Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays
Abstract
1. Introduction
2. Results
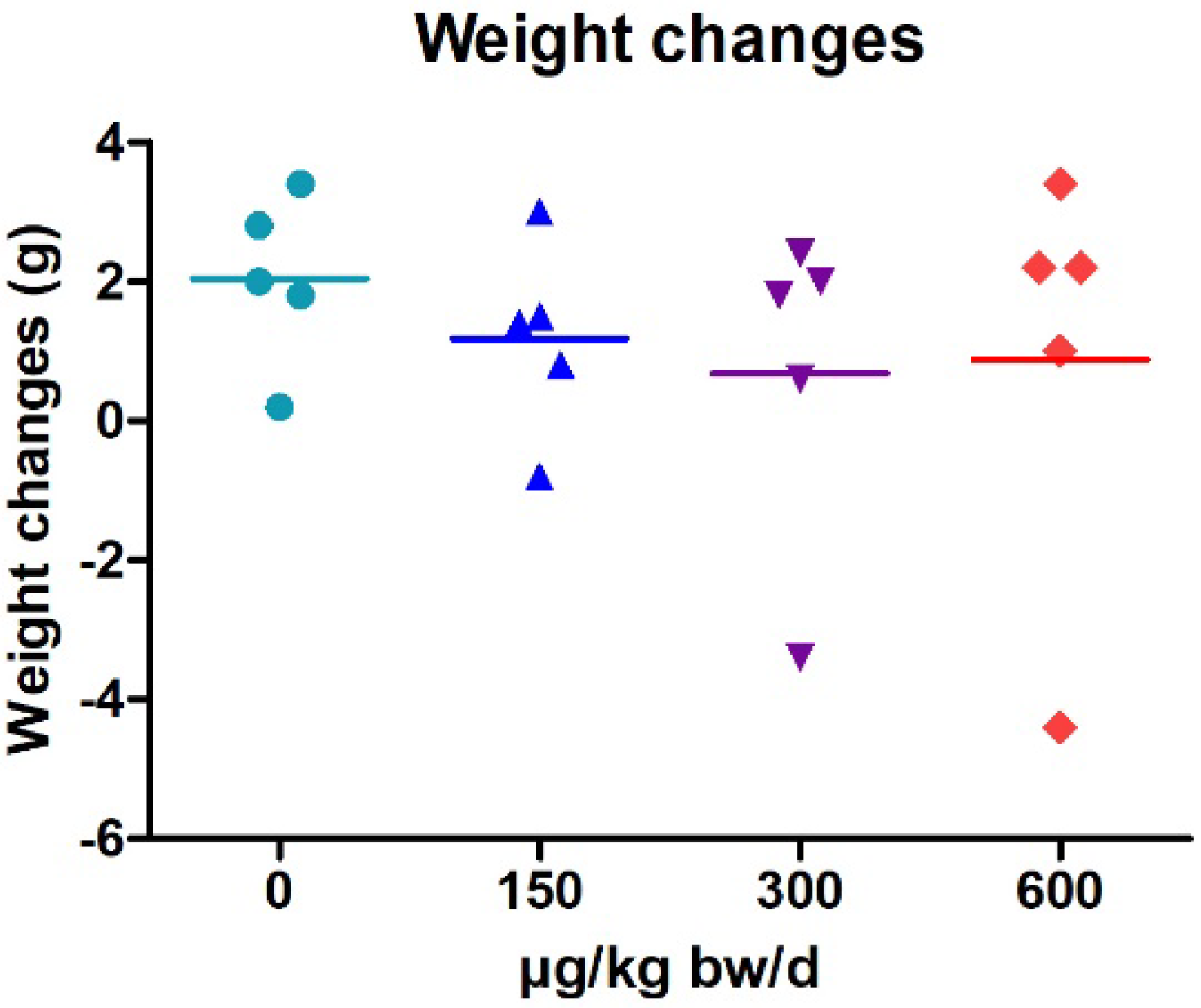
2.1. Weight Changes
2.2. Comet Assay
2.3. Bone Marrow Micronucleus Test (BMMN)
2.4. Histopathological Observations
3. Discussion
4. Materials and Methods
4.1. Shellfish Sampling
4.2. Chemicals
4.3. Animal Experimentation
4.4. Selection of Dose Levels and Treatment
4.5. Standard Comet Assay Protocol
4.6. Fpg-Modified Comet Assay Protocol
4.7. Bone Marrow Micronucleus Assay (BMMN)
4.8. Histopathological Observations
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and Biotransformation in Animal Cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Misiou, O.; Koutsoumanis, K. Climate Change and Its Implications for Food Safety and Spoilage. Trends Food Sci. Technol. 2021, 126, 142–152. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic Effects of Mycotoxins. Toxicon 2020, 185, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Kanižai Šarić, G.; Krstanović, V.; Mastanjević, K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef]
- Bennett, J.W. Mycotoxins, Mycotoxicoses, Mycotoxicology and Mycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Tepšič, K.; Gunde-Cimerman, N.; Frisvad, J.C. Growth and Mycotoxin Production by Aspergillus fumigatus Strains Isolated from a Saltern. FEMS Microbiol. Lett. 1997, 157, 9–12. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New Mycotoxins from Marine-Derived Fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and Their Consequences in Aquaculture: A Review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Grovel, O.; Pouchus, Y.F.; Verbist, J.-F. Accumulation of Gliotoxin, a Cytotoxic Mycotoxin from Aspergillus fumigatus, in Blue Mussel (Mytilus edulis). Toxicon 2003, 42, 297–300. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Esteves, A.C.; Alves, A. Marine Fungi: Opportunities and Challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Amzil, Z.; Marcaillou-Le Baut, C.; Bohec, M. Unexplained Toxicity in Molluscs Gathered during Phytoplankton Monitoring. Harmful Toxic Algal Blooms 1996, 543–546. [Google Scholar] [CrossRef]
- Sallenave-Namont, C.; Pouchus, Y.F.; Robiou du Pont, T.; Lassus, P.; Verbist, J.F. Toxigenic Saprophytic Fungi in Marine Shellfish Farming Areas. Mycopathologia 2000, 149, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.L.; de Medeiros, J.V.F.; Grault, C.E.; Santos, M.J.S.; Souza, A.L.A.; de Carvalho, R.W. The Fungus Pestalotiopsis sp., Isolated from Perna perna (Bivalvia:Mytilidae) Cultured on Marine Farms in Southeastern Brazil and Destined for Human Consumption. Mar. Pollut. Bull. 2020, 153, 110976. [Google Scholar] [CrossRef]
- Kovačić, I.; Pustijanac, E.; Ramšak, A.; Šebešćen, D.; Lipić, S. Variation of Parasite and Fungi Infection between Farmed and Wild Mussels (Mytilus galloprovincialis Lamarck, 1819) from the Adriatic Sea. J. Mar. Biol. Assoc. U. K. 2018, 98, 1871–1879. [Google Scholar] [CrossRef]
- Borzykh, O.G.; Zvereva, L.V. Fungal Assemblages Associated with Commercial Bivalve Species in Coastal Waters of the Sea of Japan, Russia. Bot. Mar. 2018, 61, 355–363. [Google Scholar] [CrossRef]
- Marrouchi, R.; Benoit, E.; Le Caer, J.-P.; Belayouni, N.; Belghith, H.; Molgó, J.; Kharrat, R. Toxic C17-Sphinganine Analogue Mycotoxin, Contaminating Tunisian Mussels, Causes Flaccid Paralysis in Rodents. Mar. Drugs 2013, 11, 4724–4740. [Google Scholar] [CrossRef]
- OCDE. Test No. 489: In vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4. In Éditions OCDE; OCDE: Paris, France, 2016. [Google Scholar] [CrossRef]
- OCDE. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4. In Éditions OCDE; OCDE: Paris, France, 2016. [Google Scholar] [CrossRef]
- Mayne, S.T. Antioxidant Nutrients and Chronic Disease: Use of Biomarkers of Exposure and Oxidative Stress Status in Epidemiologic Research. J. Nutr. 2003, 133, 933S–940S. [Google Scholar] [CrossRef]
- Collins, A.; Vettorazzi, A.; Azqueta, A. The Role of the Enzyme-Modified Comet Assay in In vivo Studies. Toxicol. Lett. 2020, 327, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 Induces Oxidative Stress in Oesophageal (SNO) Cancer Cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Radić, S.; Domijan, A.-M.; Glavaš Ljubimir, K.; Maldini, K.; Ivešić, M.; Peharec Štefanić, P.; Krivohlavek, A. Toxicity of Nanosilver and Fumonisin B1 and Their Interactions on Duckweed (Lemna minor L.). Chemosphere 2019, 229, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.-M.; Gajski, G.; Novak Jovanović, I.; Gerić, M.; Garaj-Vrhovac, V. In Vitro Genotoxicity of Mycotoxins Ochratoxin A and Fumonisin B1 Could Be Prevented by Sodium Copper Chlorophyllin—Implication to Their Genotoxic Mechanism. Food Chem. 2015, 170, 455–462. [Google Scholar] [CrossRef]
- Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive Oxygen Species Sources and Biomolecular Oxidative Damage Induced by Aflatoxin B1 and Fumonisin B1 in Rat Spleen Mononuclear Cells. Toxicology 2012, 302, 299–307. [Google Scholar] [CrossRef]
- Claudino-Silva, S.C.; Lala, B.; Mora, N.H.A.P.; Schamber, C.R.; Nascimento, C.S.; Pereira, V.V.; Hedler, D.L.; Gasparino, E. Fumonisins B1 + B2 Change the Expression of Genes in Apoptosis Balance in Nile Tilapia Fingerlings. Aquaculture 2018, 488, 155–160. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Theumer, M.G.; Cánepa, M.C.; López, A.G.; Mary, V.S.; Dambolena, J.S.; Rubinstein, H.R. Subchronic Mycotoxicoses in Wistar Rats: Assessment of the In vivo and In Vitro Genotoxicity Induced by Fumonisins and Aflatoxin B1, and Oxidative Stress Biomarkers Status. Toxicology 2010, 268, 104–110. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Abel, S.; Gelderblom, W.C. Oxidative Damage and Fumonisin B1-Induced Toxicity in Primary Rat Hepatocytes and Rat Liver in Vivo. Toxicology 1998, 131, 121–131. [Google Scholar] [CrossRef]
- Domijan, A.-M.; Abramov, A.Y. Fumonisin B1 Inhibits Mitochondrial Respiration and Deregulates Calcium Homeostasis—Implication to Mechanism of Cell Toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.-M.; Zeljezić, D.; Milić, M.; Peraica, M. Fumonisin B(1): Oxidative Status and DNA Damage in Rats. Toxicology 2007, 232, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mobio, T.A.; Anane, R.; Baudrimont, I.; Carratú, M.-R.; Shier, T.W.; Dano, S.D.; Ueno, Y.; Creppy, E.E. Epigenetic Properties of Fumonisin B1: Cell Cycle Arrest and DNA Base Modification in C6 Glioma Cells. Toxicol. Appl. Pharmacol. 2000, 164, 91–96. [Google Scholar] [CrossRef]
- IARC Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556.
- Schuchardt, S.; Ziemann, C.; Hansen, T. Combined Toxicokinetic and In vivo Genotoxicity Study on Alternaria Toxins. EFSA Support. Publ. 2014, 11, 679E. [Google Scholar] [CrossRef]
- Tardieu, D.; Tran, S.T.; Auvergne, A.; Babilé, R.; Benard, G.; Bailly, J.D.; Guerre, P. Effects of Fumonisins on Liver and Kidney Sphinganine and the Sphinganine to Sphingosine Ratio during Chronic Exposure in Ducks. Chem. Biol. Interact. 2006, 160, 51–60. [Google Scholar] [CrossRef]
- Riley, R.T.; Showker, J.L.; Owens, D.L.; Ross, P.F. Disruption of Sphingolipid Metabolism and Induction of Equine Leukoencephalomalacia by Fusarium proliferatum Culture Material Containing Fumonisin B2 or B3. Environ. Toxicol. Pharmacol. 1997, 3, 221–228. [Google Scholar] [CrossRef]
- Merrill, A.H.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid Metabolism: Roles in Signal Transduction and Disruption by Fumonisins. Environ. Health Perspect. 2001, 109, 283–289. [Google Scholar]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C.; Plattner, R.D.; Collins, T.F.; Hansen, D.K.; Porter, J.K. An Overview of Rodent Toxicities: Liver and Kidney Effects of Fumonisins and Fusarium moniliforme. Environ. Health Perspect. 2001, 109, 259–266. [Google Scholar]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin Toxicosis in Swine: An Overview of Porcine Pulmonary Edema and Current Perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar]
- Howard, P.C.; Eppley, R.M.; Stack, M.E.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Kovach, R.M.; Bucci, T.J. Fumonisin B1 Carcinogenicity in a Two-Year Feeding Study Using F344 Rats and B6C3F1 Mice. Environ. Health Perspect. 2001, 109 (Suppl. 2), 277–282. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.C.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Thurman, J.D.; Kovach, R.M.; Bucci, T.J. Compensatory Regeneration as a Mechanism for Renal Tubule Carcinogenesis of Fumonisin B1 in the F344/N/Nctr BR Rat. Environ. Health Perspect. 2001, 109 (Suppl. 2), 309–314. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Galendo, D.; Abel, S.; Swanevelder, S.; Marasas, W.F.O.; Wild, C.P. Cancer Initiation by Fumonisin B1 in Rat Liver—Role of Cell Proliferation. Cancer Lett. 2001, 169, 127–137. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of Sphingolipid Biosynthesis by Fumonisins. Implications for Diseases Associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Norred, W.P.; Showker, J.; Riley, R.T. Elevated Sphingoid Bases and Complex Sphingolipid Depletion as Contributing Factors in Fumonisin-Induced Cytotoxicity. Toxicol. Appl. Pharmacol. 1996, 138, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.M.; Dombrink-Kurtzman, M.A.; Roberts, P.C.; Kozutsumi, Y.; Kawasaki, T.; Merrill, A.H. Induction of Apoptosis by Fumonisin B1in HT29 Cells Is Mediated by the Accumulation of Endogenous Free Sphingoid Bases. Toxicol. Appl. Pharmacol. 1998, 148, 252–260. [Google Scholar] [CrossRef]
- Tarantini, A.; Huet, S.; Jarry, G.; Lanceleur, R.; Poul, M.; Tavares, A.; Vital, N.; Louro, H.; João Silva, M.; Fessard, V. Genotoxicity of Synthetic Amorphous Silica Nanoparticles in Rats following Short-Term Exposure. Part 1: Oral Route. Environ. Mol. Mutagen. 2015, 56, 218–227. [Google Scholar] [CrossRef]
- Jalili, P.; Huet, S.; Lanceleur, R.; Jarry, G.; Hegarat, L.L.; Nesslany, F.; Hogeveen, K.; Fessard, V. Genotoxicity of Aluminum and Aluminum Oxide Nanomaterials in Rats Following Oral Exposure. Nanomaterials 2020, 10, 305. [Google Scholar] [CrossRef]
- Maranghi, F.; Tassinari, R.; Narciso, L.; Tait, S.; Rocca, C.L.; Felice, G.D.; Butteroni, C.; Corinti, S.; Barletta, B.; Cordelli, E.; et al. In vivo Toxicity and Genotoxicity of Beauvericin and Enniatins. Combined Approach to Study In vivo Toxicity and Genotoxicity of Mycotoxins Beauvericin (BEA) and Enniatin B (ENNB). EFSA Support. Publ. 2018, 15, 1406E. [Google Scholar] [CrossRef]




| Organ | Treatment | Dose (µg/kg b.w) | % Hedgehogs |
|---|---|---|---|
| Duodenum | Control | 0 | 16.78 ± 4.67 |
| C17-SAMT | 150 | 17.94 ± 1.44 | |
| 300 | 17.71 ± 6.53 | ||
| 600 | 17.86 ± 2.64 | ||
| MMS | 80,000 | 49.3 ± 7.11 *** | |
| Spleen | Control | 0 | 7.23 ± 3.88 |
| C17-SAMT | 150 | 5.04 ± 2.15 | |
| 300 | 7.85 ± 4.46 | ||
| 600 | 5.5 ± 2.48 | ||
| MMS | 80,000 | 38.84 ± 7.41 *** | |
| Liver | Control | 0 | 4.92 ± 1.98 |
| C17-SAMT | 150 | 5.24 ± 2.46 | |
| 300 | 4.77 ± 1.47 | ||
| 600 | 4.7 ± 2.23 | ||
| MMS | 80,000 | 100 *** | |
| Spleen Fpg+ | Control | 0 | 13.89 ± 9.66 |
| C17-SAMT | 150 | 9.44 ± 4.81 | |
| 300 | 13.22 ± 5.92 | ||
| 600 | 11.72 ± 8.92 | ||
| MMS | 80,000 | 100 *** |
| MNPCEs/1000 PCEs | % PCEs | ||
|---|---|---|---|
| Doses (µg/kg b.w) | Mean ± SD | Mean ± SD | |
| Control | 0 | 1.2 ± 0.6 | 33 ± 0.07 |
| C17-SAMT | 150 | 1 ± 0.9 | 32 ± 0.07 |
| 300 | 0.8 ± 0.9 | 36 ± 0.02 | |
| 600 | 1.6 ± 1.3 | 31 ± 0.03 | |
| MMS | 80,000 | 13 ± 5.8 *** | 27 ± 0.04 |
| Treatment | Dose (µg/kg b.w) | Inflammation a | Clarification a | Necrosis | Apoptosis | Mitosis b |
|---|---|---|---|---|---|---|
| CTRL | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 2 | ||
| 0 | 0 | 0 | 0 | 0 | ||
| 0 | 1 | 0 | 0 | 2 | ||
| MMS | 80,000 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 1 | ||
| 0 | 0 | 0 | 0 | 1 | ||
| C17-SAMT | 150 | 0 | 3 | 0 | 0 | 5 |
| 1 | 2 | 0 | 0 | >20 | ||
| 0 | 2 | 0 | 0 | >15 | ||
| 0 | 2 | 0 | 0 | 5 | ||
| 300 | ns | ns | ns | ns | ns | |
| 0 | 0 | 0 | 0 | 0 | ||
| 0 | 3 | 0 | 0 | 5 | ||
| 0 | 1 | 0 | 0 | 2 | ||
| 1 | 2 | 0 | 0 | 2 | ||
| 600 | 0 | 0 | 0 | 0 | 1 | |
| 0 | 3 | 0 | 0 | 3 | ||
| 0 | 3 | 0 | 0 | 4 | ||
| 0 | 3 | 0 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzougui, Z.; Huet, S.; Blier, A.-L.; Hégarat, L.L.; Tounsi-Kettiti, H.; Kharrat, R.; Marrouchi, R.; Fessard, V. Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays. Mar. Drugs 2022, 20, 619. https://doi.org/10.3390/md20100619
Marzougui Z, Huet S, Blier A-L, Hégarat LL, Tounsi-Kettiti H, Kharrat R, Marrouchi R, Fessard V. Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays. Marine Drugs. 2022; 20(10):619. https://doi.org/10.3390/md20100619
Chicago/Turabian StyleMarzougui, Zeineb, Sylvie Huet, Anne-Louise Blier, Ludovic Le Hégarat, Haïfa Tounsi-Kettiti, Riadh Kharrat, Riadh Marrouchi, and Valérie Fessard. 2022. "Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays" Marine Drugs 20, no. 10: 619. https://doi.org/10.3390/md20100619
APA StyleMarzougui, Z., Huet, S., Blier, A.-L., Hégarat, L. L., Tounsi-Kettiti, H., Kharrat, R., Marrouchi, R., & Fessard, V. (2022). Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays. Marine Drugs, 20(10), 619. https://doi.org/10.3390/md20100619







