Marine Compounds, Mitochondria, and Malignancy: A Therapeutic Nexus
Abstract
:1. Introduction
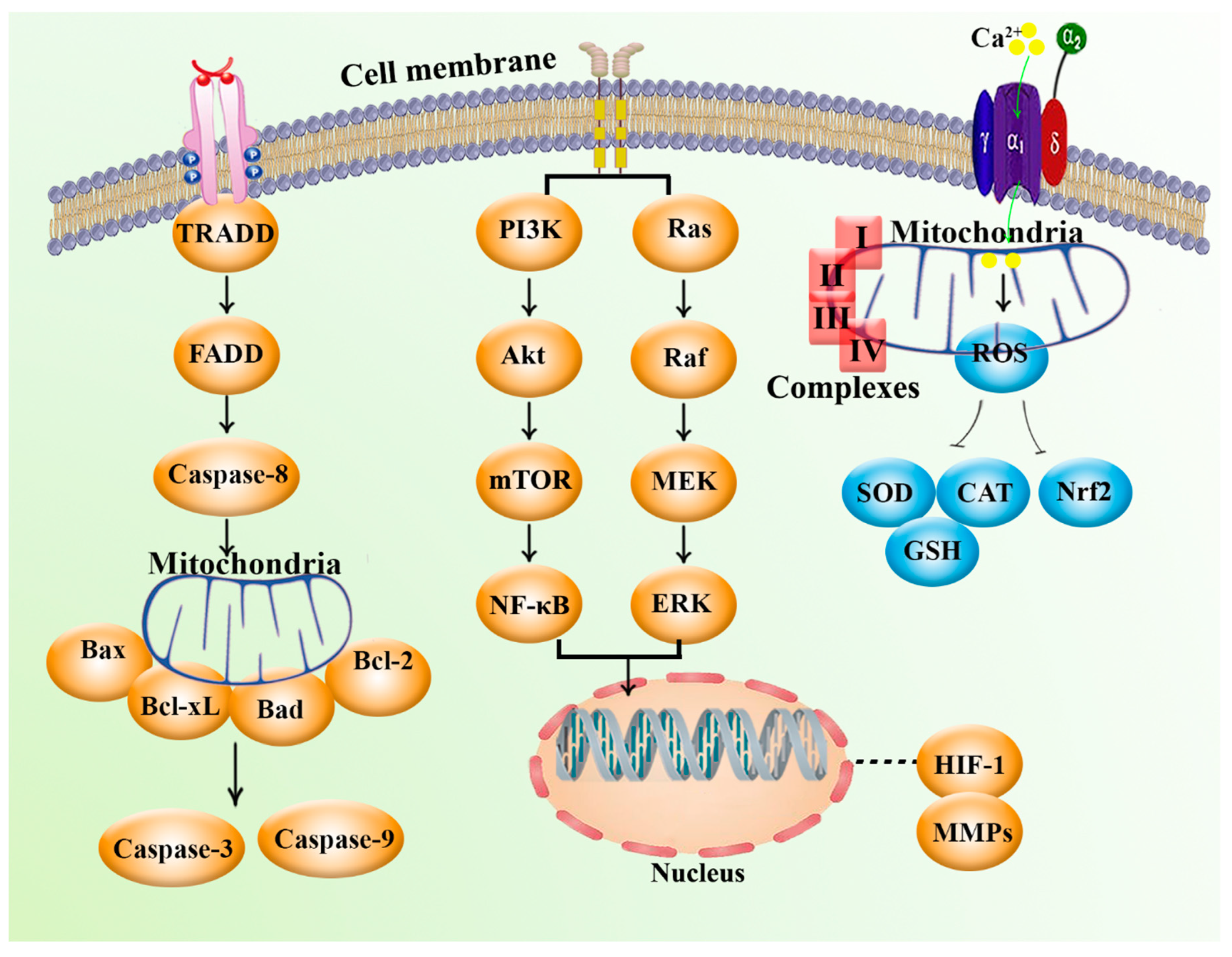
2. Mitochondria and Cancer: Biology and Cellular Signaling
3. Synthetic Agents and Candidate Phytocompounds in Modulation of Cancer through the Regulation of Mitochondria Function
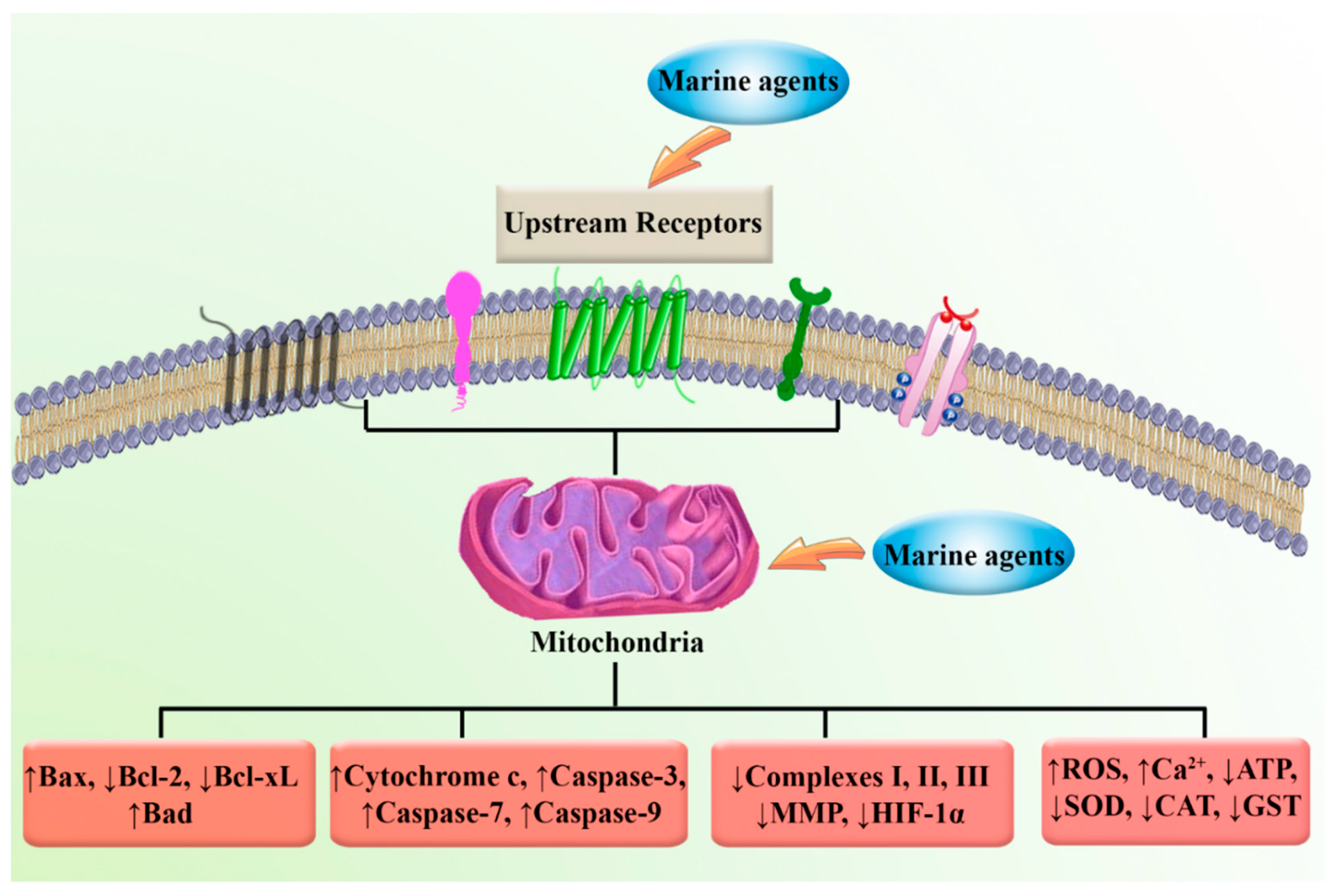
4. Marine Compounds Suppress Cancer through the Modulation of Mitochondria Function
5. Conclusion, Challenges, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Cancer causes and treatments. Int. J. Pharm. Sci. Res. 2020, 11, 3109. [Google Scholar]
- Fakhri, S.; Moradi, S.Z.; Ash-Rafzadeh, A.; Bishayee, A. Targeting cellular senescence in cancer by plant secondary metabolites: A systematic review. Pharmacol. Res. 2022, 177, 105961. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer Biol. 2022, 80, 276–305. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Yarmohammadi, A.; Narimani, F.; Wallace, C.E.; Bishayee, A. Modulation of TLR/NF-κB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy. Front Oncol 2022, 12, 834072. [Google Scholar] [CrossRef]
- Fakhri, S.; Zachariah Moradi, S.; DeLiberto, L.K.; Bishayee, A. Cellular senescence signaling in cancer: A novel therapeutic target to combat human malignancies. Biochem. Pharmacol. 2022, 199, 114989. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Nowroozi, A.; Sadrjavadi, K.; Moradi, S.; Mansouri, K.; Hosseinzadeh, L.; Shahlaei, M. Direct evidences for the groove binding of the Clomifene to double stranded DNA. Int. J. Biol. Macromol. 2018, 114, 40–53. [Google Scholar] [CrossRef]
- Adeyinka, A.; Bashir, K. Tumor lysis syndrome. JAMA Oncol. 2018, 4, 895. [Google Scholar]
- Tan, X.; Fu, J.; Yuan, Z.; Zhu, L.; Fu, L. ACNPD: The Database for Elucidating the Relationships Between Natural Products, Compounds, Molecular Mechanisms, and Cancer Types. Front. Pharmacol. 2021, 2214. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Pomeroy, A.E.; Schmidt, E.V.; Sorger, P.K.; Palmer, A.C. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer 2022. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Meerson, A.; Khatib, S.; Mahajna, J. Flavonoids Targeting Cancer Stem Cells for Augmenting Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 13044. [Google Scholar] [CrossRef]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. A Survey of Marine Natural Compounds and Their Derivatives with Anti-Cancer Activity Reported in 2010. Molecules 2011, 16, 5629–5646. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Goel, B.; Tripathi, N.; Sahu, B.; Jain, S.K. A comprehensive review on chemistry and pharmacology of marine bioactives as antimetastatic agents. Eur. J. Med. Chem. Rep. 2022, 4, 100023. [Google Scholar] [CrossRef]
- Chinen, T.; Nagumo, Y.; Watanabe, T.; Imaizumi, T.; Shibuya, M.; Kataoka, T.; Kanoh, N.; Iwabuchi, Y.; Usui, T. Irciniastatin A induces JNK activation that is involved in caspase-8-dependent apoptosis via the mitochondrial pathway. Toxicol. Lett. 2010, 199, 341–346. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Chen, A.; Li, Y.; Xia, M.; Guo, P.; Yao, S.; Chen, S. Fucosterol exhibits selective antitumor anticancer activity against HeLa human cervical cell line by inducing mitochondrial mediated apoptosis, cell cycle migration inhibition and downregulation of m-TOR/PI3K/Akt signalling pathway. Oncol. Lett. 2018, 15, 3458–3463. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 10–3389. [Google Scholar] [CrossRef]
- Sugumaran, A.; Pandiyan, R.; Kandasamy, P.; Antoniraj, M.G.; Navabshan, I.; Sakthivel, B.; Dharmaraj, S.; Chinnaiyan, S.K.; Ashokkumar, V.; Ngamcharussrivichai, C. Marine biome-derived secondary metabolites, a class of promising antineoplastic agents: A systematic review on their classification, mechanism of action and future perspectives. Sci. Total Environ. 2022, 836, 155445. [Google Scholar] [CrossRef]
- Florean, C.; Dicato, M.; Diederich, M. Immune-modulating and anti-inflammatory marine compounds against cancer. Semin. Cancer Biol. 2022, 80, 58–72. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Atanasov, A.G.; Bishayee, A.; Abdel-Mogib, M.; Hooper, J.N.A.; Al-Mourabit, A. Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas. Nutrients 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor Potential of Marine and Freshwater Lectins. Mar. Drugs 2019, 18, 11. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites-A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Current Status of Marine-Derived Compounds as Warheads in Anti-Tumor Drug Candidates. Mar Drugs 2017, 15, 99. [Google Scholar] [CrossRef]
- Fimognari, C.; Lenzi, M.; Ferruzzi, L.; Turrini, E.; Scartezzini, P.; Poli, F.; Gotti, R.; Guerrini, A.; Carulli, G.; Ottaviano, V. Mitochondrial pathway mediates the antileukemic effects of Hemidesmus indicus, a promising botanical drug. PLoS ONE 2011, 6, e21544. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Passaniti, A.; Kim, M.S.; Polster, B.M.; Shapiro, P. Targeting mitochondrial metabolism for metastatic cancer therapy. Mol. Carcinog. 2022, 61, 827–838. [Google Scholar] [CrossRef]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting mitochondria for cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef]
- Xu, J.; Shamul, J.G.; Kwizera, E.A.; He, X. Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials 2022, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, D.; Avolio, R.; Matassa, D.S.; Esposito, F. Targeting Mitochondrial Protein Expression as a Future Approach for Cancer Therapy. Front. Oncol. 2021, 11, 797265. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.F.; Chang, Y.W.; Huang, H.C.; Juan, H.F. Targeting protein interaction networks in mitochondrial dynamics for cancer therapy. Drug Discov. Today 2022, 27, 1077–1087. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Singh, K.K. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020, 21, 6992. [Google Scholar] [CrossRef]
- Qi, W.J.; Sheng, W.S.; Peng, C.; Xiaodong, M.; Yao, T.Z. Investigating into anti-cancer potential of lycopene: Molecular targets. Biomed. Pharmacother. 2021, 138, 111546. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Grasso, D.; Zampieri, L.X.; Capelôa, T.; Van de Velde, J.A.; Sonveaux, P. Mitochondria in cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Canter, J.A.; Kallianpur, A.R.; Parl, F.F.; Millikan, R.C. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005, 65, 8028–8033. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.; Wang, Y.; Hui-Juan, Y.; Tsang, P.C.; Ng, T.-Y.; Ling-Chui, W.; Nagley, P.; Ngan, H.Y. Mitochondrial DNA variant 16189T> C is associated with susceptibility to endometrial cancer. Hum. Mutat. 2003, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Chang, L.; Zhang, Q.; Liu, B.; Wu, Y. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion 2011, 11, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Bardella, C.; Pollard, P.J.; Tomlinson, I. SDH mutations in cancer. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Aghamir, S.M.K.; Heshmat, R.; Ebrahimi, M.; Ketabchi, S.E.; Dizaji, S.P.; Khatami, F. The impact of succinate dehydrogenase gene (SDH) mutations in renal cell carcinoma (RCC): A systematic review. OncoTargets Ther. 2019, 12, 7929. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Nickerson, M.L.; Wei, M.-H.; Warren, M.B.; Glenn, G.M.; Turner, M.L.; Stewart, L.; Duray, P.; Tourre, O.; Sharma, N. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am. J. Hum. Genet. 2003, 73, 95–106. [Google Scholar] [CrossRef]
- Yim, M.-S.; Ha, Y.-S.; Kim, I.Y.; Yun, S.-J.; Choi, Y.H.; Kim, W.-J. HMOX1 is an important prognostic indicator of nonmuscle invasive bladder cancer recurrence and progression. J. Urol. 2011, 185, 701–705. [Google Scholar] [CrossRef]
- Reitman, Z.J.; Yan, H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: Alterations at a crossroads of cellular metabolism. J. Natl. Cancer Inst. 2010, 102, 932–941. [Google Scholar] [CrossRef]
- Thompson, C.B. Metabolic enzymes as oncogenes or tumor suppressors. New Engl. J. Med. 2009, 360, 813. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Ooi, A.; Furge, K.A. Fumarate hydratase inactivation in renal tumors: HIF1α, NRF2, and “cryptic targets” of transcription factors. Chin. J. Cancer 2012, 31, 413. [Google Scholar] [CrossRef] [PubMed]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.A.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.; Lu, C.; Ward, P.S. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010, 5, 297. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Wan, Y.; Xie, Y.; Li, S.; Tao, D.; Wang, C.; Wu, Y.-Z.; Sui, J.-D. Apurinic/apyrimidinic endonuclease 1/reduction-oxidation effector factor-1 (APE1) regulates the expression of NLR family pyrin domain containing 3 (NLRP3) inflammasome through modulating transcription factor NF-κB and promoting the secretion of inflammatory mediators in macrophages. Ann. Transl. Med. 2021, 9, 145. [Google Scholar]
- Jones, R.G.; Thompson, C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef]
- Fukuda, R.; Zhang, H.; Kim, J.-w.; Shimoda, L.; Dang, C.V.; Semenza, G.L. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007, 129, 111–122. [Google Scholar] [CrossRef]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020, 34, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Iranpanah, A.; Gravandi, M.M.; Moradi, S.Z.; Ranjbari, M.; Majnooni, M.B.; Echeverría, J.; Qi, Y.; Wang, M.; Liao, P. Natural products attenuate PI3K/Akt/mTOR signaling pathway: A promising strategy in regulating neurodegeneration. Phytomedicine 2021, 91, 153664. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of polyphenols on oxidative stress, inflammation, and interconnected pathways during spinal cord injury. Oxidative Med. Cell. Longev. 2022, 2022, 1–34. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Akkol, E.K.; Piri, S.; Sobarzo-Sanchez, E.; Farzaei, M.H.; Echeverría, J. Targeting multiple signal transduction pathways of SARS-CoV-2: Approaches to COVID-19 therapeutic candidates. Molecules 2021, 26, 2917. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef]
- Fakhri, S.; Piri, S.; Moradi, S.Z.; Khan, H. Phytochemicals Targeting Oxidative Stress, Interconnected Neuroinflammatory, and Neuroapoptotic Pathways Following Radiation. Curr. Neuropharmacol. 2022, 20, 836–856. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Moradi, S.Z.; Akbari, V.; Aghaz, F.; Farzaei, M.H. Nanoformulated herbal bioactives for the treatment of neurodegenerative disorders. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–391. [Google Scholar]
- Fakhri, S.; Abdian, S.; Zarneshan, S.N.; Moradi, S.Z.; Farzaei, M.H.; Abdollahi, M. Nanoparticles in combating neuronal dysregulated signaling pathways: Recent approaches to the nanoformulations of phytochemicals and synthetic drugs against neurodegenerative diseases. Int. J. Nanomed. 2022, 17, 299. [Google Scholar] [CrossRef]
- Hou, J.; Yu, X.; Shen, Y.; Shi, Y.; Su, C.; Zhao, L. Triphenyl phosphine-functionalized chitosan nanoparticles enhanced antitumor efficiency through targeted delivery of doxorubicin to mitochondria. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Huang, L.; Xu, C.; Zhang, J.; Li, D.; Ding, L.; Liu, L.; Dong, Y.; Wang, W.; Duan, Y. Highly biocompatible thermosensitive nanocomposite gel for combined therapy of hepatocellular carcinoma via the enhancement of mitochondria related apoptosis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102062. [Google Scholar] [CrossRef] [PubMed]
- Suganya, M.; Gnanamangai, B.M.; Govindasamy, C.; Elsadek, M.F.; Pugazhendhi, A.; Chinnadurai, V.; Selvaraj, A.; Ravindran, B.; Chang, S.W.; Ponmurugan, P. Mitochondrial dysfunction mediated apoptosis of HT-29 cells through CS-PAC-AgNPs and investigation of genotoxic effects in zebra (Danio rerio) fish model for drug delivery. Saudi J. Biol. Sci. 2019, 26, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Marisiddaiah, R.; Zaripheh, S.; Wiener, D.; Rubin, L.P. Mitochondrial β-carotene 9′, 10′ oxygenase modulates prostate cancer growth via NF-κB inhibition: A lycopene-independent function. Mol. Cancer Res. 2016, 14, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar] [CrossRef]
- Yang, Y.; He, P.-Y.; Zhang, Y.; Li, N. Natural products targeting the mitochondria in cancers. Molecules 2020, 26, 92. [Google Scholar] [CrossRef]
- Li, J.; Mahdi, F.; Du, L.; Datta, S.; Nagle, D.G.; Zhou, Y.-D. Mitochondrial respiration inhibitors suppress protein translation and hypoxic signaling via the hyperphosphorylation and inactivation of translation initiation factor eIF2α and elongation factor eEF2. J. Nat. Prod. 2011, 74, 1894–1901. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; He, K.; Cao, T.; Song, D.; Yang, H.; Li, L.; Lin, J. The Apoptosis of Liver Cancer Cells Promoted by Curcumin/TPP-CZL Nanomicelles With Mitochondrial Targeting Function. Front. Bioeng. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Y.; Fu, S.; Zhang, J.; Wang, W.; Yan, Z.; Guo, H.; Liu, A. Seleno-short-chain chitosan induces apoptosis in human breast cancer cells through mitochondrial apoptosis pathway in vitro. Cell Cycle 2018, 17, 1579–1590. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Ma, J.; Zhao, J.; Li, D.; Cao, Y.; Liu, P. Silver nanotriangles and chemotherapeutics synergistically induce apoptosis in glioma cells via a ROS-dependent mitochondrial pathway. Int. J. Nanomed. 2020, 15, 7791. [Google Scholar] [CrossRef]
- Piplani, H.; Vaish, V.; Rana, C.; Sanyal, S.N. Up-regulation of p53 and mitochondrial signaling pathway in apoptosis by a combination of cox-2 inhibitor, celecoxib and dolastatin 15, a marine mollusk linear peptide in experimental colon carcinogenesis. Mol. Carcinog. 2013, 52, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P. Folate-chitosan nanoparticles loaded with ursolic acid confer anti-breast cancer activities in vitro and in vivo. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.M.; Martiny, J.B.; Sogin, M.; Boetius, A.; Ramette, A. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar]
- Fakhri, S.; Yarmohammadi, A.; Yarmohammadi, M.; Farzaei, M.H.; Echeverria, J. Marine Natural Products: Promising Candidates in the Modulation of Gut-Brain Axis towards Neuroprotection. Mar. Drugs 2021, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Kudryashova, E.K.; Kaune, M.; Makarieva, T.N.; Shubina, L.K.; Busenbender, T.; Denisenko, V.A.; Popov, R.S.; Hauschild, J.; Fedorov, S.N. Urupocidin C: A new marine guanidine alkaloid which selectively kills prostate cancer cells via mitochondria targeting. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Shi, S.; Yao, L.; Guo, K.; Wang, X.; Wang, Q.; Li, W. Hepatocellular toxicity of oxalicumone A via oxidative stress injury and mitochondrial dysfunction in healthy human liver cells. Mol. Med. Rep. 2018, 17, 743–752. [Google Scholar] [CrossRef]
- Freitas, S.; Martins, R.; Costa, M.; Leão, P.N.; Vitorino, R.; Vasconcelos, V.; Urbatzka, R. Hierridin B isolated from a marine cyanobacterium alters VDAC1, mitochondrial activity, and cell cycle genes on HT-29 colon adenocarcinoma cells. Mar. Drugs 2016, 14, 158. [Google Scholar] [CrossRef]
- Velatooru, L.R.; Baggu, C.B.; Janapala, V.R. Spatane diterpinoid from the brown algae, Stoechospermum marginatum induces apoptosis via ROS induced mitochondrial mediated caspase dependent pathway in murine B16F10 melanoma cells. Mol. Carcinog. 2016, 55, 2222–2235. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Induction of apoptosis by low-molecular-weight fucoidan through calcium-and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013, 77, 235–242. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.-Y.; Song, G.; Lim, W. Fucosterol suppresses the progression of human ovarian cancer by inducing mitochondrial dysfunction and endoplasmic reticulum stress. Mar. Drugs 2020, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef]
- Podunavac, M.; Mailyan, A.K.; Jackson, J.J.; Lovy, A.; Farias, P.; Huerta, H.; Molgó, J.; Cardenas, C.; Zakarian, A. Scalable Total Synthesis, IP3R Inhibitory Activity of Desmethylxestospongin B, and Effect on Mitochondrial Function and Cancer Cell Survival. Angew. Chem. 2021, 133, 11378–11382. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, J.; Liu, Z.; Zhao, S. Antitumor activity of a sulfated polysaccharide from Enteromorpha intestinalis targeted against hepatoma through mitochondrial pathway. Tumor Biol. 2014, 35, 1641–1647. [Google Scholar] [CrossRef]
- Mahdi, F.; Falkenberg, M.; Ioannou, E.; Roussis, V.; Zhou, Y.-D.; Nagle, D.G. Thyrsiferol inhibits mitochondrial respiration and HIF-1 activation. Phytochem. Lett. 2011, 4, 75–78. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Guerra, F.; Felline, S.; Rimoli, M.G.; Mollo, E.; Zara, V.; Terlizzi, A. Metabolites from invasive pests inhibit mitochondrial complex II: A potential strategy for the treatment of human ovarian carcinoma? Biochem. Biophys. Res. Commun. 2016, 473, 1133–1138. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Tsai, C.-T.; Chuang, W.-L.; Chao, Y.-H.; Pan, I.; Chen, Y.-K.; Lin, C.-C.; Wang, B.-Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Huang, X.; Huang, W.; Li, L.; Sun, X.; Song, S.; Xu, Q.; Zhang, L.; Wei, B.-G.; Deng, X. Structure determinants of lagunamide A for anticancer activity and its molecular mechanism of mitochondrial apoptosis. Mol. Pharm. 2016, 13, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-M.; Tseng, C.-C.; Chen, N.-F.; Tai, M.-H.; Hung, H.-C.; Feng, C.-W.; Cheng, S.-Y.; Huang, S.-Y.; Jean, Y.-H.; Wen, Z.-H. MSP-4, an antimicrobial peptide, induces apoptosis via activation of extrinsic Fas/FasL-and intrinsic mitochondria-mediated pathways in one osteosarcoma cell line. Mar. Drugs 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Rahimitabar, N.; Vazirizadeh, A.; Adhami, V.; Pourahmad, J. Persian Gulf Snail Crude Venom (Conus textile): A potential source of anti-cancer therapeutic agents for glioblastoma through mitochondrial-mediated apoptosis. Asian Pac. J. Cancer Prev. 2021, 22, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Shin, D.; Kamiya, K.; Ishida, R.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Marine spongean polybrominated diphenyl ethers, selective growth inhibitors against the cancer cells adapted to glucose starvation, inhibits mitochondrial complex II. J. Nat. Med. 2017, 71, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Arast, Y.; Razi, N.S.; Seydi, E.; Naserzadeh, P.; Nazemi, M.; Pourahmad, J. Selective toxicity of non polar bioactive compounds of persian gulf sea squirt Phallusia nigra on skin mitochondria isolated from rat model of melanoma. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 811. [Google Scholar] [PubMed]
- Humeniuk, R.; Menon, L.; Mishra, P.; Saydam, G.; Longo-Sorbello, G.; Elisseyeff, Y.; Lewis, L.; Aracil, M.; Jimeno, J.; Bertino, J. Aplidin synergizes with cytosine arabinoside: Functional relevance of mitochondria in Aplidin-induced cytotoxicity. Leukemia 2007, 21, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; Liu, Y.; Coothankandaswamy, V.; Mahdi, F.; Jekabsons, M.B.; Gerwick, W.H.; Valeriote, F.A.; Zhou, Y.-D.; Nagle, D.G. Kalkitoxin inhibits angiogenesis, disrupts cellular hypoxic signaling, and blocks mitochondrial electron transport in tumor cells. Mar. Drugs 2015, 13, 1552–1568. [Google Scholar] [CrossRef]
- Arepalli, S.; Sridhar, V.; Venkateswara Rao, J.; Kavin Kennady, P.; Venkateswarlu, Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: Induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apoptosis 2009, 14, 729–740. [Google Scholar] [CrossRef]
- Vaikundamoorthy, R.; Sundaramoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Marine steroid derived from Acropora formosa enhances mitochondrial-mediated apoptosis in non-small cell lung cancer cells. Tumor Biol. 2016, 37, 10517–10531. [Google Scholar] [CrossRef]
- Zangeneh, F.; Vazirizadeh, A.; reza Mirshamsi, M.; Fakhri, A.; Faizi, M.; Pourahmad, J. Induction of apoptosis by extract of Persian Gulf Marine Mollusk, Turbo Coronatus through the ROS-mediated mitochondrial targeting on human epithelial ovarian cancer cells. Iran. J. Pharm. Res. IJPR 2019, 18, 263. [Google Scholar]
- Kuang, S.; Liu, G.; Cao, R.; Zhang, L.; Yu, Q.; Sun, C. Mansouramycin C kills cancer cells through reactive oxygen species production mediated by opening of mitochondrial permeability transition pore. Oncotarget 2017, 8, 104057. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chang, X.; Luo, X.; Zhao, Y.; Wang, W.; Kang, X. Fucoxanthin induces prostate cancer PC-3 cell apoptosis by causing mitochondria dysfunction and oxidative stress. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2021, 41, 953–959. [Google Scholar]
- Terasaki, M.; Asai, A.; Zhang, H.; Nagao, A. A highly polar xanthophyll of 9′-cis-neoxanthin induces apoptosis in HCT116 human colon cancer cells through mitochondrial dysfunction. Mol. Cell. Biochem. 2007, 300, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Iyappan, P.; Bala, M.D.; Sureshkumar, M.; Veeraraghavan, V.P.; Palanisamy, A. Fucoxanthin induced apoptotic cell death in oral squamous carcinoma (KB) cells. Bioinformation 2021, 17, 181. [Google Scholar] [CrossRef]
- Shin, J.; Nile, A.; Saini, R.K.; Oh, J.-W. Astaxanthin Sensitizes Low SOD2-Expressing GBM Cell Lines to TRAIL Treatment via Pathway Involving Mitochondrial Membrane Depolarization. Antioxidants 2022, 11, 375. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Lee, Y.W.; Kang, H.W. Cellulose nanocrystals/nanofibrils loaded astaxanthin nanoemulsion for the induction of apoptosis via ROS-dependent mitochondrial dysfunction in cancer cells under photobiomodulation. Int. J. Biol. Macromol. 2020, 149, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, S.; Zhu, J.; Huang, W.; Luo, Y.; Shi, H.; Yu, D.; Chen, L.; Song, L.; Yu, R. A Novel Peptide Derived from Arca inflata Induces Apoptosis in Colorectal Cancer Cells through Mitochondria and the p38 MAPK Pathway. Mar. Drugs 2022, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Liu, H.; Wang, F.; Zheng, L.; Zhao, J.; Chu, E.; Lin, X. A novel polypeptide extracted from Ciona savignyi induces apoptosis through a mitochondrial-mediated pathway in human colorectal carcinoma cells. Clin. Color. Cancer 2012, 11, 207–214. [Google Scholar] [CrossRef]
- Ballot, C.; Kluza, J.; Martoriati, A.; Nyman, U.; Formstecher, P.; Joseph, B.; Bailly, C.; Marchetti, P. Essential role of mitochondria in apoptosis of cancer cells induced by the marine alkaloid Lamellarin DMitochondria, DNA damage, and Lamellarin D. Mol. Cancer Ther. 2009, 8, 3307–3317. [Google Scholar] [CrossRef]
- Kluza, J.; Gallego, M.-A.; Loyens, A.; Beauvillain, J.-C.; Sousa-Faro, J.-M.F.; Cuevas, C.; Marchetti, P.; Bailly, C. Cancer cell mitochondria are direct proapoptotic targets for the marine antitumor drug lamellarin D. Cancer Res. 2006, 66, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Sopha, P.; Phutubtim, N.; Chantrathonkul, B.; Ploypradith, P.; Ruchirawat, S.; Chittchang, M. Roles of autophagy in relation to mitochondrial stress responses of HeLa cells to lamellarin cytotoxicity. Toxicology 2021, 462, 152963. [Google Scholar] [CrossRef] [PubMed]
- Ballot, C.; Kluza, J.; Lancel, S.; Martoriati, A.; Hassoun, S.M.; Mortier, L.; Vienne, J.-C.; Briand, G.; Formstecher, P.; Bailly, C. Inhibition of mitochondrial respiration mediates apoptosis induced by the anti-tumoral alkaloid lamellarin D. Apoptosis 2010, 15, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Thangam, R.; Senthilkumar, D.; Suresh, V.; Sathuvan, M.; Sivasubramanian, S.; Pazhanichamy, K.; Gorlagunta, P.K.; Kannan, S.; Gunasekaran, P.; Rengasamy, R. Induction of ROS-dependent mitochondria-mediated intrinsic apoptosis in MDA-MB-231 cells by glycoprotein from codium decorticatum. J. Agric. Food Chem. 2014, 62, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-cancer activity of biosynthesized silver nanoparticles using Avicennia marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef]
- Wang, A.T.; Prinsep, M.R.; Martinus, R.D. Pterocellin A isolated from marine bryozoan Pterocella vesiculosa is cytotoxic to human HeLa cells via mitochondrial apoptotic processes. Springerplus 2016, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jeong, Y.; Roe, J.-S.; Huh, H.; Paik, S.H.; Song, J. Mitochondrial dysfunction induced by callyspongiolide promotes autophagy-dependent cell death. BMB Rep. 2021, 54, 227. [Google Scholar] [CrossRef]
- Chang, W.-T.; Bow, Y.-D.; Fu, P.-J.; Li, C.-Y.; Wu, C.-Y.; Chang, Y.-H.; Teng, Y.-N.; Li, R.-N.; Lu, M.-C.; Liu, Y.-C. A marine terpenoid, heteronemin, induces both the apoptosis and ferroptosis of hepatocellular carcinoma cells and involves the ROS and MAPK pathways. Oxidative Med. Cell. Longev. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Alonso, E.; Alvariño, R.; Duarte, A.; Marmitt, D.; Goettert, M.I.; Gaspar, H.; Alfonso, A. Cytotoxic Mechanism of Sphaerodactylomelol, an Uncommon Bromoditerpene Isolated from Sphaerococcus coronopifolius. Molecules 2021, 26, 1374. [Google Scholar] [CrossRef]
- Bai, L.-Y.; Su, J.-H.; Chiu, C.-F.; Lin, W.-Y.; Hu, J.-L.; Feng, C.-H.; Shu, C.-W.; Weng, J.-R. Antitumor effects of a sesquiterpene derivative from marine sponge in human breast cancer cells. Mar. Drugs 2021, 19, 244. [Google Scholar] [CrossRef]
- Wang, K.-C.; Lu, M.-C.; Hsu, K.-C.; El-Shazly, M.; Shih, S.-P.; Lien, S.-T.; Kuo, F.-W.; Yang, S.-C.; Chen, C.-L.; Yang, Y.-C.S. The antileukemic effect of xestoquinone, a marine-derived polycyclic quinone-type metabolite, is mediated through ros-induced inhibition of hsp-90. Molecules 2021, 26, 7037. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Sabutskii, Y.E.; Khmelevskaya, E.A.; Krisp, C.; Kaune, M.; Venz, S.; Borisova, K.L.; Busenbender, T. Inspired by sea urchins: Warburg effect mediated selectivity of novel synthetic non-glycoside 1, 4-naphthoquinone-6S-glucose conjugates in prostate cancer. Mar. Drugs 2020, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Namikoshi, M.; Kobayashi, H.; Yao, X.; Cai, G. Sesquiterpene quinones from a marine sponge Hippospongia sp. that inhibit maturation of starfish oocytes and induce cell cycle arrest with HepG2 cells. Pharm. Biol. 2006, 44, 522–527. [Google Scholar] [CrossRef]
- Sahayanathan, G.J.; Padmanaban, D.; Raja, K.; Chinnasamy, A. Anticancer effect of purified polysaccharide from marine clam Donax variabilis on A549 cells. J. Food Biochem. 2020, 44, e13486. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Kuo, T.-T.; Chang, H.-Y.; Liu, W.-S.; Hsia, S.-M.; Huang, T.-C. Manzamine A exerts anticancer activity against human colorectal cancer cells. Mar. Drugs 2018, 16, 252. [Google Scholar] [CrossRef]
- Song, S.; Kim, S.; El-Sawy, E.R.; Cerella, C.; Orlikova-Boyer, B.; Kirsch, G.; Christov, C.; Dicato, M.; Diederich, M. Anti-leukemic properties of aplysinopsin derivative ee-84 alone and combined to bh3 mimetic a-1210477. Mar. Drugs 2021, 19, 285. [Google Scholar] [CrossRef]
- Abdel-Naime, W.A.; Kimishima, A.; Setiawan, A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S.; Arai, M. Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp. Mar. Drugs 2020, 18, 555. [Google Scholar] [CrossRef]
- Seydi, E.; Motallebi, A.; Dastbaz, M.; Dehghan, S.; Salimi, A.; Nazemi, M.; Pourahmad, J. Selective toxicity of Persian Gulf sea cucumber (Holothuria parva) and sponge (Haliclona oculata) methanolic extracts on liver mitochondria isolated from an animal model of hepatocellular carcinoma. Hepat. Mon. 2015, 15, e33073. [Google Scholar] [CrossRef]
- Mao, S.-C.; Liu, Y.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.-D.; Nagle, D.G. Lipophilic 2, 5-disubstituted pyrroles from the marine sponge Mycale sp. inhibit mitochondrial respiration and HIF-1 activation. J. Nat. Prod. 2009, 72, 1927–1936. [Google Scholar] [CrossRef]
- Kanno, S.-I.; Yomogida, S.; Tomizawa, A.; Yamazaki, H.; Ukai, K.; Mangindaan, R.E.; Namikoshi, M.; Ishikawa, M. Papuamine causes autophagy following the reduction of cell survival through mitochondrial damage and JNK activation in MCF-7 human breast cancer cells. Int. J. Oncol. 2013, 43, 1413–1419. [Google Scholar] [CrossRef]
- Shih, H.-C.; El-Shazly, M.; Juan, Y.-S.; Chang, C.-Y.; Su, J.-H.; Chen, Y.-C.; Shih, S.-P.; Chen, H.-M.; Wu, Y.-C.; Lu, M.-C. Cracking the cytotoxicity code: Apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunction. Mar. Drugs 2014, 12, 3072–3090. [Google Scholar] [CrossRef] [PubMed]
- Aghvami, M.; Keshavarz, A.; Nazemi, M.; Zarei, M.H.; Pourahmad, J. Selective Cytotoxicity of α-Santonin from the Persian Gulf Sponge Dysidea Avara on Pediatric ALL B-lymphocytes via Mitochondrial Targeting. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2149. [Google Scholar]
- Morgan, J.B.; Mahdi, F.; Liu, Y.; Coothankandaswamy, V.; Jekabsons, M.B.; Johnson, T.A.; Sashidhara, K.V.; Crews, P.; Nagle, D.G.; Zhou, Y.-D. The marine sponge metabolite mycothiazole: A novel prototype mitochondrial complex I inhibitor. Bioorganic Med. Chem. 2010, 18, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Karanam, G.; Arumugam, M.K. Reactive oxygen species generation and mitochondrial dysfunction for the initiation of apoptotic cell death in human hepatocellular carcinoma HepG2 cells by a cyclic dipeptide Cyclo (-Pro-Tyr). Mol. Biol. Rep. 2020, 47, 3347–3359. [Google Scholar] [CrossRef]
- Magalhães, P.R.; Reis, P.B.; Vila-Viçosa, D.; Machuqueiro, M.; Victor, B.L. Identification of Pan-Assay INterference compoundS (PAINS) Using an MD-Based Protocol. In Computational Design of Membrane Proteins; Springer: Berlin/Heidelberg, Germany, 2021; pp. 263–271. [Google Scholar]
- De Matos, A.M.; Blázquez-Sánchez, M.T.; Sousa, C.; Oliveira, M.C.; de Almeida, R.F.; Rauter, A.P. C-Glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. PTR 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and-12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Gueven, N.; Spring, K.J.; Holmes, S.; Ahuja, K.; Eri, R.; Park, A.Y.; Fitton, J.H. Micro RNA Expression after Ingestion of Fucoidan; A Clinical Study. Mar. Drugs 2020, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Ichihara, E.; Hotta, K.; Sone, N.; Murakami, T.; Harada, D.; Oze, I.; Kubo, T.; Tanaka, H.; Kuyama, S.; et al. Three-Arm Randomized Trial of Sodium Alginate for Preventing Radiation-Induced Esophagitis in Locally Advanced Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: The OLCSG1401 Study Protocol. Clin. Lung Cancer 2017, 18, 245–249. [Google Scholar] [CrossRef]
- Baird, R.D.; Kitzen, J.; Clarke, P.A.; Planting, A.; Reade, S.; Reid, A.; Welsh, L.; López Lázaro, L.; de las Heras, B.; Judson, I.R.; et al. Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Mol. Cancer Ther. 2009, 8, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Rosing, H.; Hillebrand, M.J.; López-Lázaro, L.; Jimeno, J.M.; Manzanares, I.; van Kesteren, C.; Cvitkovic, E.; van Oosterom, A.T.; Schellens, J.H.; et al. Search for metabolites of ecteinascidin 743, a novel, marine-derived, anti-cancer agent, in man. Anti-Cancer Drugs 2001, 12, 653–666. [Google Scholar] [CrossRef]
- Van Kesteren, C.; Twelves, C.; Bowman, A.; Hoekman, K.; López-Lázaro, L.; Jimeno, J.; Guzman, C.; Mathôt, R.A.; Simpson, A.; Vermorken, J.B.; et al. Clinical pharmacology of the novel marine-derived anticancer agent Ecteinascidin 743 administered as a 1- and 3-h infusion in a phase I study. Anti-Cancer Drugs 2002, 13, 381–393. [Google Scholar] [CrossRef]
- Faivre, S.; Chièze, S.; Delbaldo, C.; Ady-Vago, N.; Guzman, C.; Lopez-Lazaro, L.; Lozahic, S.; Jimeno, J.; Pico, F.; Armand, J.P.; et al. Phase I and pharmacokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7871–7880. [Google Scholar] [CrossRef]
- Madden, T.; Tran, H.T.; Beck, D.; Huie, R.; Newman, R.A.; Pusztai, L.; Wright, J.J.; Abbruzzese, J.L. Novel marine-derived anticancer agents: A phase I clinical, pharmacological, and pharmacodynamic study of dolastatin 10 (NSC 376128) in patients with advanced solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1293–1301. [Google Scholar]
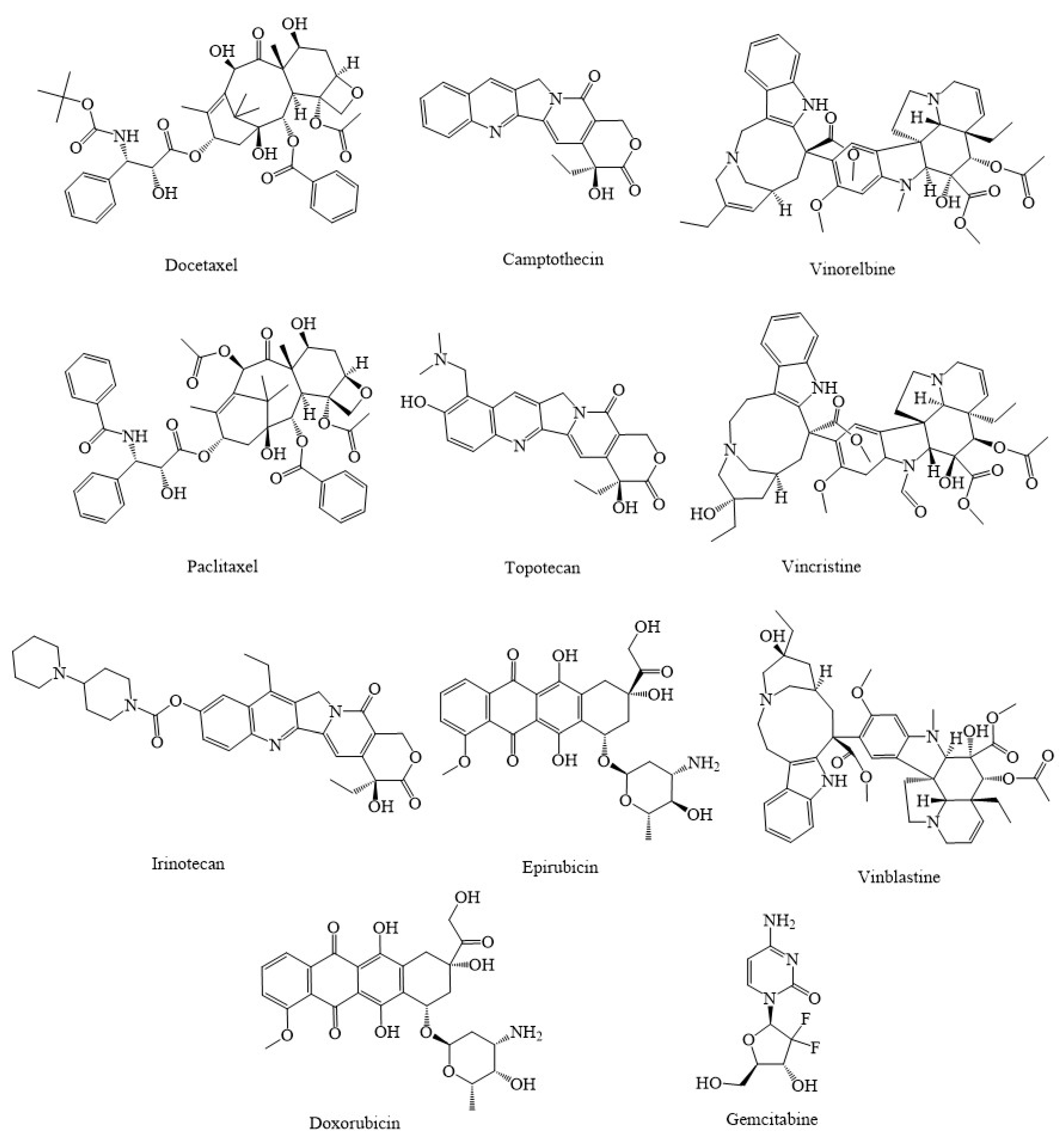
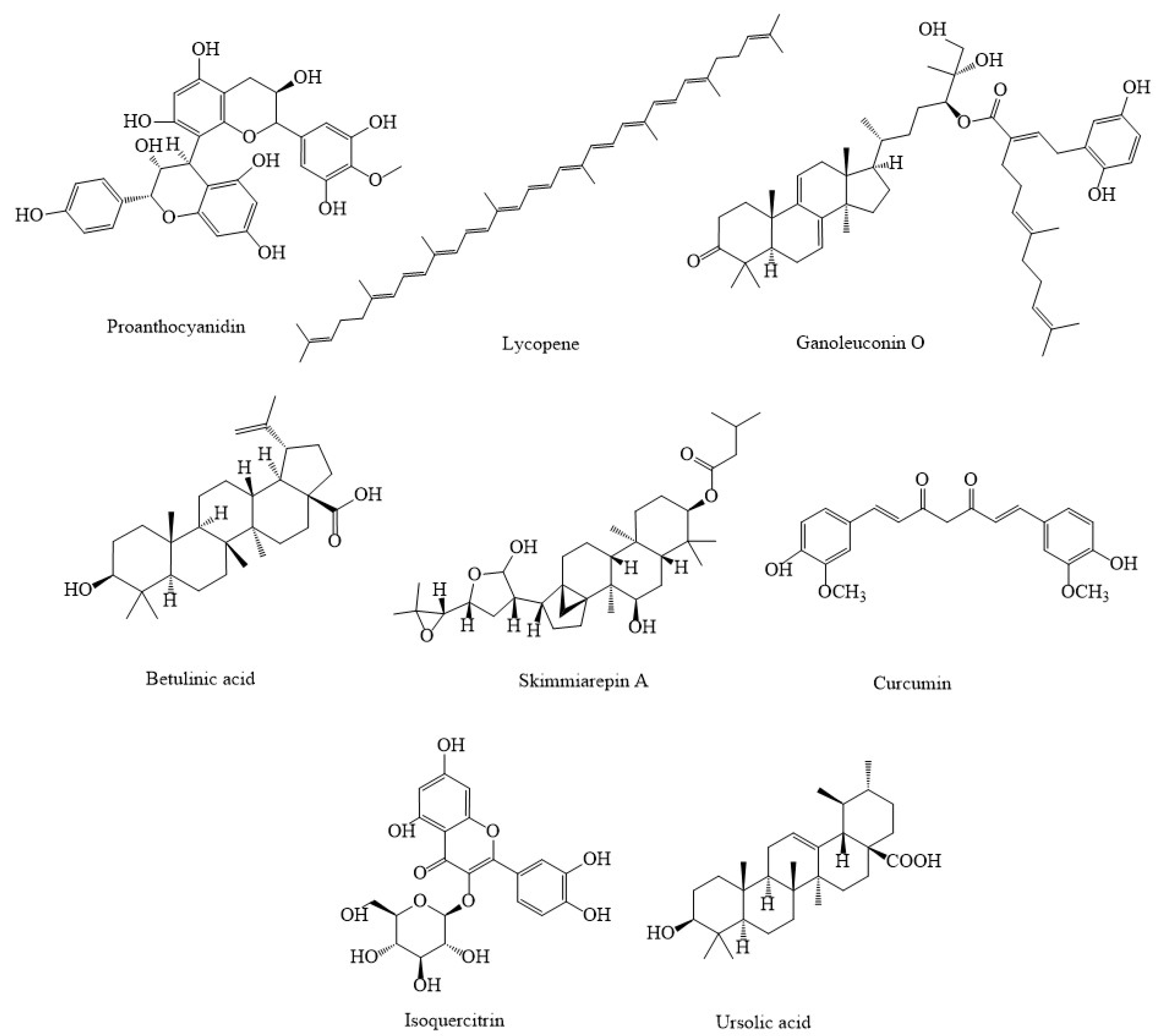
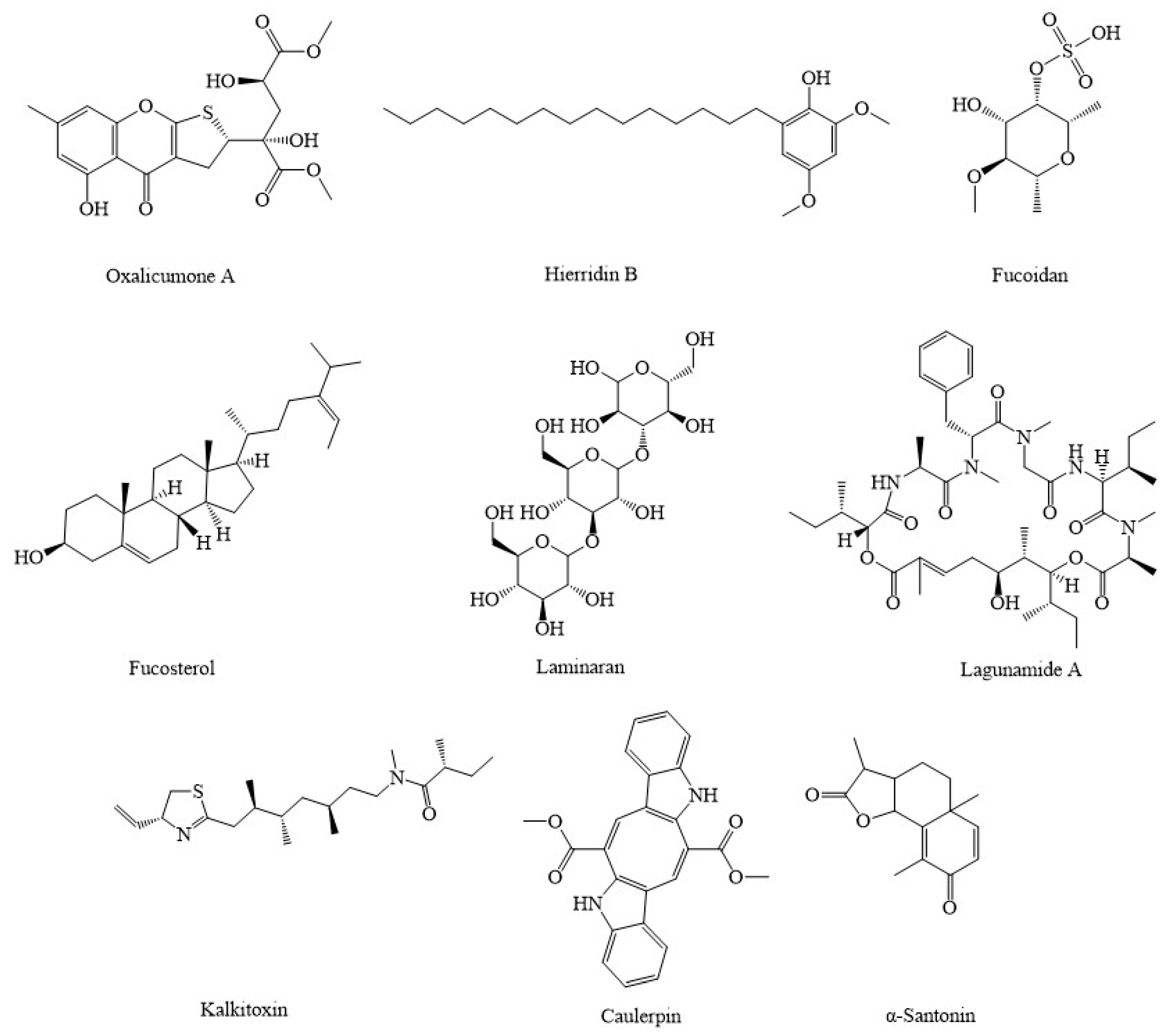
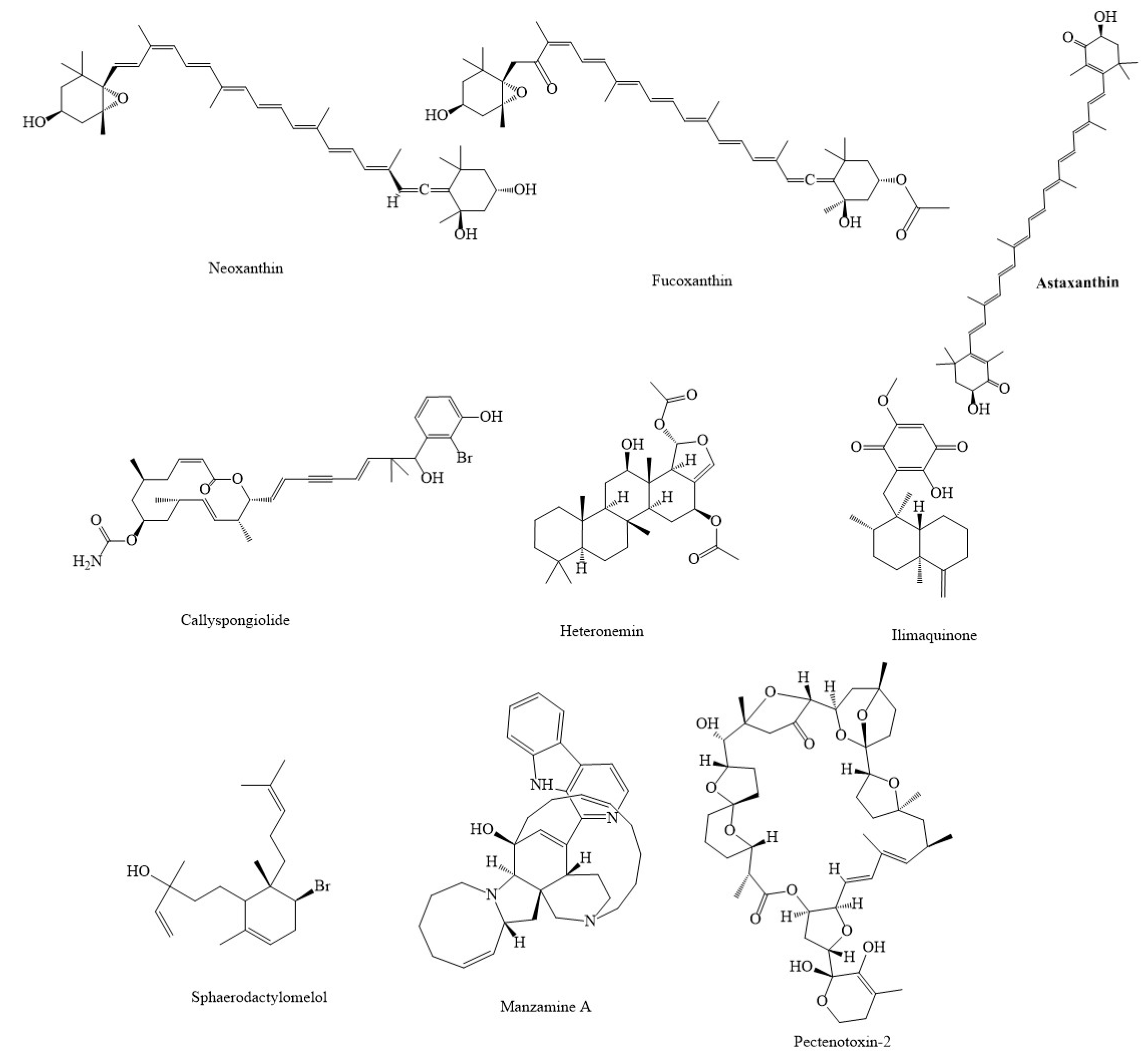
| Compound(s) | Source(s)/Class | In Vitro/In Vivo Models | Mechanisms & Outcomes | References |
|---|---|---|---|---|
| SSCC | Synthetic | Breast cancer cells (MCF-7, BT-20) | ↓Cell size; ↑ROS; ↓MMP; ↑cyt. c | [80] |
| CS-PAC-AgNPs | Synthetic | Colorectal carcinoma cells (HT-29) | ↓Cell proliferation ↑caspase-3; ↑caspase-9; ↓Bcl-2; ↓Bcl-xL; ↑cyt. c | [74] |
| PD-LPs | Synthetic | Hepatocellular carcinoma cells (SMMC-7721) | ↑Caspase-3; ↑Bax; ↓Bcl-2 | [73] |
| Gemcitabine and AgNTs | Synthetic | Glioma cells (U87) | ↑ROS; ↓MMP; ↑cyt. c | [81] |
| Celecoxib and dolastatin 15 | Synthetic + natural | Sprague-Dawley rats | ↓Bcl-2; ↑caspase-3; ↑caspase-9; ↑cyt. c; ↑p53; ↓MMP | [82] |
| Ganoleuconin O | Terpenoid | Hepatocellular carcinoma (Huh7.5) | ↑Bax; ↑caspase-9; ↑p53; ↑cyt. c; ↓ATP; ↓Bcl-2 | [77] |
| Betulinic acid | Terpenoid | Cervical carcinoma cells (HeLa); Renal cell carcinoma (PC12, and ACHN) | ↑Bax; ↑caspase-3; ↑caspase-9; ↑ROS; ↓Bcl-2 | [77] |
| Skimmiarepin A and C | Terpenoid | Breast cancer cells (T47D) | ↓HIF-1; ↓complex I | [78] |
| Ursolic acid | Terpenoid | Breast cancer cells (MCF-7) | ↓Cell proliferation; ↓MMP; ↑ROS | [83] |
| PhCsNPs | Phenolic compound | Oral squamous carcinoma cells (KB) | ↑ROS; ↓MMP; ↑cyt. c; ↑Bax; ↑caspase-3; ↑caspase-9; ↓Bcl-2 | [84] |
| Curcumin | Phenolic compound | Hepatocellular carcinoma cells (HepG2) | ↑Bax; ↑caspase-3; ↑cyt. c; ↓Bcl-2 | [79] |
| Isoquercitrin | Phenolic compound | Breast cancer cells (MDA-MB-231) | ↓MMP; ↓Bcl-2; ↑Bax | [77] |
| Compound(s) | Source(s) | In Vitro/In Vivo Models | Mechanisms & Outcomes | References |
|---|---|---|---|---|
| Oxalicumone A | Fungus Penicillium oxalicum | Cervical adenocarcinoma cells (L-02) | ↓ATP; ↑MPTP; ↑Size of mitochondria; ↓matrix density | [88] |
| Hierridin B | Marine cyanobacterium Cyanobium sp. | Colorectal carcinoma cells (HT-29) | ↓VDAC1; ↑Ca2+; ↑cyt. c | [89] |
| DDSD | Brown marine algae Stoechospermum marginatum | Mouse melanoma cells (B16F10) | ↑ROS; ↑cyt. c; ↓Bcl-2/Bax | [90] |
| Fucoidan | Brown algae | Breast cancer cells (MCF-7 MCF-7, MDA-MB-231); Hepatocellular carcinoma cells (SMMC-7721) | ↓Bcl-2; ↑Bax; ↑cyt. c; ↑Bad; ↑ROS | [91,92] |
| Fucosterol | Brown algae | Cervical carcinoma cells (HeLa); Ovarian cancer cells (ES2, OV90) | ↑ROS; ↑caspase-3; ↑caspase-9; ↑cyt. c; ↓MMP | [17,94] |
| Laminarin | Brown algae | Ovarian cancer cells (ES2, OV90); Colon cancer cells (LOVO) | ↓MMP; ↑ROS; ↑Ca2+; ↑cyt. c; ↑DNA destruction; ↑caspase-3; ↑Bax | [95] |
| Astaxanthin | Microalgae Chlorella zofingiensis, Chlorococcum sp., red yeast Phaffia rhodozyma, and the marine Agrobacterium aurantiacum | Glioblastoma multiforme cells | ↑Bax; ↑Bad; ↓Bcl-2 | [118] |
| Astaxanthin Nanoemulsion | Murine fibroblast cells (L929, NIH3T3) | ↑ROS | [118] | |
| 9′-cis-neoxanthin and fucoxanthin | Green leafy vegetables, brown algae | Colon cancer cells (HL-60) | ↑Bax; ↑cyt. c; ↓ATP; ↑AIF | [113,114,115] |
| Caulerpin | Marine green algae Caulerpa cylindracea | Leukemia cells (THP-1) | ↓Complex II | [100] |
| Lagunamide A | Marine cyanobacterium Lyngbya majuscule | NSCLC (A549); Cervical carcinoma cells (HeLa); Hepatocellular carcinoma cell (HepG2); Colorectal carcinoma cells (HCT116); Osteosarcoma cells (U2OS) | ↓Cell proliferation; ↑ROS; ↓MMP; ↑caspase-3; ↓Bcl-2; ↓Bcl-xL; ↑Bax | [102] |
| MSP-4-peptide | Fish Nile tilapia (Oreochromis niloticus) | Osteosarcoma cells (MG63) | ↓Bcl-2; ↑Bax; ↑Bid; ↑cyt. c; ↑caspase-3; ↑caspase-9 | [103] |
| 18B-15-3 | Marine fungus Aspergillus sp. | Pancreatic cancer cells (PANC-1) | ↓MMP; ↓complex III; ↓intake of oxygen | [138] |
| EI-SP | Marine algae Enteromorpha intestinalis | Hepatocellular carcinoma cells (HepG2) | ↓Bcl-2; ↑Bax; ↑cyt. c; ↑caspase-9 | [98] |
| Thyrsiferol | Red algae Laurencia thyrsifera J.Agardh | Breast cancer cells (T47D) | ↓Complex I; ↓HIF-1; ↓oxygen utilization | [99] |
| Conus textile | Marine cone snails | Glioma cells (U87MG) | ↑Caspase-3; ↑caspase-9; ↑cyt. c; ↑ROS; ↑Bax/Bcl-2 | [104] |
| Phallusia nigra | Marine tunicate | Albino\Wistar rats | ↑ROS; ↑cyt. c | [106] |
| Aplidin | Marine tunicate Aplidium albicans | leukemia lymphoma models | ↑ROS; ↓ATP; ↓MMP | [107] |
| Holothuria parva and Haliclona oculata | Sea cucumber | Sprague-Dawley rats | ↑ROS; ↑cyt. c; ↑caspase-3 | [139] |
| Kalkitoxin | Marine cyanobacterium Lyngbya majuscula | Breast cancer cells (T47D) | ↓Complex I; ↓HIF-1 | [108] |
| MDTFC | Soft coral | Leukemia cells (THP-1) | ↑Caspase-3; caspase-9; ↑Bax/Bcl-2; ↑cyt. c; ↓MMP | [109] |
| ECHC | Coral Acropora formosa | NSCLC (A549) | ↑ROS; ↑cyt. c; ↑Bax; ↓Bcl-2; ↓TNF-α; ↓IL-8; ↓MMP2; ↓MMP9 | [110] |
| Turbo coronatus | Marine mollusk | Ovarian cancer cells (EOC) | ↑ROS; ↑cyt. c; ↑caspase-3; ↓MMP | [111] |
| Mansouramycin C | Marine streptomycete | NSCLC (A549) | ↑ROS; ↓ATP; ↓MMP; ↑mitochondrial swelling | [112] |
| Arca inflata | Bivalve mollusk | Colorectal cancer cells | ↑ROS; ↑Ca2+; ↑cyt. c; ↓MMP; ↑caspase-3; ↓cell growth | [119] |
| CS5931 | Sea squirt Ciona savignyi | Colorectal carcinoma cells (HCT116) | ↑Caspase-3; ↑caspase-9; ↑cyt. c; ↑Bax; ↓MMP | [120] |
| Lamellarin D | Marine mollusk Lamellaria | Leukemia cells (p388) | ↑Bax; ↑caspase-3; ↑caspase-9; ↓Bcl-2 | [121,122,123] |
| Irciniastatin A | Marine sponge Ircinia ramosa | Pancreatic cancer cells (PANC-1) | ↑JNK; ↑p38 | [16] |
| GLP | Green alga Codium decorticatum | Breast cancer cells (MDA-MB-231) | ↑ROS; ↑cyt c; ↑caspase-3; ↑caspase-9; ↓MMP | [125] |
| Avicennia marina combined with Ag NPs | Marine mangrove plant | NSCLC (A549) | ↑ROS; ↓MMP | [126] |
| Pterocellin A | Marine bryozoan Pterocella vesiculosa | Cervical carcinoma cells (HeLa) | ↑Caspase-3; ↑nucleus condensation | [127] |
| Chlorella sorokiniana | Green algae | NSCLC | ↑Caspase-3; ↑caspase-9; ↑Bax; ↓MMP; ↑cyt. c; ↓Bcl-2 | [101] |
| Heteronemin | Marine spong Hippospongia sp. | Hepatocellular carcinoma cells | ↑ROS; ↑Bax; ↑SOD2; ↑caspase 8; ↓Bcl-2; ↓SOD1 | [129] |
| Sphaerodactylomelo | Red alga Sphaerococcus coronopifolius | Breast cancer cells (MCF-7) | ↑ROS; ↑caspase-3; caspase-9; ↑H2O2; ↓MMP; ↑cyt. c; ↓Bcl-2 | [130] |
| Ilimaquinone | Marine sponges Halichondria sp. | Breast cancer cells (MCF-7, MDA-MB-231) | ↑ROS; ↑caspase-3; ↑caspase-9; ↓MMP | [131] |
| Manzamine A | Sponges Haliclona sp., Xestospongia sp., and Pellina sp. | Colorectal carcinoma cells (HCT116) | ↑Caspase-3; ↑caspase-7; ↓MMP; ↓Bcl-2; ↓Bcl-xL | [136] |
| Aplysinopsins | Genera of sponges and scleractinian corals | Leukemia cells (K562) | ↓Cell proliferation; ↓Bcl-2; ↓MMP; ↓oxygen utilization; | [137] |
| Lipophilic 2,5-disubstituted pyrroles | Marine sponge Mycale sp. | Breast cancer cells (T47D) | ↓HIF-1; ↓complex I; ↑ROS | [140] |
| Urupocidin A | Marine sponges | Prostate cancer cells (22Rv1, LNCaP and MRC-9) | ↑ROS; ↓MMP; ↓Bcl-2 | [87] |
| Papuamine | Marine sponge Neopetrosia chaliniformis | Breast cancer cells (MCF-7) | ↓MMP; ↑cyt. c; ↑Bax | [141] |
| 10-acetylirciformonin B | Marine sponge | Acute myeloid leukemia cells (HL 60) | ↑ROS; ↑cyt. c; ↑Bax; ↓Bcl-2; ↓Bcl-xL | [142] |
| α-Santonin | Sponge Dysidea avara | ALL B-lymphocytes | ↑ROS; ↑caspase-3; ↓MMP; ↑cyt. c | [143] |
| DP | Marine sponge Callyspongia fistularis | Hepatocellular carcinoma cell (HepG2) | ↑Bax/Bcl-2; ↑caspase-3; ↓MMP; ↑ROS; ↑cyt. c | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhri, S.; Abdian, S.; Moradi, S.Z.; Delgadillo, B.E.; Fimognari, C.; Bishayee, A. Marine Compounds, Mitochondria, and Malignancy: A Therapeutic Nexus. Mar. Drugs 2022, 20, 625. https://doi.org/10.3390/md20100625
Fakhri S, Abdian S, Moradi SZ, Delgadillo BE, Fimognari C, Bishayee A. Marine Compounds, Mitochondria, and Malignancy: A Therapeutic Nexus. Marine Drugs. 2022; 20(10):625. https://doi.org/10.3390/md20100625
Chicago/Turabian StyleFakhri, Sajad, Sadaf Abdian, Seyed Zachariah Moradi, Blake E. Delgadillo, Carmela Fimognari, and Anupam Bishayee. 2022. "Marine Compounds, Mitochondria, and Malignancy: A Therapeutic Nexus" Marine Drugs 20, no. 10: 625. https://doi.org/10.3390/md20100625
APA StyleFakhri, S., Abdian, S., Moradi, S. Z., Delgadillo, B. E., Fimognari, C., & Bishayee, A. (2022). Marine Compounds, Mitochondria, and Malignancy: A Therapeutic Nexus. Marine Drugs, 20(10), 625. https://doi.org/10.3390/md20100625









