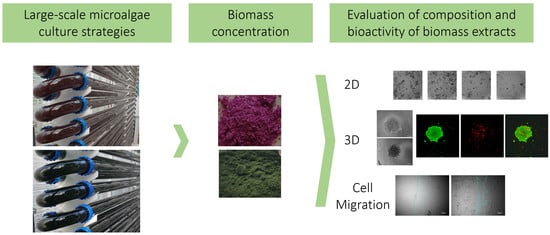
Therapeutic Potential of Microalgae-Derived Bioactive Metabolites Is Influenced by Different Large-Scale Culture Strategies
Abstract
:1. Introduction
2. Results
2.1. Microalgae Strain Performance in Photobioreactors in Standard and Stressed Conditions
2.2. Macromolecule Composition in Microalgae Strains Is Influenced by Culture Conditions
2.3. Microalgae Biomass Extracts from Standard and Stressed Cultures Exhibit Antiproliferative Activity in Two- and Three-Dimensional OC Cell Culture Models
2.4. Microalgae Extracts from Stressed Cultures Reduce the Migration Capacity of OC Cells
2.5. Microalgae Extraction Methods and Culture Strategy Influence the Antioxidant Activity of Microalgae Biomass Extracts
3. Discussion
4. Materials and Methods
4.1. Microalgae Culture Experimental Design
4.2. Microalgae Cultivation
4.3. Microalgal Analysis
4.4. Dry Weight (DW)
4.5. Growth Rate (µ) and Duplication Time (DT)
4.6. Microalgae Biomass Proximal Analysis
4.6.1. Fatty Acids
4.6.2. Proteins
4.6.3. Carbohydrates
4.7. Cell Culture
4.8. Preparation of Microalgae Extracts
4.9. Antiproliferative Activity Assays
4.10. 3D Spheroid Culture and 3D Live/Dead Staining
4.11. Migration Assay
4.12. DPPH Activity
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Pierce, M.; Glaser, K.B.; Newman, J.; Jaspars, M.; Jimenez, C.; Tagliatela-Scafati, O.; Yang, J. The Global Marine Pharmaceuticals Pipeline. Available online: https://www.marinepharmacology.org/ (accessed on 18 July 2022).
- Rumin, J.; Nicolau, E.; Junior, R.G.O.; Fuentes-Grunewald, C.; Flynn, K.J.; Picot, L. A Bibliometric Analysis of Microalgae Research in the World, Europe, and the European Atlantic Area. Mar. Drugs 2020, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Rumin, J.; Nicolau, E.; Junior, R.G.O.; Fuentes-Grunewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef] [PubMed]
- Vieira Costa, J.B.M.J.; Schneider Fanka, L.; Kosinski, R.C.; Greque de Morais, M. Microalgal biotechnology applied in biomedicine. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 429–439. [Google Scholar]
- Camacho, F.G.; Rodriguez, J.G.; Miron, A.S.; Garcia, M.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef]
- Severo, I.A.; Dias, R.R.; do Nascimento, T.C.; Depra, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae-derived polysaccharides: Potential building blocks for biomedical applications. World J. Microbiol. Biotechnol. 2022, 38, 150. [Google Scholar] [CrossRef]
- Martinez-Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [Green Version]
- de Vera, C.R.; Diaz Crespin, G.; Hernandez Daranas, A.; Montalvao Looga, S.; Lillsunde, K.E.; Tammela, P.; Perala, M.; Hongisto, V.; Virtanen, J.; Rischer, H.; et al. Marine Microalgae: Promising Source for New Bioactive Compounds. Mar. Drugs 2018, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Grunewald, C.; Bayliss, C.; Zanain, M.; Pooley, C.; Scolamacchia, M.; Silkina, A. Evaluation of batch and semi-continuous culture of Porphyridium purpureum in a photobioreactor in high latitudes using Fourier Transform Infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour. Technol. 2015, 189, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Gallardo Rodríguez, J.J.; Mirón, S.A.; García Camacho, F.; Cerón García, M.C.; Belarbi, E.H.; Molina Grima, E. Culture of dinoflagellates in a fed-batch and continuous stirred-tank photobioreactors: Growth, oxidative stress and toxin production. Process Biochem. 2010, 45, 660–666. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Avello, A.; Llanos-Rivera, A.; Krock, B.; Agurto-Muñoz, C.; Sánchez-Mirón, A.; García-Camacho, F. Production of extracts with anaesthetic activity from the culture of Heterosigma akashiwo in pilot-scale photobioreactors. Algal Res. 2020, 45, 101760. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 18 July 2022).
- Howard, D.; Garcia-Parra, J.; Healey, G.D.; Amakiri, C.; Margarit, L.; Francis, L.W.; Gonzalez, D.; Conlan, R.S. Antibody-drug conjugates and other nanomedicines: The frontier of gynaecological cancer treatment. Interface Focus 2016, 6, 20160054. [Google Scholar] [CrossRef]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9, 5515. [Google Scholar] [CrossRef]
- Fuentes-Grunewald, C.; Garces, E.; Alacid, E.; Sampedro, N.; Rossi, S.; Camp, J. Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J. Ind. Microbiol. Biotechnol. 2012, 39, 207–216. [Google Scholar] [CrossRef]
- Healey, G.D.; Pan-Castillo, B.; Garcia-Parra, J.; Davies, J.; Roberts, S.; Jones, E.; Dhar, K.; Nandanan, S.; Tofazzal, N.; Piggott, L.; et al. Antibody drug conjugates against the receptor for advanced glycation end products (RAGE), a novel therapeutic target in endometrial cancer. J. Immunother. Cancer 2019, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional In Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, O.; Mitlo, T.; Hesley, J.; Luke, S.; Owens, W.; Cromwell, E.F. High-content assays for characterizing the viability and morphology of 3D cancer spheroid cultures. Assay Drug Dev. Technol. 2015, 13, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.S.; Costa, E.C.; Nunes, A.S.; de Melo-Diogo, D.; Correia, I.J. Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models. Int. J. Pharm. 2018, 551, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Coward, T.; Fuentes-Grunewald, C.; Silkina, A.; Oatley-Radcliffe, D.L.; Llewellyn, G.; Lovitt, R.W. Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour. Technol. 2016, 221, 607–615. [Google Scholar] [CrossRef]
- Sosa-Hernandez, J.E.; Romero-Castillo, K.D.; Parra-Arroyo, L.; Aguilar-Aguila-Isaias, M.A.; Garcia-Reyes, I.E.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H.M.N. Mexican Microalgae Biodiversity and State-Of-The-Art Extraction Strategies to Meet Sustainable Circular Economy Challenges: High-Value Compounds and Their Applied Perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef]
- Lu, X.; Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Liu, X.; Xie, S. Effects of Different Environmental Factors on the Growth and Bioactive Substance Accumulation of Porphyridium purpureum. Int. J. Environ. Res. Public Health 2020, 17, 2221. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef]
- Wali, A.F.; Al Dhaheri, Y.; Ramakrishna Pillai, J.; Mushtaq, A.; Rao, P.G.M.; Rabbani, S.A.; Firdous, A.; Elshikh, M.S.; Farraj, D.A.A. LC-MS Phytochemical Screening, In Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- Elkhateeb, W.; El-Sayed, H.; Fayad, W.; Al Kolaibe, A.G.; Daba, M.E.a.G. In vitro Anti-breast cancer and antifungal Bio-efficiency of some microalgal extracts. Egypt. J. Aquat. Biol. Fish. 2020, 24, 263–279. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Samarakoon, K.W.; Chaminda Lakmal, H.H.; Kim, E.-A.; Kwon, O.-M.; Dilshara, M.G.; Lee, J.B.; Jeon, Y.-J. Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae 2016, 31, 277–287. [Google Scholar] [CrossRef]
- Hanaa Ali Hussein, H.M.; Maziah Mohd Ghazaly, A.A. Laith, Mohd Azmuddin Abdullah Anticancer and antioxidant activities of Nannochloropsis oculata and Chlorella sp. extracts in co-application with silver nanoparticle. J. King Saud Univ. Sci. 2020, 32, 3486–3494. [Google Scholar] [CrossRef]
- Nikolova, B.; Semkova, S.; Tsoneva, I.; Antov, G.; Ivanova, J.; Vasileva, I.; Kardaleva, P.; Stoineva, I.; Christova, N.; Nacheva, L.; et al. Characterization and potential antitumor effect of a heteropolysaccharide produced by the red alga Porphyridium sordidum. Eng. Life Sci. 2019, 19, 978–985. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de Los Angeles Arrojo-Agudo, M.; Perez-Manriquez, C.; Abdala-Diaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nageli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Wang, W.-N.; Li, Y.; Zhang, Y.; Xiang, W.-Z.; Li, A.-F.; Li, T. Comparison on characterization and antioxidant activity of exopolysaccharides from two Porphyridium strains. J. Appl. Phycol. 2021, 33, 2983–2994. [Google Scholar] [CrossRef]
- Abolhasani, M.H.; Safavi, M.; Goodarzi, M.T.; Kassaee, S.M.; Azin, M. Identification and anti-cancer activity in 2D and 3D cell culture evaluation of an Iranian isolated marine microalgae Picochlorum sp. RCC486. Daru 2018, 26, 105–116. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Invest. 2013, 93, 528–542. [Google Scholar]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschlager, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal Prospects of Antioxidants from Algal Sources in Cancer Therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; de Oliveira Junior, R.G.; Fleury, A.; Oudinet, C.; Pytowski, L.; Bérard, J.B.; Nicolau, E.; Thiéry, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from Porphyridium purpureum induces apoptosis in human melanoma cells expressing the oncogenic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Rev. Bras. Farmacogn. 2018, 28, 457–467. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grunewald, C.; Pastrana, L.M.; Fucinos, P.; Wojdylo, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef] [Green Version]








| Microalgae Extract (mg/mL) | % Inhibition of DPPH | |||
|---|---|---|---|---|
| Methanol Extraction | H2O Extraction | |||
| Standard | Stressed | Standard | Stressed | |
| 0.0625 | 2.76 | 0 | 7.30 | 5.40 |
| 0.125 | 4.60 | 0.92 | 4.44 | 7.30 |
| 0.25 | 15.95 * | 1.84 | 0 | 5.71 |
| 0.5 | 31.60 * | 5.21 | 6.67 | 10.79 * |
| 1 | 50.31 * | 17.79 * | 0 | 19.36 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Parra, J.; Fuentes-Grünewald, C.; Gonzalez, D. Therapeutic Potential of Microalgae-Derived Bioactive Metabolites Is Influenced by Different Large-Scale Culture Strategies. Mar. Drugs 2022, 20, 627. https://doi.org/10.3390/md20100627
Garcia-Parra J, Fuentes-Grünewald C, Gonzalez D. Therapeutic Potential of Microalgae-Derived Bioactive Metabolites Is Influenced by Different Large-Scale Culture Strategies. Marine Drugs. 2022; 20(10):627. https://doi.org/10.3390/md20100627
Chicago/Turabian StyleGarcia-Parra, Jezabel, Claudio Fuentes-Grünewald, and Deyarina Gonzalez. 2022. "Therapeutic Potential of Microalgae-Derived Bioactive Metabolites Is Influenced by Different Large-Scale Culture Strategies" Marine Drugs 20, no. 10: 627. https://doi.org/10.3390/md20100627
APA StyleGarcia-Parra, J., Fuentes-Grünewald, C., & Gonzalez, D. (2022). Therapeutic Potential of Microalgae-Derived Bioactive Metabolites Is Influenced by Different Large-Scale Culture Strategies. Marine Drugs, 20(10), 627. https://doi.org/10.3390/md20100627