A Multi-Species Investigation of Sponges’ Filtering Activity towards Marine Microalgae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessement of Reproducibility
2.2. Effect of Cell Size on Cleaning Capacity
2.3. Effect of Initial Cell Concentration on Cleaning Capacity
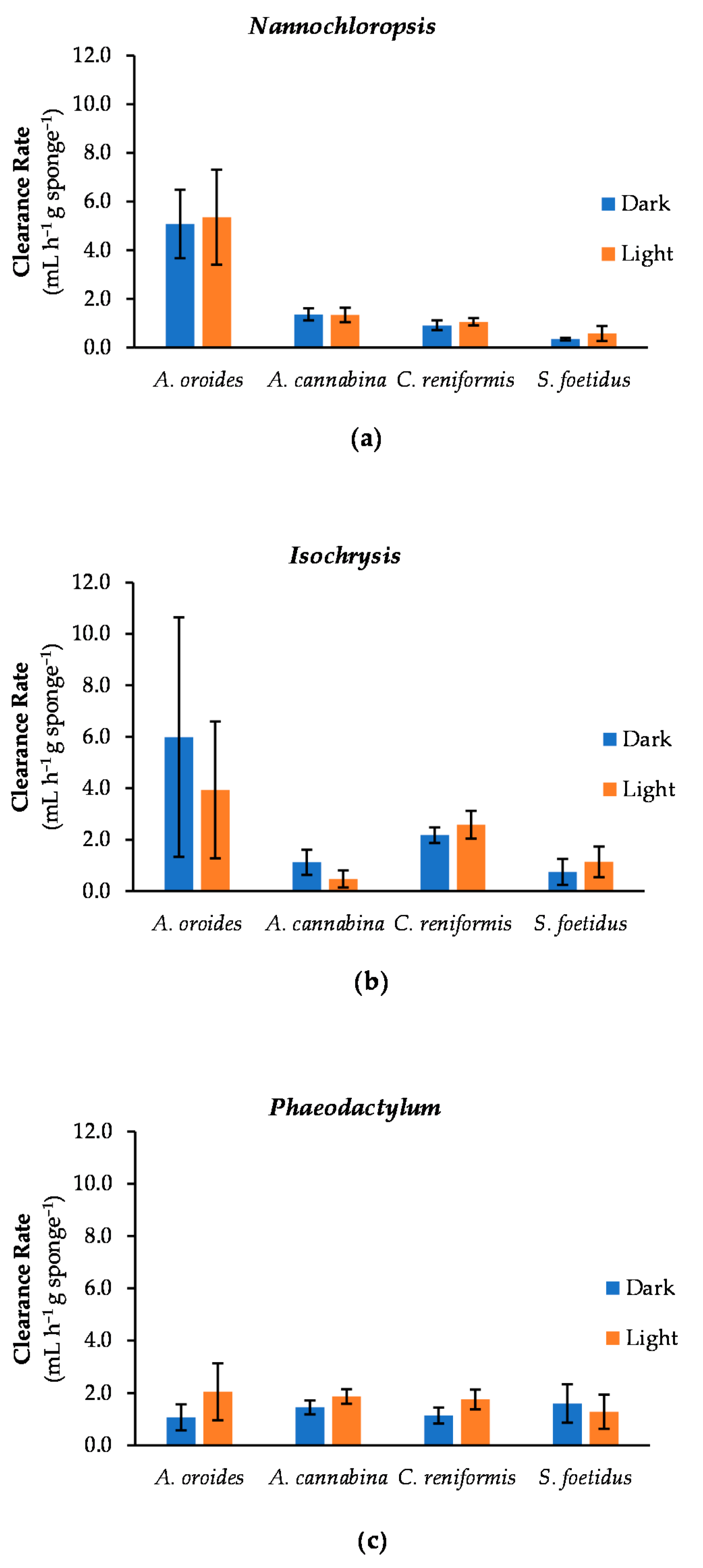
2.4. Effect of Light Intensity on Cleaning Capacity
3. Materials and Methods
3.1. Sponge Species Studied
3.2. Sponge Sampling
3.3. Biological Substrates
3.4. Experimental Procedures
3.5. Preliminary Experiments
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Wu, R.S.S. The Environmental Impact of Marine Fish Culture: Towards a Sustainable Future. Mar. Pollut. Bull. 1995, 31, 159–166. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Hernández-González, M.C.; Aranda, C.; Chopin, T.; Neori, A.; Halling, C.; Troell, M. Mariculture waste management. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2211–2217. [Google Scholar] [CrossRef]
- Belias, C.V.; Bikas, V.G.; Dassenakis, M.J.; Scoullos, M.J. Environmental Impacts of Coastal Aquaculture in Eastern Mediterranean Bays: The Case of Astakos Gulf, Greece. Environ. Sci. Pollut. Res. Int. 2003, 10, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated Aquaculture: Rationale, Evolution and State of the Art Emphasizing Seaweed Biofiltration in Modern Mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated Multi-Trophic Aquaculture (IMTA) in marine temperate waters. In Integrated Mariculture: A Global Review; Food & Agriculture Organization: Rome, Italy, 2009; pp. 7–46. [Google Scholar]
- Reitner, J.; Wörheide, G. Non-lithistid fossil demospongiae—Origins of their palaeobiodiversity and highlights in history of preservation. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Willenz, P., Eds.; Springer: Boston, MA, USA, 2002; pp. 52–68. [Google Scholar] [CrossRef]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sarà, A.; Sidri, M.; Pronzato, R. The Marine Sponge Chondrilla Nucula Schmidt, 1862 as an Elective Candidate for Bioremediation in Integrated Aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering Activity of Spongia Officinalis Var. Adriatica (Schmidt) (Porifera, Demospongiae) on Bacterioplankton: Implications for Bioremediation of Polluted Seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef]
- Osinga, R.; Tramper, J.; Wijffels, R.H. Cultivation of Marine Sponges. Mar. Biotechnol. 1999, 1, 509–532. [Google Scholar] [CrossRef]
- Reiswig, H.M. In Situ Pumping Activities of Tropical Demospongiae. Mar. Biol. 1971, 9, 38–50. [Google Scholar] [CrossRef]
- Vogel, S. Current-Induced Flow through Living Sponges in Nature. Proc. Natl. Acad. Sci. USA 1977, 74, 2069–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, P.; Riisgård, H.U. The Sponge Pump. J. Theor. Biol. 1994, 168, 53–63. [Google Scholar] [CrossRef]
- Pile, A.; Witman, J. In Situ Grazing on Plankton <10 μm by the Boreal Sponge Mycale Lingua. Mar. Ecol. Prog. Ser. 1996, 141, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ribes, M.; Coma, R.; Gili, J.-M. Natural Diet and Grazing Rate of the Temperate Sponge Dysidea Avara (Demospongiae, Dendroceratida) throughout an Annual Cycle. Mar. Ecol. Prog. Ser. 1999, 176, 179–190. [Google Scholar] [CrossRef]
- Reiswig, H. Bacteria as Food for Temperate-Water Marine Sponges. Can. J. Zool. 1975, 53, 582–589. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Garrone, R.; Vacelet, J. Marine Sponges Discriminate between Food Bacteria and Bacterial Symbionts: Electron Microscope Radioautography and in Situ Evidence. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1984, 220, 519–528. [Google Scholar]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The Sponge Holobiont in a Changing Ocean: From Microbes to Ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Goeij, J.; Berg, H.; Oostveen, M.; Epping, E.; Duyl, F. Major Bulk Dissolved Organic Carbon (DOC) Removal by Encrusting Coral Reef Cavity Sponges. Mar. Ecol.—Prog. Ser. 2008, 357, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-Scale Production of Pharmaceuticals by Marine Sponges: Sea, Cell, or Synthesis? Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine Sponge Collagen: Isolation, Characterization and Effects on the Skin Parameters Surface-PH, Moisture and Sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Brümmer, F.; Nickel, M. Sustainable use of marine resources: Cultivation of sponges. In Sponges (Porifera); Müller, W.E.G., Ed.; Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 143–162. [Google Scholar] [CrossRef]
- Frost, T.M. In Situ Measurements of Clearance Rates for the Freshwater Sponge Spongilla lacustris. Limnol. Oceanogr. 1978, 23, 1034–1039. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Thomassen, S.; Jakobsen, H.; Larsen, P. Suspension-Feeding in Marine Sponges Halichondria-Panicea and Haliclona-Urceolus—Effects of Temperature on Filtration-Rate and Energy-Cost of Pumping. Mar. Ecol. Prog. Ser. 1993, 96, 177–188. [Google Scholar] [CrossRef]
- Osinga, R.; Kleijn, R.; Groenendijk, E.; Niesink, P.; Tramper, J.; Wijffels, R.H. Development of In Vivo Sponge Cultures: Particle Feeding by the Tropical Sponge Pseudosuberites Aff. Andrewsi. Mar. Biotechnol. 2001, 3, 0544–0554. [Google Scholar] [CrossRef]
- Fu, W.; Sun, L.; Zhang, X.; Zhang, W. Potential of the Marine Sponge Hymeniacidon Perleve as a Bioremediator of Pathogenic Bacteria in Integrated Aquaculture Ecosystems. Biotechnol. Bioeng. 2006, 93, 1112–1122. [Google Scholar] [CrossRef]
- Ledda, F.D.; Pronzato, R.; Manconi, R. Mariculture for Bacterial and Organic Waste Removal: A Field Study of Sponge Filtering Activity in Experimental Farming. Aquac. Res. 2014, 45, 1389–1401. [Google Scholar] [CrossRef]
- Longo, C.; Corriero, G.; Licciano, M.; Stabili, L. Bacterial Accumulation by the Demospongiae Hymeniacidon Perlevis: A Tool for the Bioremediation of Polluted Seawater. Mar. Pollut. Bull. 2010, 60, 1182–1187. [Google Scholar] [CrossRef]
- Coughlan, J. The Estimation of Filtering Rate from the Clearance of Suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- Trani, R.; Corriero, G.; de Pinto, M.C.; Mercurio, M.; Pazzani, C.; Pierri, C.; Scrascia, M.; Longo, C. Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus Spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. J. Mar. Sci. Eng. 2021, 9, 178. [Google Scholar] [CrossRef]
- Azov, Y. Eastern Mediterranean—A Marine Desert? Mar. Pollut. Bull. 1991, 23, 225–232. [Google Scholar] [CrossRef]
- Turon, X.; Galera, J.; Uriz, M.J. Clearance Rates and Aquiferous Systems in Two Sponges with Contrasting Life-History Strategies. J. Exp. Zool. 1997, 278, 22–36. [Google Scholar] [CrossRef]
- Duckworth, A.; Brück, W.; Janda, K.; Pitts, T.; Mccarthy, P. Retention Efficiencies of the Coral Reef Sponges Aplysina lacunosa, Callyspongia vaginalis and Niphates digitalis Determined by Coulter Counter and Plate Culture Analysis. Mar. Ecol. Prog. Ser. 2006, 2, 243–248. [Google Scholar] [CrossRef]
- Fu, W.; Wu, Y.; Sun, L.; Zhang, W. Efficient Bioremediation of Total Organic Carbon (TOC) in Integrated Aquaculture System by Marine Sponge Hymeniacidon Perleve. Biotechnol. Bioeng. 2007, 97, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R. Sponges; University of California Press: Oakland, CA, USA, 1978. [Google Scholar]
- Hill, M.S.; Hill, A.L. Porifera (sponges). In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 423–432. [Google Scholar] [CrossRef]
- Weissenfels, N. The Filtration Apparatus for Food Collection in Freshwater Sponges (Porifera, Spongillidae). Zoomorphology 1992, 112, 51–55. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P. Filter-Feeding in Marine Macro-Invertebrates: Pump Characteristics, Modelling and Energy Cost. Biol. Rev. Camb. Philos. Soc. 1995, 70, 67–106. [Google Scholar] [CrossRef]
- Simpson, T.L. The Cell Biology of Sponges; Springer: New York, NY, USA, 1984. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Microbial Associations in Sponges. I. Ecology, Physiology and Microbial Populations of Coral Reef Sponges. Mar. Biol. 1978, 49, 161–167. [Google Scholar] [CrossRef]
- Maldonado, M.; Zhang, X.; Cao, X.; Xue, L.; Cao, H.; Zhang, W. Selective Feeding by Sponges on Pathogenic Microbes: A Reassessment of Potential for Abatement of Microbial Pollution. Mar. Ecol. Prog. Ser. 2010, 403, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Wehrl, M.; Steinert, M.; Hentschel, U. Bacterial Uptake by the Marine Sponge Aplysina Aerophoba. Microb. Ecol. 2007, 53, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffers, S.; Nieuwland, G.; RPM, B.; Duyl, F. Removal of Bacteria and Nutrient Dynamics within the Coral Framework of Curacao (Netherlands Antilles). Coral Reefs 2004, 23, 413–422. [Google Scholar] [CrossRef]
- Gerrodette, T.; Flechsig, A.O. Sediment-Induced Reduction in the Pumping Rate of the Tropical Sponge Verongia Lacunosa. Mar. Biol. 1979, 55, 103–110. [Google Scholar] [CrossRef]
- Idan, T.; Goren, L.; Shefer, S.; Ilan, M. Sponges in a Changing Climate: Survival of Agelas oroides in a Warming Mediterranean Sea. Front. Mar. Sci. 2020, 7, 603593. [Google Scholar] [CrossRef]
- Manconi, R.; Cadeddu, B.; Ledda, F.; Pronzato, R. An Overview of the Mediterranean Cave-Dwelling Horny Sponges (Porifera, Demospongiae). ZooKeys 2013, 281, 1–68. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Dimitriadis, C.; Arvanitidis, C.; Voultsiadou, E. Taxonomic and Functional Surrogates of Sessile Benthic Diversity in Mediterranean Marine Caves. PLoS ONE 2017, 12, e0183707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voultsiadou, E. Sponge Diversity in the Aegean Sea: Check List and New Information. Ital. J. Zool. 2005, 72, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Garces, V.G.; Salazar-Oropeza, O.; Cordero-Esquivel, B.; O’Donnell, K.A. Induced Deflagellation of Isochrysis Microalgae in a Near-Infrared Optical Trap. Appl. Opt. 2015, 54, 1827–1833. [Google Scholar] [CrossRef]
- Quarmby, L.M. Cellular Deflagellation. Int. Rev. Cytol. 2004, 233, 47–91. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa Ishiwata, Y.; Ota, T.; Sasaki, H.; Taguchi, S. Diel Variation in Motility of Prymnesiophyte Isochrysis galbana under Different Irradiance. Plankton Benthos Res. 2019, 14, 271–275. [Google Scholar] [CrossRef]
- Fassini, D.; Parma, L.; Wilkie, I.C.; Bavestrello, G.; Bonasoro, F.; Candia Carnevali, M.D. Ecophysiology of Mesohyl Creep in the Demosponge Chondrosia Reniformis (Porifera: Chondrosida). J. Exp. Mar. Biol. Ecol. 2012, 428, 24–31. [Google Scholar] [CrossRef]
- Lazoski, C.; Solé-Cava, A.; Boury-Esnault, N.; Klautau, M.; Russo, C. Cryptic Speciation in a High Gene Flow Scenario in the Oviparous Marine Sponge Chondrosia Reniformis. Mar. Biol. 2001, 139, 421–429. [Google Scholar] [CrossRef]
- Morganti, T.M.; Ribes, M.; Yahel, G.; Coma, R. Size Is the Major Determinant of Pumping Rates in Marine Sponges. Front. Physiol. 2019, 10, 1474. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, C.; Vacca, S.; Ciucis, C.D.; Marengo, B.; Duckworth, A.R.; Manconi, R.; Pronzato, R.; Domenicotti, C. Growth Dynamics and Bioactivity Variation of the Mediterranean Demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol. 2009, 30, 327–336. [Google Scholar] [CrossRef]
- Koukouras, A.; Russo, A.; Voultsiadou-Koukoura, E.; Arvanitidis, C.; Stefanidou, D. Macrofauna Associated with Sponge Species of Different Morphology. Mar. Ecol. 1996, 17, 569–582. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Chintiroglou, C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voultsiadou, E. Census of Biodiversity in Marine Caves of the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 245–265. [Google Scholar] [CrossRef] [Green Version]
- Nickel, M.; Brümmer, F. In Vitro Sponge Fragment Culture of Chondrosia Reniformis (Nardo, 1847). J. Biotechnol. 2003, 100, 147–159. [Google Scholar] [CrossRef]
- Bonasoro, F.; Wilkie, I.C.; Bavestrello, G.; Cerrano, C.; Carnevali, M.D.C. Dynamic Structure of the Mesohyl in the Sponge Chondrosia Reniformis (Porifera, Demospongiae). Zoomorphology 2001, 121, 109–121. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Steinert, G.; Nielsen, S.; Hardoim, C.C.P.; Wu, Y.-C.; McCormack, G.P.; López-Legentil, S.; Marchant, R.; Webster, N.; Thomas, T.; et al. Predicting the HMA-LMA Status in Marine Sponges by Machine Learning. Front. Microbiol. 2017, 8, 752. [Google Scholar] [CrossRef] [Green Version]
- Enrichetti, F.; Bavestrello, G.; Betti, F.; Coppari, M.; Toma, M.; Pronzato, R.; Canese, S.; Bertolino, M.; Costa, G.; Pansini, M.; et al. Keratose-Dominated Sponge Grounds from Temperate Mesophotic Ecosystems (NW Mediterranean Sea). Mar. Ecol. 2020, 41, e12620. [Google Scholar] [CrossRef]
- Grenier, M.; Ruiz, C.; Fourt, M.; Santonja, M.; Dubois, M.; Klautau, M.; Vacelet, J.; Boury-Esnault, N.; PÉrez, T. Sponge Inventory of the French Mediterranean Waters, with an Emphasis on Cave-Dwelling Species. Zootaxa 2018, 4466, 205–228. [Google Scholar] [CrossRef]
- Katagan, T.; Tokaç, A.; Besiktepe, S.; Öztürk, B. The Aegean Sea Marine Biodiversity, Fisheries, Conservation and Governance; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2015; Publication No: 41. [Google Scholar]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fernández, E.; Acosta-Salmón, H.; Rangel-Dávalos, C. Ingestion and Digestion of 10 Species of Microalgae by Winged Pearl Oyster Pteria sterna (Gould, 1851) Larvae. Aquaculture 2004, 230, 417–423. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical Structure and Biological Activity of a Highly Branched (1 → 3,1 → 6)-β-D-Glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Gomes, A.; Campos, A.M.; Falé, P.; Afonso, C.; Bandarra, N.M. Bioprospection of Isochrysis galbana and Its Potential as a Nutraceutical. Food Funct. 2019, 10, 7333–7342. [Google Scholar] [CrossRef]
- Tesson, B.; Gaillard, C.; Martin-Jézéquel, V. Insights into the Polymorphism of the Diatom Phaeodactylum Tricornutum Bohlin. Bot. Mar. 2009, 52, 104–116. [Google Scholar] [CrossRef]
- Mandalakis, M.; Stravinskaitė, A.; Lagaria, A.; Psarra, S.; Polymenakou, P. Ultrasensitive and High-Throughput Analysis of Chlorophyll a in Marine Phytoplankton Extracts Using a Fluorescence Microplate Reader. Anal. Bioanal. Chem. 2017, 409, 4539–4549. [Google Scholar] [CrossRef]
- Moncheva, S.; Gotsis-Skretas, O.; Pagou, K.; Krastev, A. Phytoplankton Blooms in Black Sea and Mediterranean Coastal Ecosystems Subjected to Anthropogenic Eutrophication: Similarities and Differences. Estuar. Coast. Shelf Sci. 2001, 53, 281–295. [Google Scholar] [CrossRef] [Green Version]
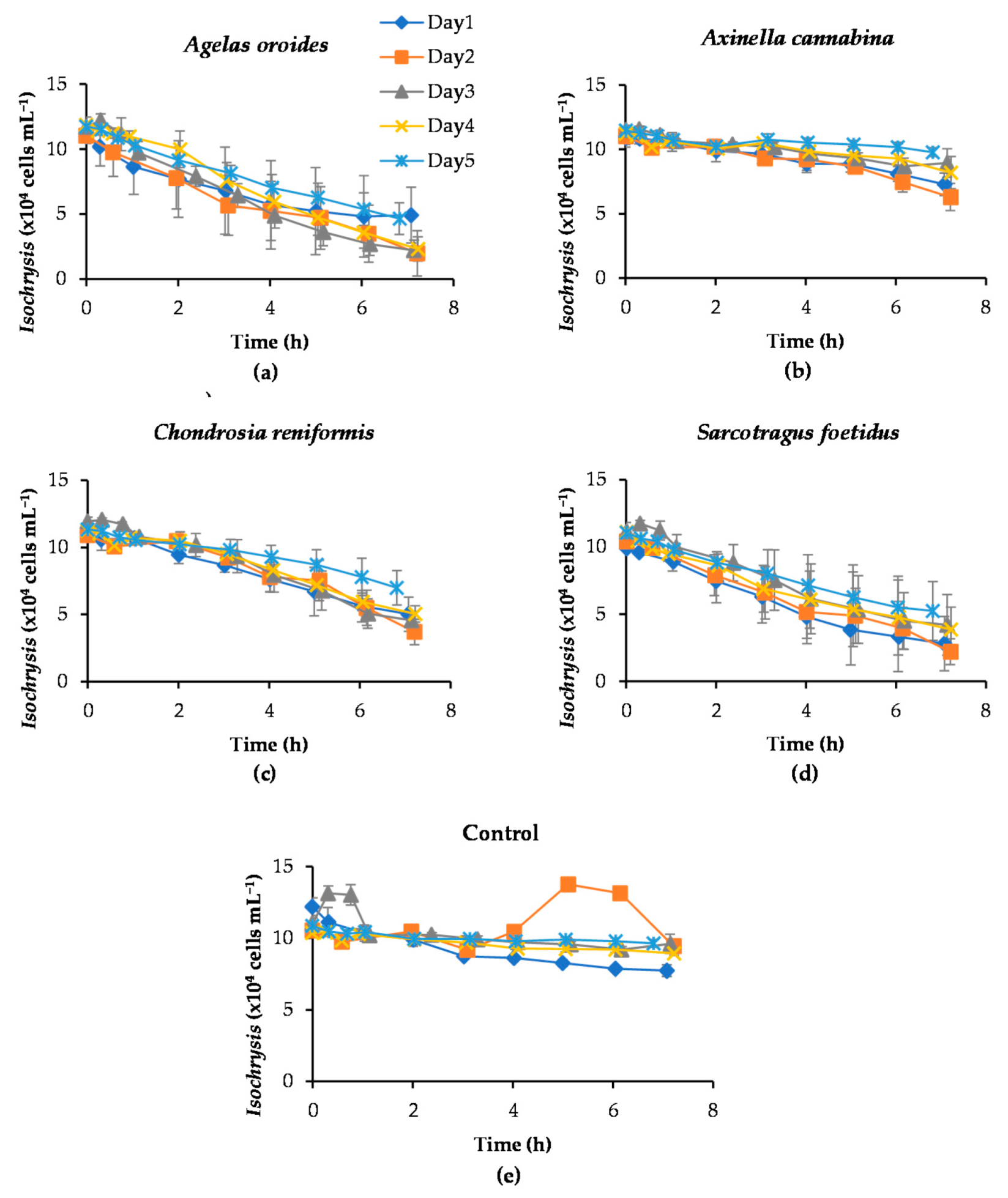
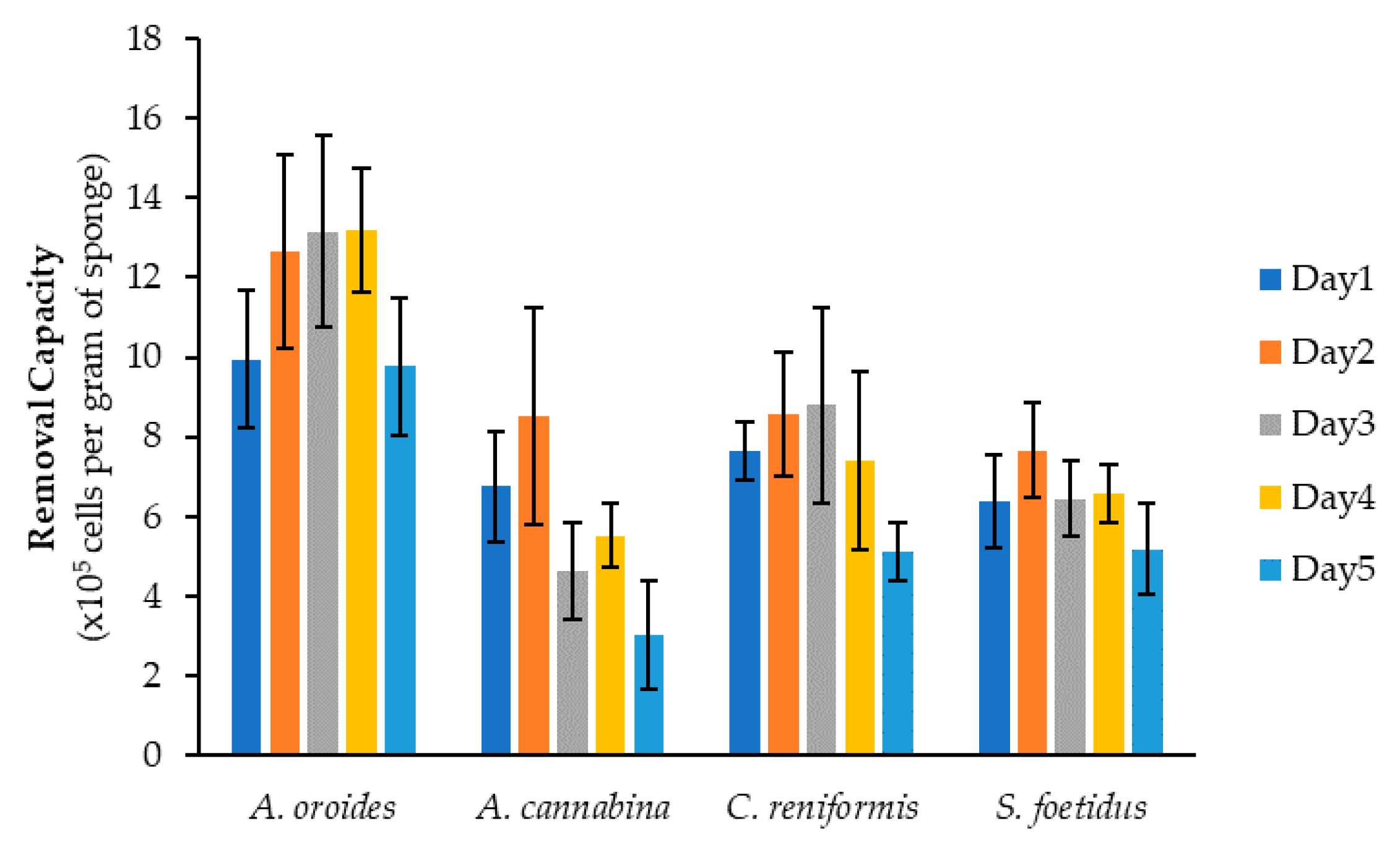
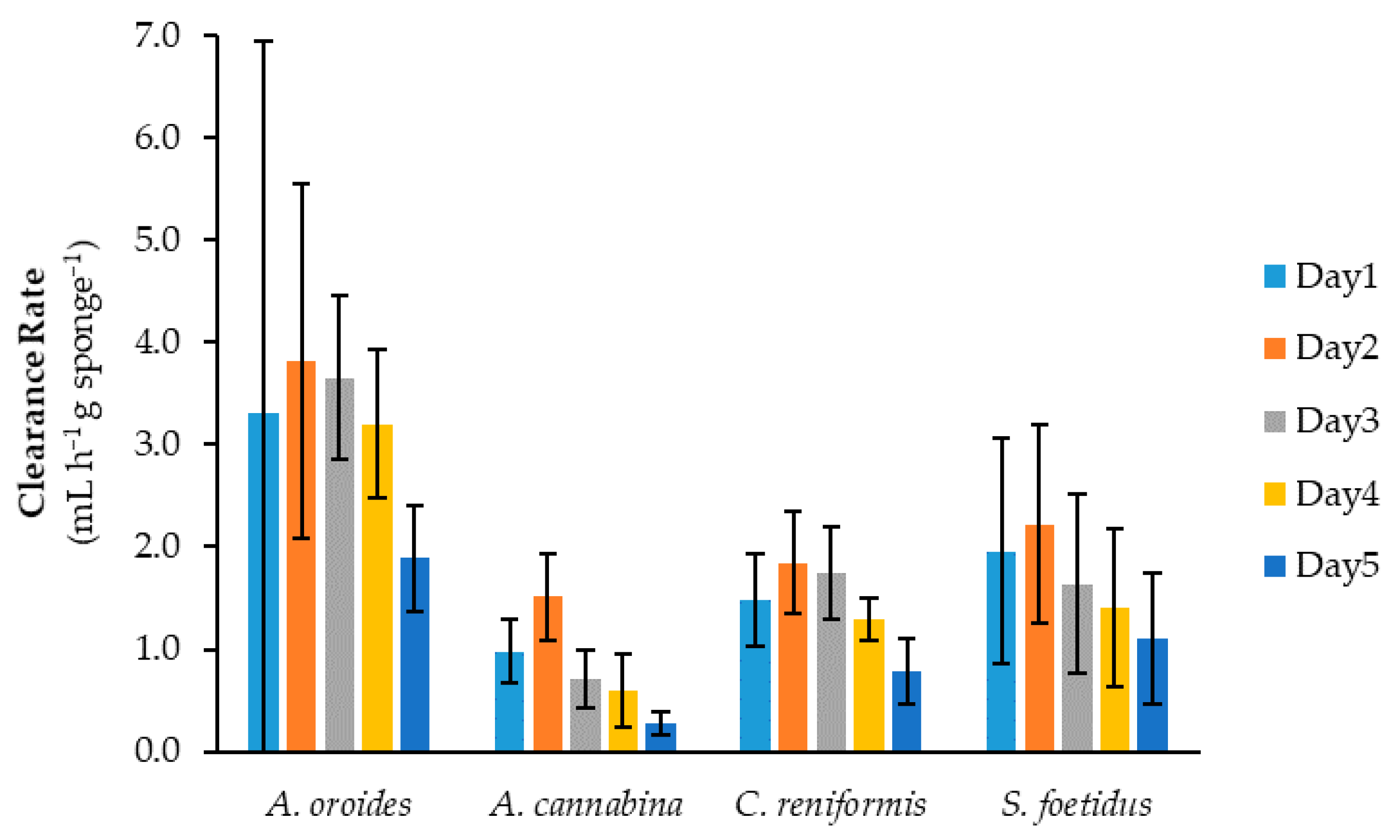
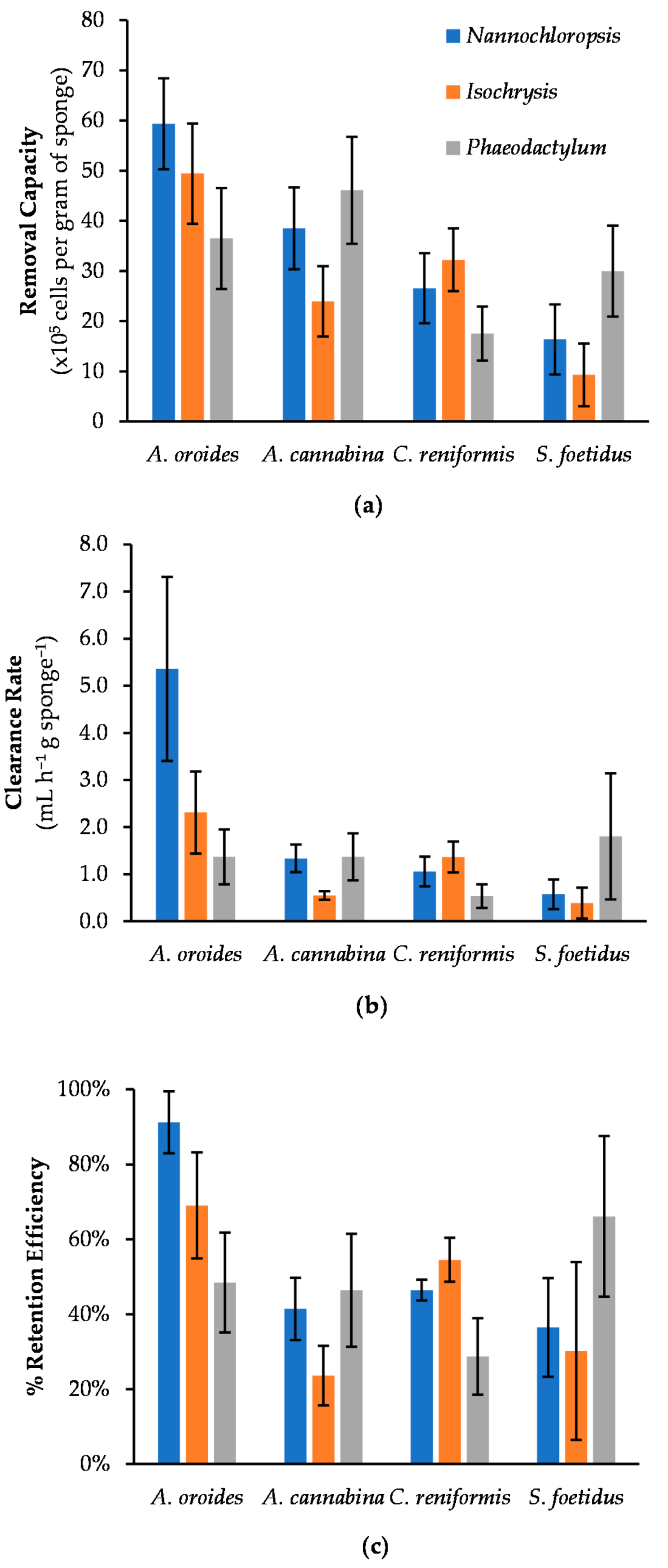
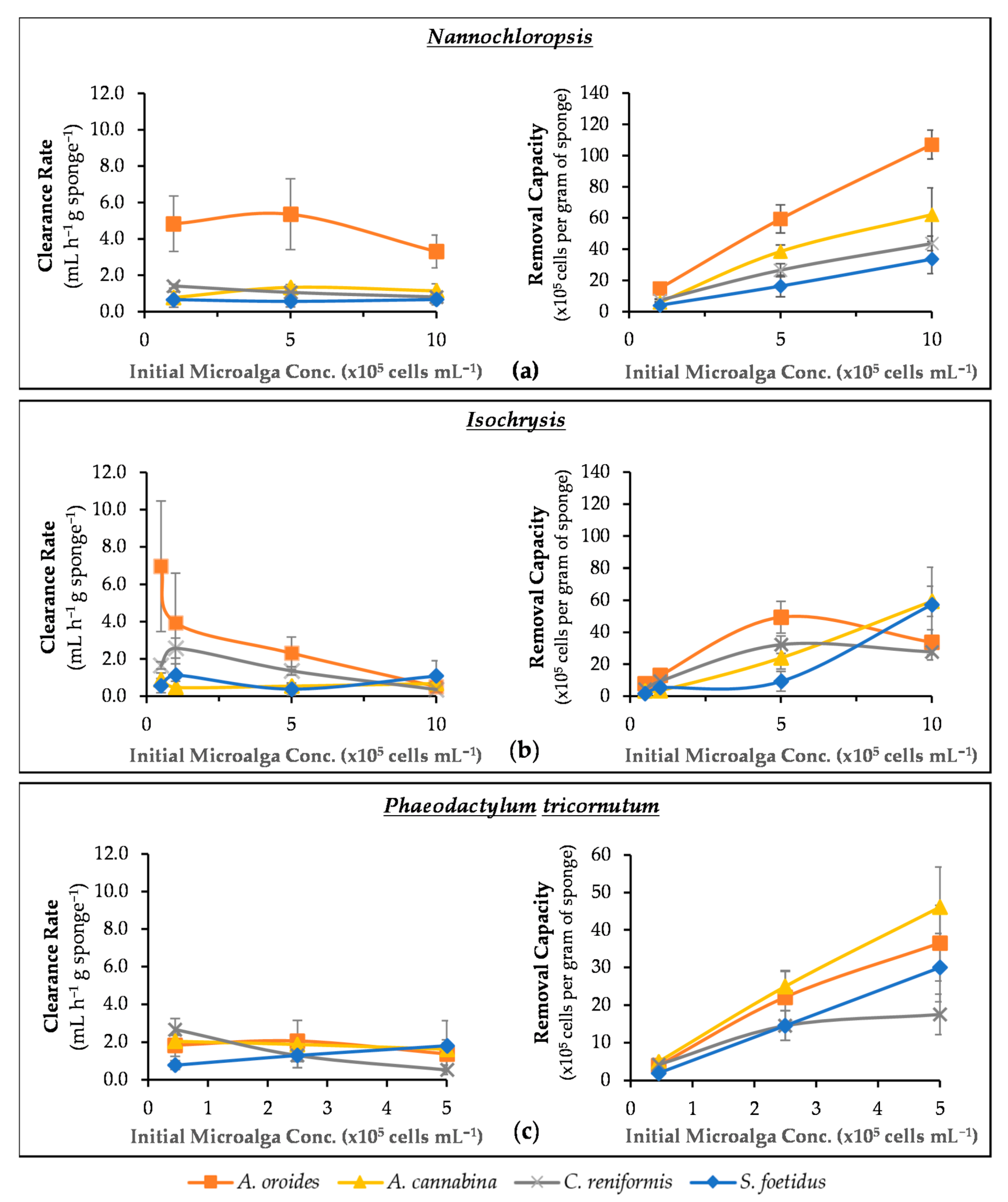
| Microalgae | Cell Size (μm) | Sponge Species | Wet Weight (g) | Retention Rate (×105 cells h−1 g Sponge−1) | Clearance Rate (mL h−1 g Sponge−1) | R2 | Removal Capacity (×105 cells g Sponge−1) | Retention Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| Nannochloropsis | 3.2 (0.2) | A. oroides | 67.9 (5.4) | 25.4 (5.4) | 5.4 (2.0) | 0.98 | 59.4 (9.1) | 91 (8) |
| A. cannabina | 50.4 (7.6) | 9.9 (3.6) | 1.3(0.3) | 0.96 | 38.5 (8.2) | 41 (8) | ||
| C. reniformis | 84.2 (12.4) | 5.0 (1.2) | 1.1 (0.2) | 0.99 | 26.6 (4.2) | 46 (3) | ||
| S. foetidus | 106.5 (26.8) | 3.1 (1.2) | 0.6 (0.3) | 0.97 | 16.4 (6.7) | 36 (13) | ||
| Isochrysis | 3.8 (0.4) | A. oroides | 67.9 (5.4) | 10.4 (3.8) | 2.3 (0.9) | 0.97 | 49.4 (10.0) | 69 (14) |
| A. cannabina | 50.4 (7.6) | 2.5 (0.3) | 0.6 (0.1) | 0.80 | 23.9 (7.0) | 24 (8) | ||
| C. reniformis | 84.2 (12.4) | 6.2 (1.4) | 1.4 (0.2) | 1.00 | 32.2 (5.5) | 55(6) | ||
| S. foetidus | 106.5 (26.8) | 1.8 (1.3) | 0.4 (0.3) | 0.96 | 9.3 (6.3) | 30 (24) | ||
| Phaeodactylum | 21.7 (1.2) | A. oroides | 67.9 (5.4) | 8.3 (4.3) | 1.4 (0.6) | 0.96 | 36.5 (10.1) | 48 (13) |
| A. cannabina | 50.4 (7.6) | 13.7 (4.3) | 1.6 (0.5) | 0.96 | 46.1 (10.7) | 46 (15) | ||
| C. reniformis | 84.2 (12.4) | 4.5 (1.6) | 0.5 (0.3) | 0.98 | 17.5 (5.4) | 29 (10) | ||
| S. foetidus | 106.5 (26.8) | 14.3 (11.2) | 1.8 (1.3) | 0.81 | 30.0 (9.1) | 66 (21) |
| Nannochloropsis | Isochrysis | Phaeodactylum | |
|---|---|---|---|
| A. oroides | 0.196 | 0.0024 | 0.405 |
| A. cannabina | 0.051 | 0.106 | 0.245 |
| C. reniformis | 0.0001 | <0.00001 | 0.00002 |
| S. foetidus | 0.893 | 0.127 | 0.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varamogianni-Mamatsi, D.; Anastasiou, T.I.; Vernadou, E.; Papandroulakis, N.; Kalogerakis, N.; Dailianis, T.; Mandalakis, M. A Multi-Species Investigation of Sponges’ Filtering Activity towards Marine Microalgae. Mar. Drugs 2022, 20, 24. https://doi.org/10.3390/md20010024
Varamogianni-Mamatsi D, Anastasiou TI, Vernadou E, Papandroulakis N, Kalogerakis N, Dailianis T, Mandalakis M. A Multi-Species Investigation of Sponges’ Filtering Activity towards Marine Microalgae. Marine Drugs. 2022; 20(1):24. https://doi.org/10.3390/md20010024
Chicago/Turabian StyleVaramogianni-Mamatsi, Despoina, Thekla I. Anastasiou, Emmanouela Vernadou, Nikos Papandroulakis, Nicolas Kalogerakis, Thanos Dailianis, and Manolis Mandalakis. 2022. "A Multi-Species Investigation of Sponges’ Filtering Activity towards Marine Microalgae" Marine Drugs 20, no. 1: 24. https://doi.org/10.3390/md20010024
APA StyleVaramogianni-Mamatsi, D., Anastasiou, T. I., Vernadou, E., Papandroulakis, N., Kalogerakis, N., Dailianis, T., & Mandalakis, M. (2022). A Multi-Species Investigation of Sponges’ Filtering Activity towards Marine Microalgae. Marine Drugs, 20(1), 24. https://doi.org/10.3390/md20010024










