Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect
Abstract
1. Neurodegenerative Diseases
2. Alzheimer’s Disease
2.1. AD Risk Factors
2.1.1. Age
2.1.2. Genetics
2.1.3. Other Risk Factors
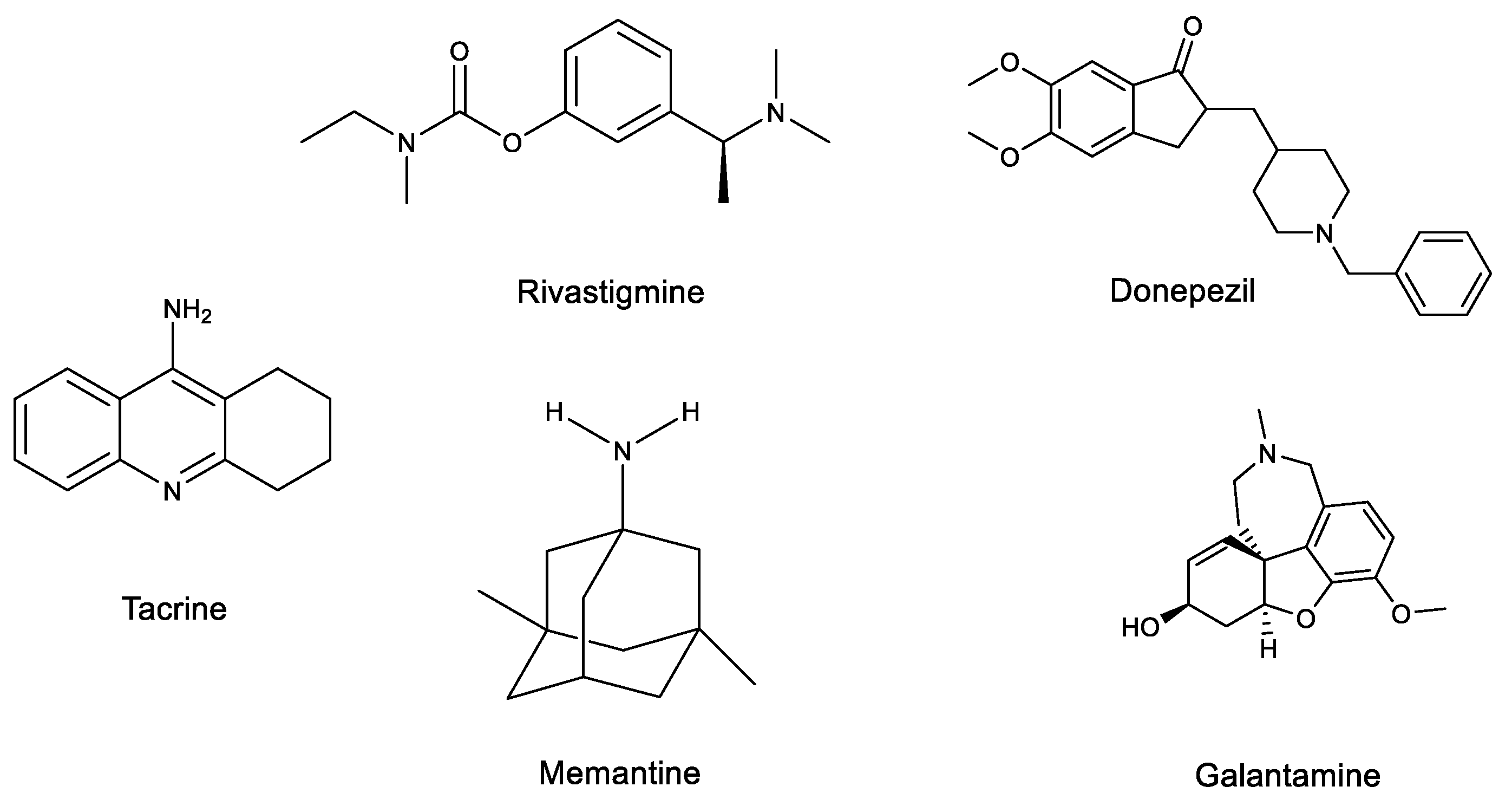
2.2. AD Treatments
2.3. Natural Marine Sources Decreasing AD Symptoms
DHA
3. DHA-Phospholipids from Marine Sources
3.1. DHA-PLs in Fish
3.2. DHA-PLs in Krill
3.3. DHA-PLs in Mollusks
3.4. DHA-PLs in Marine By-Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortada, I.; Farah, R.; Nabha, S.; Ojcius, D.M.; Fares, Y.; Almawi, W.Y.; Sadier, N.S. Immunotherapies for Neurodegenerative Diseases. Front. Neurol. 2021, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Woodruff, T.M. Neuroinflammation as a Therapeutic Target in Neurodegenerative Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Asefy, Z.; Hoseinnejhad, S.; Ceferov, Z. Nanoparticles approaches in neurodegenerative diseases diagnosis and treatment. Neurol. Sci. 2021, 42, 2653–2660. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Jeromin, A.; Bowser, R. Biomarkers in neurodegenerative diseases. Neurodegener. Dis. 2017, 15, 491–528. [Google Scholar]
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimer Res. Ther. 2020, 12, 1–11. [Google Scholar]
- Sait, A.; Angeli, C.; Doig, A.J.; Day, P.J.R. Viral Involvement in Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 1049–1060. [Google Scholar] [CrossRef]
- Möller, H.J.; Graeber, M.B. Alzheimer first case. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 111–122. [Google Scholar]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 1–21. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathobiological Subtypes of Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2021, 49, 321–333. [Google Scholar] [CrossRef]
- Gaugler, J.; James, B.; Johnson, T.; Reimer, J. Alzheimer’s disease facts and figures. Alzheimer Assoc. 2021, 17, 327–406. [Google Scholar]
- Paitel, E.R.; Samii, M.R.; Nielson, K.A. A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer’s disease. Behav. Brain Res. 2021, 396, 112904. [Google Scholar] [CrossRef]
- Gumus, M.; Multani, N.; Mack, M.L.; Tartaglia, M.C. Progression of neuropsychiatric symptoms in young-onset versus late-onset Alzheimer’s disease. GeroScience 2021, 43, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.C.; Abreu, M.J.; Machado, C.; Santos, B.; Costa, A.S. Neuropsychiatric Profile in Early Versus Late Onset Alzheimer’s Disease. Am. J. Alzheimer Dis. Dement. 2018, 33, 93–99. [Google Scholar] [CrossRef]
- Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 1–13. [Google Scholar]
- Jacobs, H.I.L.; Riphagen, J.M.; Ramakers, I.H.G.B.; Verhey, F.R.J. Alzheimer’s disease pathology: Pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol. Psychiatry 2021, 26, 897–906. [Google Scholar] [CrossRef]
- Park, K.H.; Noh, Y.; Choi, E.J.; Kim, H.; Chun, S.; Son, Y.D. Functional connectivity of the hippocampus in early- and vs. late-onset alzheimer’s disease. J. Clin. Neurol. 2017, 13, 387–393. [Google Scholar] [CrossRef]
- Kosicek, M.; Hecimovic, S. Phospholipids and Alzheimer’s disease: Alterations, mechanisms and potential biomarkers. Int. J. Mol. Sciences. 2013, 14, 1310–1322. [Google Scholar] [CrossRef]
- Vinicius, M.; Mello, C.D.; Vieira, L.; Cruz de Souza, L.; Gomes, K.; Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 1–11. [Google Scholar]
- Scollo, F.; Tempra, C.; Lolicato, F.; Sciacca, M.F.M.; Raudino, A.; Milardi, D.; Rosa, C. Phospholipids Critical Micellar Concentrations Trigger Different Mechanisms of Intrinsically Disordered Proteins Interaction with Model Membranes. J. Phys. Chem. Lett. 2018, 9, 5125–5129. [Google Scholar] [CrossRef]
- Borges, M.K.; Lopes, T.N.; Biella, M.M.; Siqueira, A.; Mauer, S.; Aprahamian, I. Early-Onset Alzheimer Disease (EOAD) With Aphasia: A Case Report. Front. Psychiatry 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Ayodele, T.; Rogaeva, E.; Kurup, J.T.; Beecham, G.; Reitz, C. Early-Onset Alzheimer’s Disease: What Is Missing in Research? Curr. Neurol. Neurosci. Rep. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Cacace, R.; Sleegers, K.; Broeckhoven, C. Van Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Hong, C.H.; Lee, K.S.; Kang, D.R.; Lee, J.D.; Choi, S.H.; Kim, S.Y.; Na, D.L.; Seo, S.W.; Kim, D.K.; et al. Mortality Risk after Diagnosis of Early-Onset Alzheimer’s Disease versus Late-Onset Alzheimer’s Disease: A Propensity Score Matching Analysis. J. Alzheimer Dis. 2017, 56, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Carpanini, S.M.; Harwood, J.C.; Baker, E.; Torvell, M.; Sims, R.; Williams, J.; Morgan, B.P. The impact of complement genes on the risk of late-onset Alzheimer’s disease. Genes 2021, 12, 443. [Google Scholar] [CrossRef]
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Yoon, C.W.; Kim, S.W.; Jeong, H.J.; Seo, S.; Na, D.L.; Noh, Y.; Seong, J.K. Effects of Alzheimer’s and Vascular Pathologies on Structural Connectivity in Early- and Late-Onset Alzheimer’s Disease. Front. Neurosci. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, M.N.; Paska, A.V.; Konjevod, M.; Kouter, K.; Strac, D.S.; Erjavec, G.N.; Pivac, N. Epigenetics of Alzheimer ’ s Disease. 2021, 11, 1–38. Biomolecules 2021, 11, 1–38. [Google Scholar]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef]
- Tomaszewski, N.; He, X.; Solomon, V.; Lee, M.; Mack, W.J.; Quinn, J.F.; Braskie, M.N.; Yassine, H.N. Effect of APOE Genotype on Plasma Docosahexaenoic Acid (DHA), Eicosapentaenoic Acid, Arachidonic Acid, and Hippocampal Volume in the Alzheimer’s Disease Cooperative Study-Sponsored DHA Clinical Trial. J. Alzheimer Dis. 2020, 74, 975–990. [Google Scholar] [CrossRef]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Sudeshna Das, B.T.H. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Coughlan, G.; Larsen, R.; Kim, M.; White, D.; Gillings, R.; Irvine, M.; Scholey, A.; Cohen, N.; Legido-Quigley, C.; Hornberger, M.; et al. APOE ε4 alters associations between docosahexaenoic acid and preclinical markers of Alzheimer’s disease. Brain Commun. 2021, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, F.K.; Al-Janabi, T.; Hardy, J.; Karmiloff-Smith, A.; Nizetic, D.; Tybulewicz, V.L.J.; Fisher, E.M.C.; Strydom, A. A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015, 16, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Maestri, M.; Spanetta, M.; Placidi, F.; Bonanni, E.; Mercuri, N.B.; Guarnieri, B. Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med. Rev. 2021, 55, 101375. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, S.; Hermkens, D.M.A.; Van Der Weerd, L.; Vries, H.E.; Daemen, M.J. Vascular Hypothesis of Alzheimer Disease: Topical Review of Mouse Models. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1265–1283. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Oxidative Stress in the Pathogenesis of Alzheimer’s Disease and Cerebrovascular Disease with Therapeutic Implications. CNS Neurol. Disord. Drug Targets 2020, 19, 94–108. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Liss, L.; Horrocks, L.A. Neurochemical aspects of Alzheimer’s disease: Involvement of membrane phospholipids. Metab. Brain Dis. 1988, 3, 19–35. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Pettegrew, J.W.; Panchalingam, K.; Hamilton, R.L.; McClure, R.J. Brain Membrane Phospholipid Alterations in Alzheimer’s Disease. Neurochem. Res. 2001, 26, 771–782. [Google Scholar] [CrossRef]
- Darreh-Shori, T.; Modiri, N.; Blennow, K.; Baza, S.; Kamil, C.; Ahmed, H.; Andreasen, N.; Nordberg, A. The apolipoprotein E ε4 allele plays pathological roles in AD through high protein expression and interaction with butyrylcholinesterase. Neurobiol. Aging 2011, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.R.; Maxwell, S.P.; Reid, G.A.; Cash, M.K.; DeBay, D.R.; Darvesh, S. Quantification of Butyrylcholinesterase Activity as a Sensitive and Specific Biomarker of Alzheimer’s Disease. J. Alzheimer Dis. 2017, 58, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Nielsen, J.A.; Villalobos, A.; Chapin, D.; Jones, S.B.; Hubbard, S.T.; Shalaby, I.A.; Ramirez, A.; Nason, D.; White, W. Pharmacology of selective acetylcholinesterase inhibitors: Implications for use in Alzheimer’s disease. Eur. J. Pharmacol. 2004, 486, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. Selectivity of Cholinesterase Inhibition Clinical Implications for the Treatment of Alzheimer’s Disease; Springer: Berlin, Germany, 1999. [Google Scholar]
- Jasiecki, J.; Wasąg, B. Butyrylcholinesterase protein ends in the pathogenesis of alzheimer’s disease—Could BCHE genotyping be helpful in alzheimer’s therapy? Biomolecules 2019, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, M.E. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Esang, M.; Gupta, M. Aducanumab as a Novel Treatment for Alzheimer’s Disease: A Decade of Hope, Controversies, and the Future. Cureus 2021, 13, 10–13. [Google Scholar] [CrossRef]
- Musiek, E.S.; Gomez-Isla, T.; Holtzman, D.M. Aducanumab for Alzheimer disease: The amyloid hypothesis moves from bench to bedside. J. Clin. Investig. 2021, 131, 1–4. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Gadhave, K.; Gehi, B.R.; Kumar, P.; Xue, B.; Uversky, V.N.; Giri, R. The Dark Side of Alzheimer’s Disease: Unstructured Biology of Proteins from the Amyloid Cascade Signaling Pathway; Springer International Publishing: New York, NY, USA, 2020. [Google Scholar]
- Lao, K.; Zhang, R.; Luan, J.; Zhang, Y.; Gou, X. Therapeutic Strategies Targeting Amyloid-β Receptors and Transporters in Alzheimer’s Disease. J. Alzheimer Dis. 2021, 79, 1429–1442. [Google Scholar] [CrossRef]
- Bertsch, M.; Franchi, B.; Meacci, L.; Primicerio, M.; Tesi, M. The amyloid cascade hypothesis and Alzheimer’s disease: A mathematical model. Eur. J. Appl. Math. 2020, 32, 749–768. [Google Scholar] [CrossRef]
- Leong, Y.Q.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab. Brain Dis. 2020, 35, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Apátiga-Pérez, R.; Soto-Rojas, L.O.; Campa-Córdoba, B.B.; Luna-Viramontes, N.I.; Cuevas, E.; Villanueva-Fierro, I.; Ontiveros-Torres, M.A.; Bravo-Muñoz, M.; Flores-Rodríguez, P.; Garcés-Ramirez, L.; et al. Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab. Brain Dis. 2021, 37, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, D.; Wu, P.; Klein, C.; Jin, C. Formaldehyde, Epigenetics, and Alzheimer’s Disease. Chem. Res. Toxicol. 2019, 32, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, A.; Keegan, R.M.; Deshmukh, R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed. Pharmacother. 2018, 98, 297–307. [Google Scholar] [CrossRef]
- Mandal, P.K.; Samkaria, A.; Maroon, J.C. AD Hypotheses and Suggested Clinical Trials. ACS Chem. Neurosci. 2021, 12, 3968–3971. [Google Scholar] [CrossRef]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future Therapeutic Perspectives into the Alzheimer’s Disease Targeting the Oxidative Stress Hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Bennett, J.P. Medical hypothesis: Neurodegenerative diseases arise from oxidative damage to electron tunneling proteins in mitochondria. Med. Hypotheses 2019, 127, 1–4. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Lin, C.-H.; Lane, H.-Y. Involvement of Cholinergic, Adrenergic, and Glutamatergic Network Modulation with Cognitive Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2283. [Google Scholar] [CrossRef]
- Yeung, J.H.Y.; Walby, J.L.; Palpagama, T.H.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Glutamatergic receptor expression changes in the Alzheimer’s disease hippocampus and entorhinal cortex. Brain Pathol. 2021, 31, e13005. [Google Scholar] [CrossRef]
- Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Sun, Z. How glutamatergic synapse loss affects the firing rhythm of DG-CA3 model related with Alzheimer’s disease. Cogn. Neurodynamics 2021, 16, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Snyder, P.J.; Giacobini, E.; Foix, L.A.P.C.; Hayden, K.; System, C.; Group, W.; Hampel, H.; Mesulam, M.M.; Cuello, C.; Khachaturian, A.S.; et al. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimer Dis. 2020, 6, 2–15. [Google Scholar]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Alzheimer’s Disease Activity of Bromophenols from a Red Alga, Symphyocladia latiuscula (Harvey) Yamada. ACS Omega 2019, 4, 12259–12270. [Google Scholar] [CrossRef] [PubMed]
- Ghoran, S.H.; Kijjoa, A. Marine-Derived Compounds with Anti-Alzheimer’s Disease Activities. Mar. Drugs 2021, 19, 410. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 62, 1242–1269. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Z.; Qian, P.; Herrup, K. Marine bacterial extracts as a new rich source of drugs against Alzheimer’s disease. J. Neurochem. 2020, 152, 493–508. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty acids, mercury, and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: Effect of culinary treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Gomes-Bispo, A.; Lourenço, H.; Matos, J.; Afonso, C.; Cardoso, C.; Castanheira, I.; Motta, C.; Prates, J.A.M.; Bandarra, N.M. The chemical composition and lipid profile of the chub mackerel (Scomber colias) show a strong seasonal dependence: Contribution to a nutritional evaluation. Biochimie 2020, 178, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal Changes in Lipid Composition of Sardine (Sardina pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kusumoto, Y.; Kanchi, N.; Kinoshita, H.; Kanegae, S.; Yamaguchi, N.; Ozawa, H. Recent trends in mental illness and omega-3 fatty acids. J. Neural Transm. 2020, 127, 1491–1499. [Google Scholar] [CrossRef]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes, M.A.H.; Wareham, N.J.; Khaw, K.T. Dietary intake and status of n−3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of α-linolenic acid to long-chain n−3 polyunsaturated fatty acids: Results from the EPIC-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1040–1051. [Google Scholar]
- Matura, S.; Prvulovic, D.; Mohadjer, N.; Fusser, F.; Oertel, V.; Reif, A.; Pantel, J.; Karakaya, T. Association of dietary fat composition with cognitive performance and brain morphology in cognitively healthy individuals. Acta Neuropsychiatr. 2021, 33, 134–140. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wei, Z. Food-grade systems for delivery of DHA and EPA: Opportunities, fabrication, characterization and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef]
- Cutuli, D.; Landolfo, E.; Decandia, D.; Nobili, A.; Viscomi, M.T.; La Barbera, L.; Sacchetti, S.; De Bartolo, P.; Curci, A.; D’amelio, M.; et al. Neuroprotective role of dietary supplementation with omega-3 fatty acids in the presence of basal forebrain cholinergic neurons degeneration in aged mice. Int. J. Mol. Sci. 2020, 21, 1741. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lim, S.J.; Ryu, Y.M.; Park, H.-R.; Suh, H.J.; Han, S.H. Absorption rate of krill oil and fish oil in blood and brain of rats. Lipids Health Dis. 2018, 17, 162. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y.; Cheon, Y.; Ma, K.; Rapoport, S.I.; Rao, J.S. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J. Psychiatr. Res. 2013, 47, 636–643. [Google Scholar] [CrossRef]
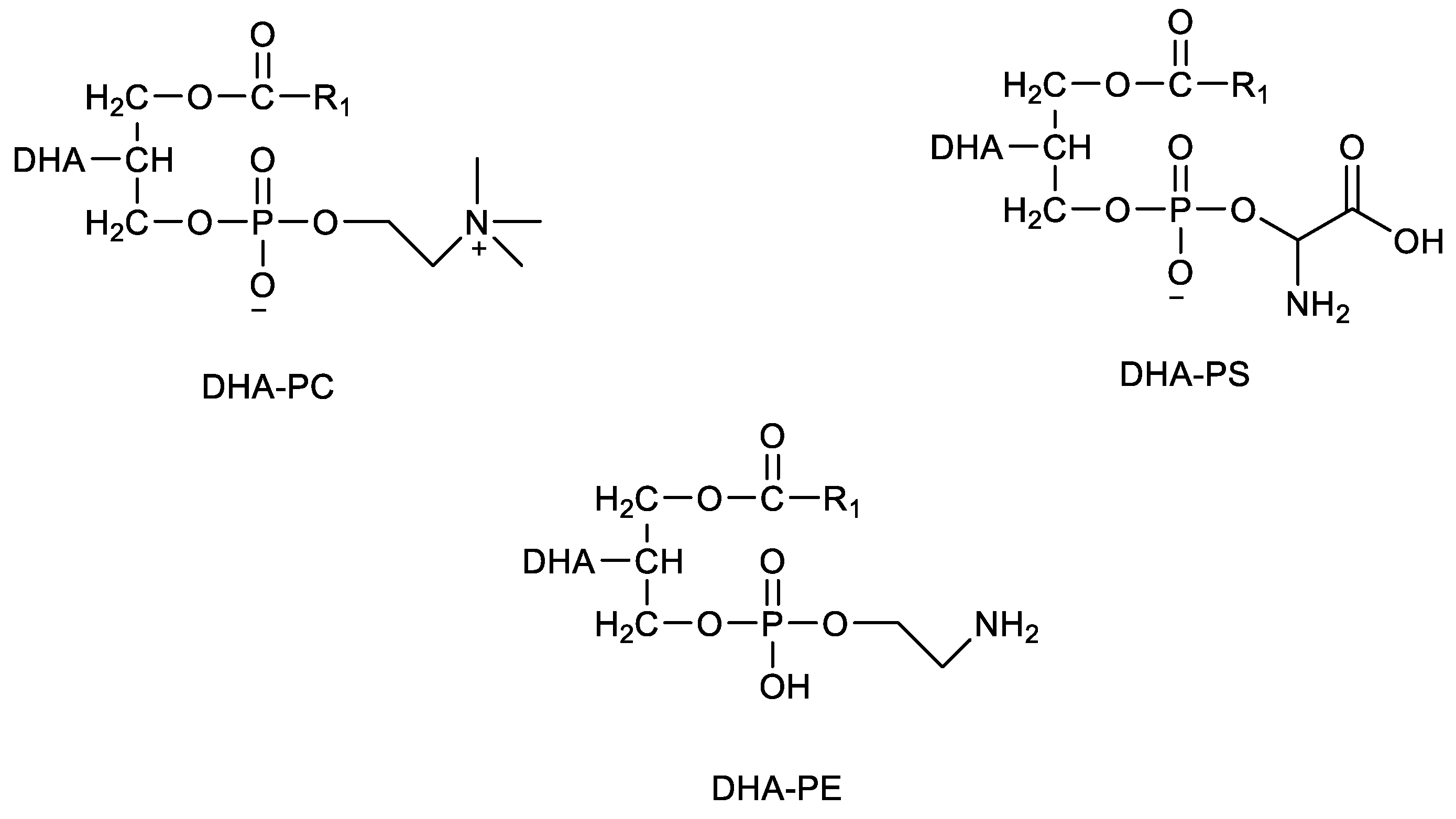
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, M.; Wang, C.; Xue, C.; Zhang, T.; Wang, Y. Comparative Analyses of DHA-Phosphatidylcholine Forage and Liposomes on Alzheimer’s Disease in SAMP8 Mice. Eur. J. Lipid Sci. Technol. 2019, 121, 1800524. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.; Evans, J.E.; Emmerich, T.; Crynen, G.; Shackleton, B.; Keegan, A.P.; Luis, C.; Tai, L.; LaDu, M.J.; Mullan, M.; et al. APOE ε4 specific imbalance of arachidonic acid and docosahexaenoic. Aging 2017, 9, 964–978. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Zhang, Y.; Wang, X.; Jin, Q.; Zhang, H. Advances in exogenous docosahexaenoic acid-containing phospholipids: Sources, positional isomerism, biological activities, and advantages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1420–1448. [Google Scholar] [CrossRef] [PubMed]
- Deinema, L.A.; Vingrys, A.; Wong, C.Y.; Jackson, D.; Chinnery, H.R.; Downie, L.E. A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Cui, J.; Wen, M.; Xu, J.; Yanagita, T.; Wang, Q.; Xue, C.; Wang, Y. Long-Term Effects of Docosahexaenoic Acid-Bound Phospholipids and the Combination of Docosahexaenoic Acid-Bound Triglyceride and Egg Yolk Phospholipid on Lipid Metabolism in Mice. J. Ocean Univ. China 2018, 17, 392–398. [Google Scholar] [CrossRef]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. Safety of phosphatidylserine containing omega3 fatty acids in ADHD children: A double-blind placebo-controlled trial followed by an open-label extension. Eur. Psychiatry 2013, 28, 386–391. [Google Scholar] [CrossRef]
- Hossain, Z.; Hosokawa, M.; Takahashi, K. Growth Inhibition and Induction of Apoptosis of Colon Cancer Cell Lines by Applying Marine Phospholipid. Nutr. Cancer 2009, 61, 123–130. [Google Scholar] [CrossRef]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Che, H.; Zhou, M.; Zhang, T.; Zhang, L.; Ding, L.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. Comparative study of the effects of phosphatidylcholine rich in DHA and EPA on Alzheimer’s disease and the possible mechanisms in CHO-APP/PS1 cells and SAMP8 mice. Food Funct. 2018, 9, 643–654. [Google Scholar] [CrossRef]
- Wen, M.; Ding, L.; Zhang, L.; Zhou, M.; Xu, J.; Wang, J.; Wang, Y.; Xue, C. DHA-PC and DHA-PS improved Aβ1–40 induced cognitive deficiency uncoupled with an increase in brain DHA in rats. J. Funct. Foods 2016, 22, 417–430. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, L.; Wen, M.; Che, H.; Huang, J.; Zhang, T.; Xue, C.; Mao, X.; Wang, Y. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.-S. Phospholipids from marine source: Extractions and forthcoming industrial applications. J. Funct. Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Farkas, T.; Kitajka, K.; Fodor, E.; Csengeri, I.; Landes, E.; Yeo, Y.K.; Krasznai, Z.; Halver, J.E. Docosahexaenoic acid-containing phospholipid molecular species in brains of vertebrates. Proc. Natl. Acad. Sci. USA 2000, 97, 6362–6366. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, Z.A.; Murzina, S.A.; Pekkoeva, S.N.; Ruokolainen, T.R.; Veselov, A.E.; Efremov, D.A.; Nemova, N.N. Lipid Profile of the Young Atlantic Salmon Salmo salar L. in the Letnyaya Zolotitsa River (Arkhangelsk Oblast, White Sea Basin). J. Ichthyol. 2019, 59, 407–413. [Google Scholar] [CrossRef]
- Egerton, S.; Mannion, D.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The proximate composition of three marine pelagic fish: Blue whiting (Micromesistius poutassou), boarfish (Capros aper) and Atlantic herring (Clupea harengus). Ir. J. Agric. Food Res. 2020, 59, 185–200. [Google Scholar] [CrossRef]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.S.; Lu, H.F.S.; Bruheim, I.; Jacobsen, C. Quality changes of Antarctic krill powder during long term storage. Eur. J. Lipid Sci. Technol. 2017, 119, 1600085. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, X.; Yang, F.; Zhang, M.; Huang, R.; Liu, J. Characterization of Molecular Species and Anti-Inflammatory Activity of Purified Phospholipids from Antarctic Krill Oil. Mar. Drugs 2021, 19, 124. [Google Scholar] [CrossRef]
- Showman, C.; Barnes, K.; Jaczynski, J.; Matak, K.E. Separation and concentration of ω-3 PUFA-rich phospholipids by hydration of krill oil. LWT 2020, 126, 109284. [Google Scholar] [CrossRef]
- Sistilli, G.; Kalendova, V.; Cajka, T.; Irodenko, I.; Bardova, K.; Oseeva, M.; Zacek, P.; Kroupova, P.; Horakova, O.; Lackner, K.; et al. Krill Oil Supplementation Reduces Exacerbated Hepatic Steatosis Induced by Thermoneutral Housing in Mice with Diet-Induced Obesity. Nutrients 2021, 13, 437. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, X.; Wang, W.; Zhou, Y.; Zhou, X.; Deng, S.; Zheng, B.; Wen, Z. Effect of Antarctic krill phospholipid (KOPL) on high fat diet-induced obesity in mice. Food Res. Int. 2021, 148, 110456. [Google Scholar] [CrossRef]
- Kroupova, P.; Keijer, J.; Bunschoten, A.; Vodicka, M.; Irodenko, I.; Oseeva, M.; Zacek, P.; Kopecky, J.; Rossmeisl, M.; Horakova, O. Omega-3 phospholipids from krill oil enhance intestinal fatty acid oxidation more effectively than omega-3 triacylglycerols in high-fat diet-fed obese mice. Nutrients 2020, 12, 2037. [Google Scholar] [CrossRef]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of krill oil and lean and fatty fish on cardiovascular risk markers: A randomised controlled trial. J. Nutr. Sci. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-J.; Shi, J.-H.; Qian, W.-B.; Cai, Z.-Z.; Li, D. Effects of Krill Oil on serum lipids of hyperlipidemic rats and human SW480 cells. Lipids Heal. Dis. 2008, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, F.; Wen, M.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. The Protective Effect of Antarctic Krill Oil on Cognitive Function by Inhibiting Oxidative Stress in the Brain of Senescence-Accelerated Prone Mouse Strain 8 (SAMP8) Mice. J. Food Sci. 2018, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar] [CrossRef]
- Zhukova, N.V. Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Tabakaev, A.V. Lipids and Fatty Acids from Soft Tissues of the Bivalve Mollusk Spisula sachalinensis. Chem. Nat. Compd. 2017, 53, 16–20. [Google Scholar] [CrossRef]
- Krishnan, S.; Chakraborty, K.; Vijayagopal, P. Nutritional profiling of selected species of edible marine molluscs from the south-west coast of India. Indian J. Fish. 2019, 66, 56–63. [Google Scholar] [CrossRef]
- Kapranova, L.L.; Nekhoroshev, M.V.; Malakhova, L.V.; Ryabushko, V.I.; Kapranov, S.V.; Kuznetsova, T.V. Fatty Acid Composition of Gonads and Gametes in the Black Sea Bivalve Mollusk Mytilus galloprovincialis Lam. at Different Stages of Sexual Maturation. J. Evol. Biochem. Physiol. 2019, 55, 448–455. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and Healthy Value of Chemical Constituents Obtained from Patagonian Squid (Doryteuthis gahi) By-Products Captured at Different Seasons. Foods 2021, 10, 2144. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; González, M.J.; Lois, S.; Medina, I. Evolution of lipid damage and volatile amine content in Patagonian squid (Doryteuthis gahi) by-products during frozen storage. Int. J. Food Sci. Technol. 2022, 57, 5409–5418. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Wang, F.; Zhang, S.; Li, H.; Zhang, Y.; Wang, X.; Liu, K.; Li, X. Separation, identification and cardiovascular activities of phospholipid classes from the head of Penaeus vannamei by lipidomics and zebrafish models. Food Funct. 2021, 12, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkelayeh, S.; Cheema, S.K.; Hawboldt, K. Evaluation of conventional solvent processes for lipid and astaxanthin extraction from shrimp processing by-products. Chem. Eng. Commun. 2022, 1–14. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Arena, R.; Renda, G.; Ficano, G.; Randazzo, M.; Fricano, S.; Sadok, S.; Santulli, A. In Vitro Bioactivity of Astaxanthin and Peptides from Hydrolisates of Shrimp (Parapenaeus longirostris) By-Products: From the Extraction Process to Biological Effect Evaluation, as Pilot Actions for the Strategy “From Waste to Profit”. Mar. Drugs 2021, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Xing, S.; Li, X.; Han, L.; Liu, K.; Wei, T.; Zhou, M. Marine Phospholipids from Fishery By-Products Attenuate Atherosclerosis. Eur. J. Lipid Sci. Technol. 2021, 123, 2000276. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Stewart, I.; Carne, A.; Tian, H.; Bekhit, A.E.D.A. Omega-3 phospholipids in Pacific blue mackerel (Scomber australasicus) processing by-products. Food Chem. 2021, 353, 129451. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Mozzoni, M.; Antoniello, N.; Cazzola, R.; Savarè, R.; Cerutti, R.; Grossi, E.; Cestaro, B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012, 15, 46–54. [Google Scholar] [CrossRef]



| Hypothesis | Explanation | References |
|---|---|---|
| Amyloid cascade | Amyloid hypothesis was developed in 1992. Amyloid cascade hypothesis is associated with the presence of soluble toxic oligomers from β-amyloid. Monomers of β-amyloid are produced through life however changes in amyloid cascade result from the unbalance between the production and the clearance of β-amyloid peptides resulting in its accumulation and aggregation in the brain. The consequences of this hypothesis are inflammation, oxidative stress, neuronal injury and death and tau pathology. | [51,52,53,54] |
| Vascular | This hypothesis was developed in 1993. Several vascular risk factors, such as atherosclerosis, hypertension, diabetes, or heart diseases are related with the increasing of β-amyloid production, dysregulation of BBB, decreased cerebral blood flow, tau hyperphosphorylation. Vascular cell dysfunction affects BBB permeability messing with the regulation of brain metabolites, accelerating β-amyloid pathology. These events cause the reduction of β-amyloid clearance, promoting the production and aggregation of β-amyloid, that leads to a lower neuronal activity and progressive neurodegeneration and neuronal death. | [38,55] |
| Epigenetics | Recently, studies regarding epigenetics (study of gene expression modification and/or chromatin structure) suggest that this hypothesis may contribute to the risk of AD development. DHA methylation and histone acetylation are responsible for gene expression at the transcriptional level. DHA methylation is catalyzed by DHA methyl transferases (DNMTs) with DNMT1 being related to faulty DHA methylation leading dementia and to neurodegeneration. | [29,56,57] |
| Oxidative stress (OS) | The brain is the human structure that uses more oxygen. OS can be defined as the imbalance (incapacity of cells to remove) of antioxidant system between reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). The decreased levels of antioxidants and excessive generation of ROS and/or RNS can lead to macromolecular damage, oxidative damage to cells (loss of function and apoptosis), molecules and biological systems.OS is considered an early event in AD. | [58,59,60,61] |
| Glutamatergic | Over 40% of neuronal synapses are glutamatergic and glutamate level is regulated by metabolite swap between neuronal, astrocytic and endothelial cells. So, β-amyloid binding to glutamate receptors provokes the overactivation of glutamate receptors causing glutamate accumulation and resulting in glutamatergic synapse loss and synapse dysfunction, leading to neuronal swelling, destruction of membrane integrity and cell death, which is related to AD. | [62,63,64,65] |
| Cholinergic | Cholinergic neurons use acetylcholine (ACh) as neurotransmitter, which is directly correlated with physiological processes. So, normal cognitive function depends on a correct cholinergic neurotransmission. ACh is essential for brain especially in memory functions. So, decrease of ACh levels, due to decrease of the enzyme that synthesizes ACh, results in cholinergic loss in cortical structures, such as cerebral cortex, basal forebrain and hippocampus, that are associated with AD. Inhibitors of AChE and of BChE increase ACh levels in the brain. | [66,67,68] |
| Sources | Tissues/Organs | DHA-PLs Concentration | PLs Classes | References |
|---|---|---|---|---|
| Fish | ||||
| Sardina pilchardus | Edible part | 3.65–11.25% | PC, PE | [77] |
| Salmo salar L. | Edible part | 3.95 ± 0.56 to 8.12 ± 1.45% | PS, PC, LPC | [105] |
| Micromesistius poutassou1 | Edible part | 24.63 ± 2.19% | - | [106] |
| Capros aper1 | Edible part | 29.35 ± 2.24% | - | [106] |
| Clupea harengus1 | Edible part | 35.38 ± 4.75% | - | [106] |
| Scomber colias1 | Edible part | 4.4 ± 0.2 to 8.4 ± 0.9% | - | [76] |
| Krill | ||||
| Euphausia superba1 | Muscle | 54.0% | - | [102] |
| Mollusks | ||||
| Spisula sachalinensis1 | Mantle, Adductor, locomotor muscle, internals | 40.3 ± 2.02% 35.0 ±1.70% 37.5 ± 1.91% 45.6 ± 2.22% | - | [120] |
| By-products | ||||
| Doriteuthis gahi | Viscera, skin, cartilage | 359.2 to 463.5 (g/kg) | PC, PE | [124] |
| Penaeus Vannamei | Head | 45.7% | PC, PE, PS | [124] |
| Scomber australasicus | roe, head, skin, male gonad | 37.6 ± 2.1% 21.7 ± 1.1% 13.4 ± 1.1% 26.9 ± 1.4% | PC, PE, LPC | [124] |
| Codfish | Roe | 58.92 ± 1.36% | PC, PE, LPC | [124] |
| Squid | Gonad | 69.71 ± 0.55% | PC, PE, LPC | [124] |
| Shrimp | Head | 66.44 ± 0.81% | PC, PE, LPC | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; Rauter, A.P.; Bandarra, N.M. Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Mar. Drugs 2022, 20, 662. https://doi.org/10.3390/md20110662
Ferreira I, Rauter AP, Bandarra NM. Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Marine Drugs. 2022; 20(11):662. https://doi.org/10.3390/md20110662
Chicago/Turabian StyleFerreira, Inês, Amélia P. Rauter, and Narcisa M. Bandarra. 2022. "Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect" Marine Drugs 20, no. 11: 662. https://doi.org/10.3390/md20110662
APA StyleFerreira, I., Rauter, A. P., & Bandarra, N. M. (2022). Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Marine Drugs, 20(11), 662. https://doi.org/10.3390/md20110662








