google Scholar] [CrossRef]
- Banskota, A.H.; McAlpine, J.B.; Sørensen, D.; Ibrahim, A.; Aouidate, M.; Piraee, M.; Alarco, A.M.; Farnet, C.M.; Zazopoulos, E. Genomic analyses lead to novel secondary metabolites: Part 3 ECO-0501, a novel antibacterial of a new class. J. Antibiot. 2006, 59, 533–542. [Google Scholar] [CrossRef]
- Stevens, C.L.; Nagarajan, K.; Haskell, T.H. The Structure of Amicetin. J. Org. Chem. 1962, 27, 2991–3005. [Google Scholar] [CrossRef]
- Haneda, K.; Shinose, M.; Seino, A.; Tabata, N.; Tomoda, H.; Iwai, Y.; Omura, S. Cytosaminomycins, new anticoccidial agents produced by Streptomyces sp. KO-8119. I. Taxonomy, production, isolation and physico-chemical and biological properties. J. Antibiot. (Tokyo) 1994, 47, 774–781. [Google Scholar] [CrossRef]
- Bu, Y.; Yamazaki, H.; Ukai, K.; Namikoshi, M. Anti-Mycobacterial Nucleoside Antibiotics from a Marine-Derived Streptomyces sp. TPU1236A. Mar. Drugs 2014, 6102–6112. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, K.; Huang, D.; Wu, W.; Xu, Y.; Xia, W. Complete genome sequence of Streptomyces spongiicola HNM0071T, a marine sponge-associated actinomycete producing staurosporine and echinomycin Marine Genomics Complete genome sequence of Streptomyces spongiicola HNM0071 T, a marine sponge-associated act. Mar. Genomics 2018, 1. [Google Scholar] [CrossRef]
- Ito, T.; Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. 2014, 67, 353–360. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef]
- Carrano, L.; Marinelli, F. The relevance of chemical dereplication in microbial natural product screening. J. Appl. Bioanal. 2015, 1, 55–67. [Google Scholar] [CrossRef]
- Chiani, M.; Akbarzadeh, A.; Farhangi, A.; Mazinani, M.; Saffari, Z.; Emadzadeh, K.; Mehrabi, M.R. Optimization of culture medium to increase the production of desferrioxamine B (Desferal) in Streptomyces pilosus. Pakistan J. Biol. Sci. 2010, 13, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Chiani, M.; Akbarzadeh, A.; Farhangi, A.; Mehrabi, M.R. Production of Desferoxamine B (Desferal) using Corn Steep Liquor in Streptomyces pilosus. Pakistan J. Biol. Sci. 2010, 13, 1151–1155. [Google Scholar] [CrossRef]
- Nicault, M.; Tidjani, A.-R.; Gauthier, A.; Dumarcay, S.; Gelhaye, E.; Bontemps, C.; Leblond, P. Mining the Biosynthetic Potential for Specialized Metabolism of a Streptomyces Soil Community. Antibiotics 2020, 9, 271. [Google Scholar] [CrossRef]
- Ishaque, N.M.; Burgsdorf, I.; Limlingan Malit, J.J.; Saha, S.; Teta, R.; Ewe, D.; Kannabiran, K.; Hrouzek, P.; Steindler, L.; Costantino, V.; et al. Isolation, Genomic and Metabolomic Characterization of Streptomyces tendae VITAKN with Quorum Sensing Inhibitory Activity from Southern India. Microorganisms 2020, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- AbuSara, N.F.; Piercey, B.M.; Moore, M.A.; Shaikh, A.A.; Nothias, L.-F.; Srivastava, S.K.; Cruz-Morales, P.; Dorrestein, P.C.; Barona-Gómez, F.; Tahlan, K. Comparative Genomics and Metabolomics Analyses of Clavulanic Acid-Producing Streptomyces Species Provides Insight Into Specialized Metabolism. Front. Microbiol. 2019, 10, 2550. [Google Scholar] [CrossRef]
- Camesi, A.B.R.; Lukito, A.; Waturangi, D.E.; Kwan, H.J. Screening of Antibiofilm Activity from Marine Bacteria against Pathogenic Bacteria. Microbiol. Indones. 2016, 10, 87–94. [Google Scholar] [CrossRef]
- Artanti, N.; Maryani, F.; Mulyani, H.; Dewi, R.; Saraswati, V.; Murniasih, T. Bioactivities Screening of Indonesian Marine Bacteria Isolated from Sponges. Ann. Bogor. 2016, 20, 23–28. [Google Scholar]
- Cristianawati, O.; Sibero, M.T.; Ayuningrum, D.; Nuryadi, H.; Syafitri, E.; Radjasa, O.K.; Riniarsih, I. Screening of antibacterial activity of seagrass-associated bacteria from the North Java Sea, Indonesia against multidrug-resistant bacteria. AACL Bioflux 2019, 12, 1054–1064. [Google Scholar]
- Nurkanto, A.; Julistiono, H.; Agusta, A.; Sjamsuridzal, W.; Aktivitas, P.; Ampat, R.; Barat, P. Screening Antimicrobial Activity of Actinomycetes Isolated from Raja Ampat, West Papua, Indonesia. Makara J. Sci. 2012, 1, 21–26. [Google Scholar] [CrossRef]
- Mahdiyah, D.; Farida, H.; Riwanto, I.; Mustofa, M.; Wahjono, H.; Laksana Nugroho, T.; Reki, W. Screening of Indonesian peat soil bacteria producing antimicrobial compounds. Saudi J. Biol. Sci. 2020, 27, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Fotso, S.; Mahmud, T.; Zabriskie, T.M.; Santosa, D.A.; Sulastri; Proteau, P.J. Angucyclinones from an Indonesian Streptomyces sp. J. Nat. Prod. 2008, 71, 61–65. [Google Scholar] [CrossRef]
- Fotso, S.; Mahmud, T.; Zabriskie, T.M.; Santosa, D.A.; Sulastri; Proteau, P.J. Rearranged and unrearranged angucyclinones from Indonesian Streptomyces spp. J. Antibiot. 2008, 61, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fotso, S.; Santosa, D.A.; Saraswati, R.; Yang, J.; Mahmud, T.; Mark Zabriskie, T.; Proteau, P.J. Modified phenazines from an indonesian Streptomyces sp. J. Nat. Prod. 2010, 73, 472–475. [Google Scholar] [CrossRef][Green Version]
- Sheng, Y.; Lam, P.W.; Shahab, S.; Santosa, D.A.; Proteau, P.J.; Zabriskie, T.M.; Mahmud, T. Identification of Elaiophylin Skeletal Variants from the Indonesian Streptomyces sp. ICBB 9297. J. Nat. Prod. 2015, 78, 2768–2775. [Google Scholar] [CrossRef]
- Fotso, S.; Zabriskie, T.M.; Proteau, P.J.; Flatt, P.M.; Santosa, D.A.; Sulastri; Mahmud, T.; Limazepines, A.-F. pyrrolo[1,4]benzodiazepine antibiotics from an Indonesian Micrococcus sp. J. Nat. Prod. 2009, 72, 690–695. [Google Scholar] [CrossRef]
- Sheng, Y.; Fotso, S.; Serrill, D.; Shahab, S.; Santosa, D.A.; Ishmael, J.E.; Proteau, P.J.; Zabriskie, T.M.; Mahmud, T. Succinylated Apoptolidins from Amycolatopsis sp. ICBB 8242. Org. Lett. 2015, 17, 2526–2529. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Labortaroy Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1989; ISBN 0879695773. [Google Scholar]
- Garg, N.; Kapono, C.A.; Lim, Y.W.; Koyama, N.; Vermeij, M.J.A.; Conrad, D.; Rohwer, F.; Dorrestein, P.C. Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. Int. J. Mass Spectrom. 2015, 377, 719–727. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).