Astaxanthin Delivery Systems for Skin Application: A Review
Abstract
:1. Introduction
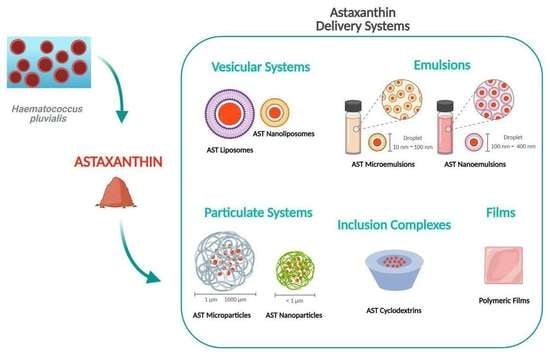
2. Astaxanthin Delivery Systems for Skin Application
2.1. Vesicular Systems
Liposomes
2.2. Emulsions
2.2.1. Microemulsions
2.2.2. Nanoemulsions
2.3. Particulate Systems
2.3.1. Microparticles
2.3.2. Nanoparticles
2.4. Inclusion Complexes
Cyclodextrin
2.5. Films
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [Green Version]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel insights into the biotechnological production of Haematococcus pluvialis-derived astaxanthin: Advances and key challenges to allow its industrial use as novel food ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Microalgal Application in Cosmetics. In Microalgae in Health and Disease Prevention; Fleurence, J., Levine, I.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 317–323. [Google Scholar]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Gürsoy, K.; Teymur, H.; Koca, G.; Tanas Işikçi, Ö.; Göktaş Demircan, F.B.; Kankaya, Y.; Koçer, U. The Effect of Astaxanthin on Random Pattern Skin Flaps. Ann. Plast. Surg. 2020, 84, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people—a randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihisa, Y.; Andoh, T.; Matsunaga, K.; Ur Rehman, M.; Maoka, T.; Shimizu, T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS ONE 2016, 11, e0152288. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. The Xanthophyll Carotenoid Astaxanthin has Distinct Biological Effects to Prevent the Photoaging of the Skin Even by its Postirradiation Treatment. Photochem. Photobiol. 2019, 95, 490–500. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Nakajima, H.; Ohtsuki, M.; Imokawa, G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J. Dermatol. Sci. 2010, 58, 136–142. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Rehman, M.u.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef]
- Seki, T.; Sueki, H.; Kono, H.; Suganuma, K.; Yamashita, E. Effects of astaxanthin from haematococcus pluvialis on human skin. Fr. J. 2001, 12, 98–103. [Google Scholar]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of astaxanthin on cutaneous wound healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Anti-inflammatory effect of Astaxanthin in phthalic anhydride- induced atopic dermatitis animal model. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surfaces B Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer-Korting, M.; Korting, H.C.; Braun-Falco, O. Liposome preparations: A step forward in topical drug therapy for skin disease? A review. J. Am. Acad. Dermatol. 1989, 21, 1271–1275. [Google Scholar] [CrossRef]
- Dopierała, K.; Karwowska, K.; Petelska, A.D.; Prochaska, K. Thermodynamic, viscoelastic and electrical properties of lipid membranes in the presence of astaxanthin. Biophys. Chem. 2020, 258, 106318. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta-Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Sung, D.K.; Kim, S.H.; Choi, W.I.; Hwang, E.T.; Choi, D.J.; Chang, J.H. Controlled release of astaxanthin from nanoporous silicified-phospholipids assembled boron nitride complex for cosmetic applications. Appl. Surf. Sci. 2017, 424, 15–19. [Google Scholar] [CrossRef]
- Hama, S.; Uenishi, S.; Yamada, A.; Ohgita, T.; Tsuchiya, H.; Yamashita, E.; Kogure, K. Scavenging of Hydroxyl Radicals in Aqueous Solution by Astaxanthin Encapsulated in Liposomes. Biol. Pharm. Bull. 2012, 35, 2238–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, J.; Yang, S.; Xue, Y.; Zhang, T.; Wang, J.; Xue, C. The effect of various antioxidants on the degradation of o/w microemulsions containing esterified astaxanthins from Haematococcus pluvialis. J. Oleo Sci. 2015, 64, 515–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Kim, D.M.; Hyun, S.S.; Yun, P.; Lee, C.H.; Byun, S.Y. Identification of an emulsifier and conditions for preparing stable nanoemulsions containing the antioxidant astaxanthin. Int. J. Cosmet. Sci. 2012, 34, 64–73. [Google Scholar] [CrossRef]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.S.; Kang, H.W. Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: Investigation on anticancer, wound healing, and antibacterial effects. Colloids Surf. B Biointerfaces 2018, 172, 170–179. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. A new alternative insight of nanoemulsion conjugated with κ-carrageenan for wound healing study in diabetic mice: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 133, 236–250. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Violleau, F.; Silvestre, F.; Durrieu, V. A new way of valorizing biomaterials: The use of sunflower protein for α-tocopherol microencapsulation. Food Res. Int. 2013, 53, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application-a review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Rubilar, M.; Jofré, I.; Villarroel, M.; Navarrete, P.; Esparza, M.; Romero, F.; Vilches, E.A.; Acevedo, V.; Shene, C. Oil bodies as a potential microencapsulation carrier for astaxanthin stabilisation and safe delivery. J. Microencapsul. 2014, 31, 488–500. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Lin, S.F.; Chen, Y.C.; Chen, R.N.; Chen, L.C.; Ho, H.O.; Tsung, Y.H.; Sheu, M.T.; Liu, D.Z. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, M.; Zhao, Z.; Lin, Q.; Wei, D.; Jiang, Y. Enhancing the stability of astaxanthin by encapsulation in poly (l-lactic acid) microspheres using a supercritical anti-solvent process. Particuology 2019, 44, 54–62. [Google Scholar] [CrossRef]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Venegas, J.B.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core-Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Boucher, J.; Cengelli, F.; Trumbic, D.; Marison, I.W. Sorption of hydrophobic organic compounds (HOC) in rapeseed oil bodies. Chemosphere 2008, 70, 1452–1458. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Magalhães, T.S.S.d.A.; Macedo, P.C.d.O.; Pacheco, S.Y.K.; da Silva, S.S.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and evaluation of antimicrobial and modulatory activity of inclusion complex of euterpe oleracea mart oil and β-cyclodextrin or HP-β-cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, J.G.d.O.; Tavares, E.d.A.; Silva, S.S.D.; Félix Silva, J.; Carvalho, Y.M.B.G.D.; Ferreira, M.R.A.; Araúj, A.A.d.S.; Barbosa, E.G.; Fernandes Pedrosa, M.d.F.; Soares, L.A.L.; et al. Inclusion complexes of copaiba (Copaifera multijuga hayne) oleoresin and cyclodextrins: Physicochemical characterization and anti-inflammatory activity. Int. J. Mol. Sci. 2017, 18, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Júnior, W.F.; Bezerra de Menezes, D.L.; de Oliveira, L.C.; Koester, L.S.; Oliveira de Almeida, P.D.; Lima, E.S.; de Azevedo, E.P.; da Veiga Júnior, V.F.; Neves de Lima, Á.A. Inclusion complexes of β and HPβ-cyclodextrin with α, β amyrin and in vitro anti-inflammatory activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.B.; da Silva Junior, W.F.; de Oliveira Pinheiro, J.G.; Da Fonseca, A.G.; Moura Lemos, T.M.A.; de Oliveira Rocha, H.A.; De Azevedo, E.P.; Mendonça Junior, F.J.B.; Neves de Lima, A.A. Characterization and antiproliferative activity of a novel 2-aminothiophene derivative-β-cyclodextrin binary system. Molecules 2018, 23, 3130. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-β, β-carotene-4,4′-dione) by Captisol® (sulfobutyl ether β-cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Kim, S.; Cho, E.; Yoo, J.; Cho, E.; Ju Choi, S.; Son, S.M.; Lee, J.M.; In, M.J.; Kim, D.C.; Kim, J.H.; et al. β-CD-mediated encapsulation enhanced stability and solubility of Astaxanthin. J. Appl. Biol. Chem. 2010, 53, 559–565. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.; Xu, X.; Zhuang, H.; Shen, W. Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 2008, 109, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jin, Z.; Xu, X. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative Stress Regulation on Endothelial Cells by Hydrophilic Astaxanthin Complex: Chemical, Biological, and Molecular Antioxidant Activity Evaluation. Oxid. Med. Cell. Longev. 2017, 2017, 8073798. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]



| Preparation Technique | Liposome Type | Characterization | Storage and Stability Data | Assays | References |
|---|---|---|---|---|---|
| Dissolution of hydrogenated lecithin and treatment by a high-pressure homogenizer to form nanoemulsions and tetraethyl orthosilicate addition to promote silification | Lecithin silicified liposomes | Brauner–Emmett–Teller isotherm, field emission scanning electron microscopy, Fourier transform infrared spectroscopy, UV–visible spectrophotometry | - | In vitro: DPPH free radical scavenging activity and drug release profile | [31] |
| Film dispersion-ultrasonic technique | Soybean phosphatidylcholine nanoliposomes | Dynamic light scattering, transmission electron microscopy, X-ray diffraction, differential scanning calorimetry, thermal gravimetric analysis, and dissolution study. | Thermal stability enhanced after encapsulation | In vitro: drug release profile | [27] |
| Lipid hydration method | Egg phosphatidylcholine liposomes | Dynamic light scattering | - | In vitro: antioxidant activity In vivo: UV treatment of mouse dorsal skin and effect of iontophoretic transdermal delivery | [18] |
| Lipid hydration method | Egg phosphatidylcholine liposomes | - | - | In vitro antioxidant activity by scavenging hydroxyl radical, and protective effect against cytotoxicity induced by hydroxyl radical | [32] |
| Preparation Technique | Emulsion Type | Characterization | Storage and Stability Data | Assays (In Vitro, In Vivo) | References |
|---|---|---|---|---|---|
| High-pressure homogenization | Oil/water nanoemulsion, glyceryl ester and hydrogenated lecithin as emulsifiers | Dynamic light scattering and transmission electron microscopy | Stability maintained for one month of storage | - | [37] |
| Low-energy emulsion phase inversion method | Oil/water nanoemulsion functionalized carboxymethyl chitosan | Droplet size, zeta potential and transmission electron microscopy | Stability without alteration for three months | In vitro: skin permeation studies, Cell viability assays on L929 cells, Cell culture and cytotoxicity assays | [38] |
| Spontaneous and ultrasonication emulsification methods | Oil/water nanoemulsion | Dynamic light scattering and transmission electron microscopy | Interference of storage conditions | In vitro: cytotoxicity (MTT assay), antimicrobial activity and scratch wound healing assay | [39] |
| Spontaneous and ultrasonication emulsification methods | Oil/water nanoemulsion | Dynamic light scattering and transmission electron microscopy, Fourier transform infrared spectroscopy, differential scanning calorimetry, X-ray diffraction, thermal gravimetric analysis, and scanning electron microscopy | - | In vitro: cytotoxicity (MTT assay), scratch wound-healing assay. In vivo: wound healing in nondiabetic and diabetic mice | [40] |
| Oil phase dispersed with AST in ethyl butyrate and homogenizing with aqueous phase in a high-speed blender and high-pressure microfluidizer | Oil/water microemulsions | Dynamic light scattering and UV-visible spectrophotometry | - | - | [35] |
| Preparation Technique | System Type | Characterization | Storage and Stability Data | Assays | References |
|---|---|---|---|---|---|
| AST microencapsulation by response surface methodology | Oil bodies (isolated from mature seeds) microcapsules | Fourier transform infrared spectroscopy (FT-IR), flow cytometry and microscopy | Oxidative stability, double half-life compared to free AST | In vitro: absorption assay | [43] |
| Multiple emulsion/ solvent evaporation | Chitosan matrix cross-linked with glutaraldehyde microparticles | AST extract analysis by high-performance liquid chromatography (HPLC) | Pigment quantity during microcapsules storage at 25, 35 and 45 °C | In vitro: storage stability evaluation | [44] |
| Extrusion | Calcium alginate microparticles | Analysis of AST content by HPLC | Various environmental conditions: light, temperature and nitrogen gas | In vitro: assay of AST content | [45] |
| Supercritical anti-solvent | Poly(L-lactic acid) microspheres | Scanning electron microscopy (SEM), transmission electron microscopy (TEM), FT-IR, X-ray diffraction (XRD), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), UV-visible spectrophotometry | 6-Month measurements by UV–vis spectrophotometry | In vitro: assay of AST content and AST release profile | [46] |
| Emulsion solvent evaporation | Poly(lactic-co-glycolic acid) (PLGA) copolymer nanoparticles | Dynamic light scattering (DLS), SEM, TEM, FT-IR, XRD, TGA, DSC | - | In vitro: anti-photodamage effect in HaCaT cells | [47] |
| Hot homogenization | Nanostructured lipid carriers | DLS, atomic force microscopy, SEM | Samples stored at 4 °C, protected from light for 1 month | In vitro: antioxidant activity by the α-tocopherol equivalent antioxidant capacity assay | [48] |
| Macromolecular co-assembly combined with solvent evaporation | Natural DNA and chitosan nanocarriers | DLS, TEM, field emission SEM, HPLC (AST content) | - | In vitro: oxidative stress, cytotoxicity (MTT assay) and cell uptake assay | [49] |
| Antisolvent precipitation method combined with electrostatic deposition method | PLGA and chitosan oligosaccharides nanoparticles | DLS, SEM, TEM, FT-IR, XRD, DSC | 72 h of storage at room temperature | In vitro: cytotoxicity and AST release profile | [50] |
| Solvent displacement process | Ethylcellulose, Poly(ethylene oxide) 4-methoycinnamoyl-phthaloylchitosan and poly(vinylalcohol-covinyl-4- methoxycinnamate nanospheres | SEM, TEM | Thermal stability | In vitro: AST release profile | [51] |
| CDs | Characterization | Storage and Stability Data | Assays | References |
|---|---|---|---|---|
| β-cyclodextrin (β-CD) | High-performance liquid chromatography (HPLC), scanning electron microscopy and Fourier transform infrared spectroscopy (FT-IR) | Stability enhanced by over 7–9 folds under various storage conditions such as pH, temperature, ultraviolet irradiation, and presence of oxygen | In vitro: water solubility | [63] |
| Sulfobutyl ether β-CD | UV-visible spectrophotometry | - | In vitro: water solubility | [62] |
| β-CD | HPLC | Storage at 4, 30, 57 °C and under light (light intensity of 1500 lux) | In vitro water solubility | [57] |
| Hydroxypropyl- β-cyclodextrin (HP-β-CD) | Thermogravimetry, UV-visible spectrophotometry, FT-IR, molecular modeling, nucleic magnetic resonance | Stability under oxygen and light at 4, 25 and 50 °C, storage at 4 and 25 °C in dark incubators | In vitro: water solubility, antioxidant capacity by reducing power, DPPH free radical scavenging activity and hydroxyl radical scavenging activity | [64,65,66] |
| HP-β-CD | FT-IR, UV-visible spectrophotometry | Storage at 6 °C under light protection for 6 months | In vitro cytoprotective activity of HP-β-CD complex. Direct biological evaluation of HP-β-CD antioxidant capacity Indirect HP-β-CD antioxidant protection against reactive oxygen species | [67] |
| Preparation Technique | Filming Agent | Characterization | Storage and Stability Data | Assays |
|---|---|---|---|---|
| Collagen solution incorporating AST and gentamicin | Biomaterials extracted from the waste material of the outer skin of the squid Doryteuthis singhalensis | Scanning electron microscopy, energy dispersive X-ray spectroscopy, X-ray diffraction | - | In vitro: biodegradation study and DPPH free radical scavenging activity In vivo: wound-healing activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, S.G.M.; Freire, M.C.L.C.; Oliveira, V.d.S.; Solisio, C.; Converti, A.; de Lima, Á.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511. https://doi.org/10.3390/md19090511
Lima SGM, Freire MCLC, Oliveira VdS, Solisio C, Converti A, de Lima ÁAN. Astaxanthin Delivery Systems for Skin Application: A Review. Marine Drugs. 2021; 19(9):511. https://doi.org/10.3390/md19090511
Chicago/Turabian StyleLima, Sarah Giovanna Montenegro, Marjorie Caroline Liberato Cavalcanti Freire, Verônica da Silva Oliveira, Carlo Solisio, Attilio Converti, and Ádley Antonini Neves de Lima. 2021. "Astaxanthin Delivery Systems for Skin Application: A Review" Marine Drugs 19, no. 9: 511. https://doi.org/10.3390/md19090511
APA StyleLima, S. G. M., Freire, M. C. L. C., Oliveira, V. d. S., Solisio, C., Converti, A., & de Lima, Á. A. N. (2021). Astaxanthin Delivery Systems for Skin Application: A Review. Marine Drugs, 19(9), 511. https://doi.org/10.3390/md19090511