Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties
Abstract
1. Introduction
2. The Beginning
3. The Biosynthesis of Psammaplyisns
4. The Chemistry of Psammaplysins
5. The Absolute Configuration of Psammaplysins
6. Purification of Psammaplysins
7. Natural Occurrence
8. Structural Variations
9. Semisynthetic Analogs of Psammaplysins
10. Pharmacological Properties
10.1. Compounds with Antimicrobial Properties
10.2. Compounds with Growth Inhibition and Cytotoxic Activities
10.3. Compounds with Antimalarial Activities
10.4. Compounds with Antifouling Activities
10.5. Compounds with Other Reported Activities
11. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Census. 2021. Available online: https://www.marinespecies.org/introduced/wiki/Number_of_marine_species (accessed on 19 July 2022).
- Bouchet, P. The magnitude of marine biodiversity. In The Exploration of Marine Biodiversity: Scientific and Technological Challenges; Duarte, C.M., Ed.; Fundación BBVA: Bilbao, Spain, 2006; pp. 31–62. [Google Scholar]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://marinlit.rsc.org/ (accessed on 19 July 2022).
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-N.; Hong, L.-L.; Gu, B.-B.; Yang-Ting Sun, Y.-T.; Wang, J.; Liu, J.-T.; Lin, H.-W. Natural Products from Sponges. In Symbiotic Microbiomes of Coral Reefs Sponges and Corals; Li, Z., Ed.; Springer Nature B.V.: Dordrecht, The Netherland, 2019; Chapter 15; pp. 329–463. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Thoms, C.; Schupp, P.J. Activated chemical defense in marine sponges—A case study on Aplysinella rhax. J. Chem. Ecol. 2008, 34, 1242–1252. [Google Scholar] [CrossRef]
- Thoms, C.; Wolff, M.; Padmakumar, K.; Ebel, R.; Proksch, P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z. Naturforsch. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Ortlepp, S.; Sjogren, M.; Dahlstrom, M.; Weber, H.; Ebel, R.; Edrada, R.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef]
- Teeyapant, R.; Woerdenbag, H.J.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch. 1993, 48, 939–945. [Google Scholar] [CrossRef]
- Lever, J.; Brkljača, R.; Rix, C.; Urban, S. Application of networking approaches to assess the chemical diversity, biogeography, and pharmaceutical potential of Verongiida natural products. Mar. Drugs 2021, 19, 582. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Hamann, M.T. The marine bromotyrosine derivatives. Alkaloids Chem. Biol. 2005, 61, 59–262. [Google Scholar]
- Lira, N.S.; Montes, R.C.; Tavares, J.F.; da Silva, M.S.; da Cunha, E.V.; de Athayde-Filho, P.F.; Rodrigues, L.C.; da Silva Dias, C.; Barbosa-Filho, J.M. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13C NMR spectral data. Mar. Drugs 2011, 9, 2316–2368. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical diversity and biological activities of marine sponges of the genus Suberea: A systematic review. Mar. Drugs 2019, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Marmann, A.; Lin, W.; Proksch, P. Sponge derived bromotyrosines: Structural diversity through natural combinatorial chemistry. Nat. Prod. Comm. 2015, 10, 219–231. [Google Scholar] [CrossRef]
- Quinoa, E.; Crews, P. Phenolic constituents of psammaplysilla. Tetrahedron Lett. 1987, 28, 3229–3232. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-derived natural lead compound disulfide-linked dimer psammaplin A: Biological activity and structural modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef]
- Kumar, M.S.L.; Ali, K.; Chaturvedi, P.; Meena, S.; Datta, D.; Panda, G. Design, synthesis and biological evaluation of oxime lacking psammaplin inspired chemical libraries as anti-cancer agents. J. Mol. Struct. 2021, 1225, 129173. [Google Scholar] [CrossRef]
- Bao, Y.; Xu, Q.; Wang, L.; Wei, Y.; Hu, B.; Wang, J.; Liu, D.; Zhao, L.; Jing, Y. Studying histone deacetylase inhibition and apoptosis induction of psammaplin A monomers with modified thiol group. ACS Med. Chem. Lett. 2021, 12, 39–47. [Google Scholar] [CrossRef]
- Lebouvier, N.; Jullian, V.; Desvignes, I.; Maurel, S.; Parenty, A.; Dorin-Semblat, D.; Doerig, C.; Sauvain, M.; Laurent, D. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a Vanuatu marine sponge Pseudoceratina sp. Mar. Drugs 2009, 7, 640–653. [Google Scholar] [CrossRef]
- Mayer, A.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Mayer, A.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2020, 18, 5. [Google Scholar]
- Shaala, L.A.; Youssef, D.T.A. Pseudoceratonic Acid and molok’iamine derivatives from the Red Sea Verongiid sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. [Google Scholar] [CrossRef]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A-C, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Borselli, D.; Di Giorgio, C.; De Meo, M.; Bolla, J.-M.; Vidal, N.; Combes, S.; Brunel, J.M. New ianthelliformisamine derivatives as antibiotic enhancers against resistant Gram-negative bacteria. J. Med. Chem. 2014, 57, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated arginine-derived alkaloids from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2011, 74, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M.I.; Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef]
- Mayer, A.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.A.; Erpenbeck, D.; Aalbersberg, W.; et al. New antiplasmodial bromotyrosine derivatives from Suberea ianthelliformis. Chem. Biodivers. 2012, 9, 1436–1451. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Xue, Y.; Oster, L.; Fex, T.; et al. Clavatadine A, a natural product with selective recognition and irreversible inhibition of factor XIa. J. Med. Chem. 2008, 51, 3583–3587. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Hooper, J.N.A.; Quinn, R.J. Clavatadines C-E, guanidine alkaloids from the Australian sponge Suberea clavata. J. Nat. Prod. 2009, 72, 973–975. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Souliman, M.; Khedr, A. Bioactive secondary metabolites from a Red Sea marine Verongid sponge Suberea species. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; El Sayed, K.A. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A.; El Sayed, K.A. Bioactive alkaloids from the Red Sea marine Verongid sponge Pseudoceratina arabica. Tetrahedron 2015, 71, 7837–7841. [Google Scholar] [CrossRef]
- Badr, J.M.; Shaala, L.A.; Abou-Shoer, M.I.; Tawfik, M.A.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Pseudoceratina arabica. J. Nat. Prod. 2008, 71, 1472–1474. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bowden, B.F.; Kapoor, V. Ianthellamide A, a selective kynurenine-3-hydroxylase inhibitor from the Australian marine sponge Ianthella quadrangulata. Bioorg. Med. Chem. Lett. 2012, 22, 3398–3401. [Google Scholar] [CrossRef]
- Tian, L.W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.; Wellington, C.; Hooper, J.N.; Quinn, R.J. Aplysinellamides A-C, bromotyrosine-derived metabolites from an Australian Aplysinella sp. marine sponge. J. Nat. Prod. 2014, 77, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Rotem, M.; Carmely, S.; Kashman, Y. Two new antibiotics from the Red Sea sponge Psammaplysilla purprea. Tetrahedron 1983, 39, 667–676. [Google Scholar] [CrossRef]
- Roll, D.M.; Chang, C.W.J.; Scheuer, P.J.; Gray, G.A.; Shoolery, J.N.; Matsumoto, G.K.; Van Duyne, G.D.; Clardy, J. Structure of the psammaplysins. J. Am. Chem. Soc. 1985, 107, 2916–2920. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic psammaplysin analogues from Red Sea sponge Aplysinella species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef]
- Mándi, A.; Mudianta, I.W.; Kurtán, T.; Garson, M.J. Absolute configuration and conformational study of psammaplysins A and B from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2015, 78, 2051–2056. [Google Scholar] [CrossRef]
- Copp, B.R.; Ireland, C.M.; Barrows, R.L. Psammaplysin C: A new cytotoxic dibromotyrosine derived metabolite from the marine sponge Druinella (= Psammplysilla) purpurea. J. Nat.Prod. 1992, 55, 822–823. [Google Scholar] [CrossRef]
- Ichiba, T.; Scheuer, P.J.; Kelly-Borges, M. Three bromotyrosine derivatives, one terminating in an unprecedented diketocyclopentenylidene enamine. J. Org. Chem. 1993, 58, 4149–4150. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Ceratinamides A and B: New antifouling dibromotyrosine derivatives from the marine sponge Pseudoceratina purpurea. Tetrahedron 1996, 52, 8181–8186. [Google Scholar] [CrossRef]
- Liu, S.; Fu, X.; Schmitz, F.J.; Kelly-Borges, M. Psammaplysin F, a new bromotyrosine derivative from a sponge, Aplysinella sp. J. Nat. Prod. 1997, 60, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Andrews, K.T.; Birrell, G.W.; Tran, T.L.; Camp, D.; Davis, R.A.; Quinn, R.J. Psammaplysin H, a new antimalarial bromotyrosine alkaloid from a marine sponge of the genus Pseudoceratina. Bioorg. Med. Chem. Lett. 2011, 21, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Peter, J.; Schupp, P.J.; Schrör, J.; Anna Engemann, A.; Rohde, S.; Dovi Kelman, D.; Voogd, N.; Carroll, A.; Motti, C.A. Twilight zone sponges from Guam yield theonellin isocyanate and psammaplysins I and J. J. Nat. Prod. 2012, 75, 502–506. [Google Scholar] [CrossRef]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.; Lee, H.S.; Kang, J.S.; Yun, J.; Sim, C.J.; Shin, H.J.; Lee, J.S. Cytotoxic psammaplysin analogues from a Suberea sp. marine sponge and the role of the spirooxepinisoxazoline in their activity. J. Nat. Prod. 2013, 76, 1731–1736. [Google Scholar] [CrossRef]
- Kurimoto, S.I.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Omura, S.; Kobayashi, J.; Kubota, T. Ceratinadins E and F, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. [Google Scholar] [CrossRef]
- Jiao, W.H.; Li, J.; Zhang, M.M.; Cui, J.; Gui, Y.H.; Zhang, Y.; Li, J.Y.; Liu, K.C.; Lin, H.W. Frondoplysins A and B, unprecedented terpene-alkaloid bioconjugates from Dysidea frondosa. Org. Lett. 2019, 21, 6190–6193. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Asfour, H.Z.; Shaala, A.L. Psammaceratin A: A cytotoxic psammaplysin dimer featuring an unprecedented (2Z,3Z)-2,3-bis(aminomethylene)succinamide backbone from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2021, 19, 433. [Google Scholar] [CrossRef]
- Kumar, R.; Bidgood, C.L.; Levrier, C.; Gunter, J.H.; Nelson, C.C.; Sadowski, M.C.; Davis, R.A. Synthesis of a unique psammaplysin F library and functional evaluation in prostate cancer cells by multiparametric quantitative single cell imaging. J. Nat. Prod. 2022, 83, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, G.M.; Eckman, L.L.; Ray, S.; Hughes, R.O.; Pfefferkorn, J.A.; Barluenga, S.; Nicolaou, K.C.; Bewley, C.A. Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg. Med. Chem. 2002, 12, 2487–2490. [Google Scholar] [CrossRef]
- Ramsey, D.M.; Amirul, I.M.; Turnbull, L.; Davis, R.A.; Whitchurch, C.B.; McAlpine, S.R. Psammaplysin F: A unique inhibitor of bacterial chromosomal partitioning. Bioorg. Med. Chem. Lett. 2013, 23, 4862–4866. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Christen, K.E.; Davis, R.A.; Kennedy, D. Psammaplysin F increases the efficacy of bortezomib and sorafenib through regulation of stress granule formation. Int. J. Biochem. Cell. Biol. 2019, 112, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Malamas, M.S.; Sredy, J.; Moxham, C.; Katz, A.; Xu, W.; McDevitt, R.; Adebayo, F.O.; Sawicki, D.R.; Seestaller, L.; Sullivan, D.; et al. Novel benzofuran and benzothiophene biphenyls as inhibitors of protein tyrosine phosphatase 1B with antihyperglycemic properties. J. Med. Chem. 2000, 43, 1293–1310. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.R.; Kafle, B.; Hwang, J.-S.; Khadka, D.; Lee, S.-M.; Kang, J.-S.; Ham, S.W.; Han, I.-O.; Park, H.; Cho, H. Thiazolidinedione derivatives as PTP1B inhibitors with antihyperglycemic and antiobesity effects. Bioorg. Med. Chem. Lett. 2009, 19, 6161–6165. [Google Scholar] [CrossRef]


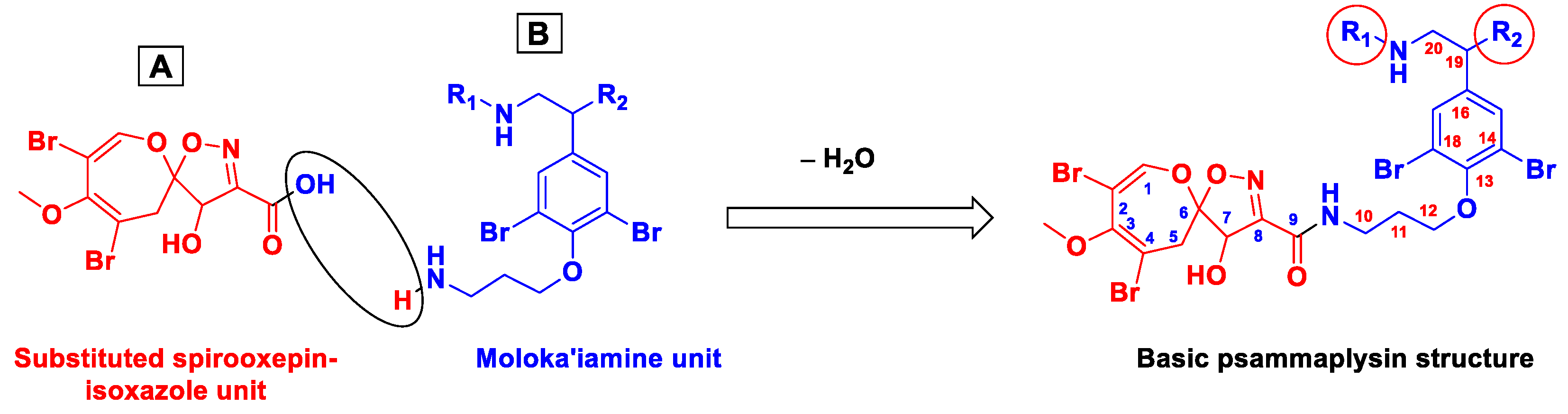
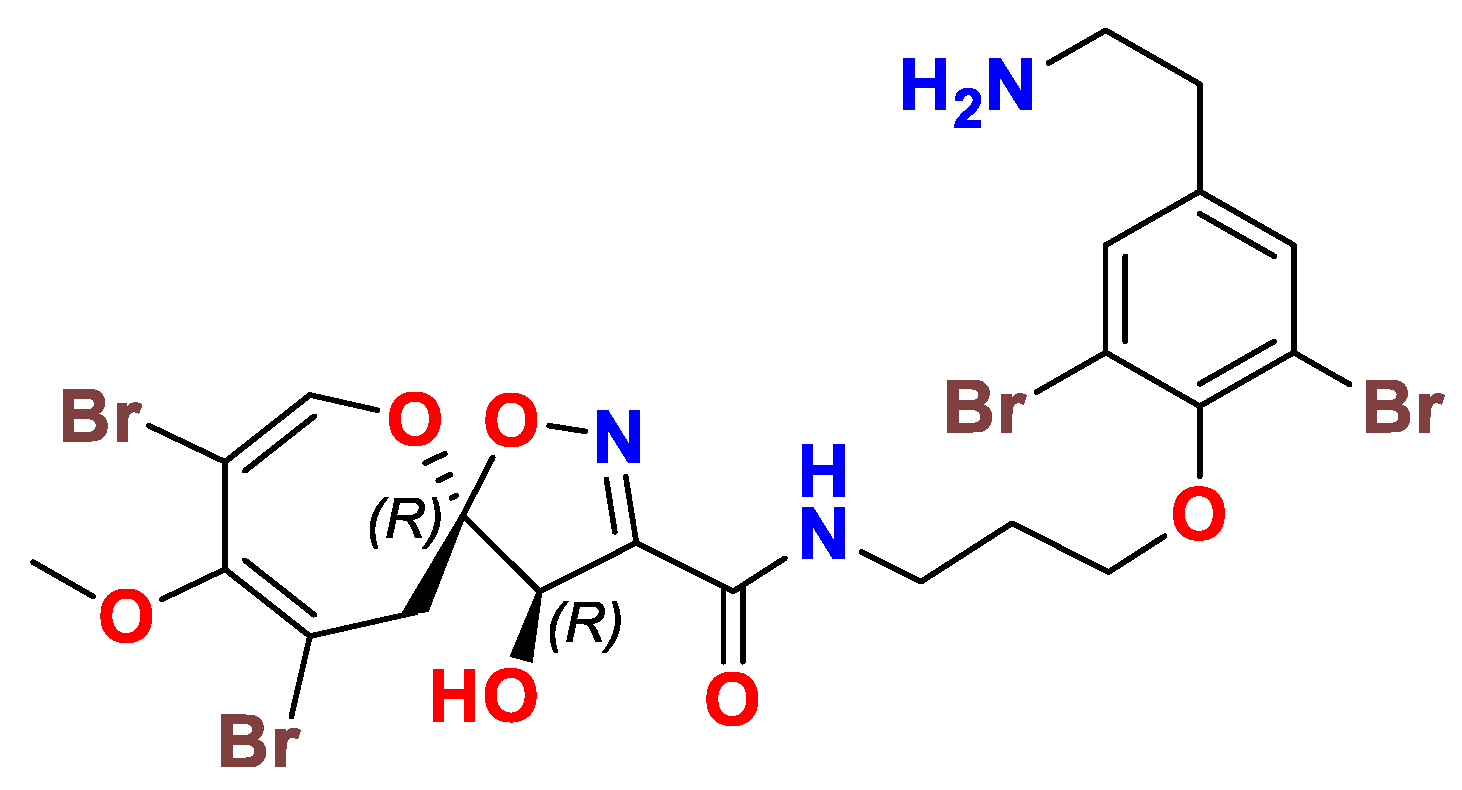
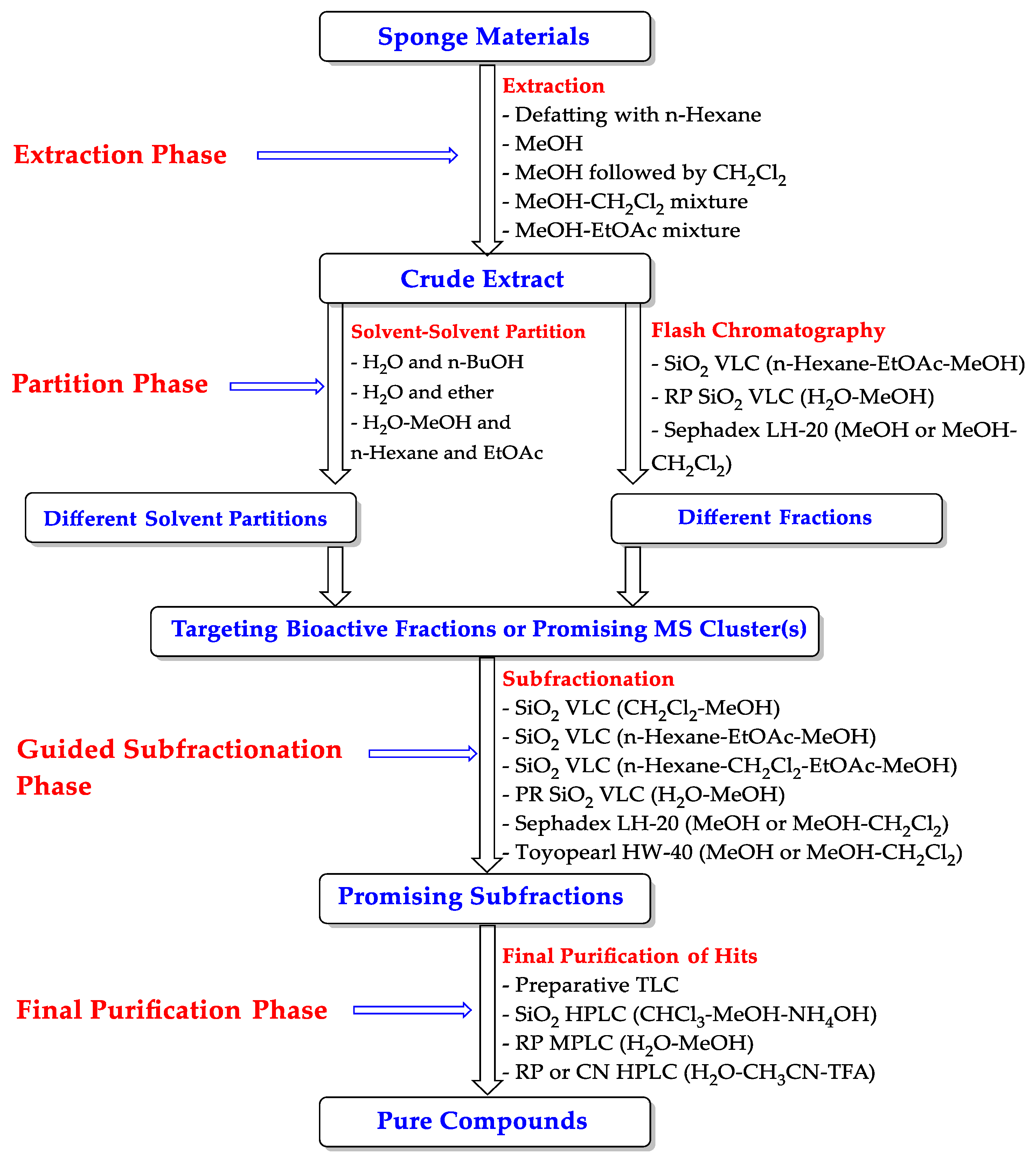
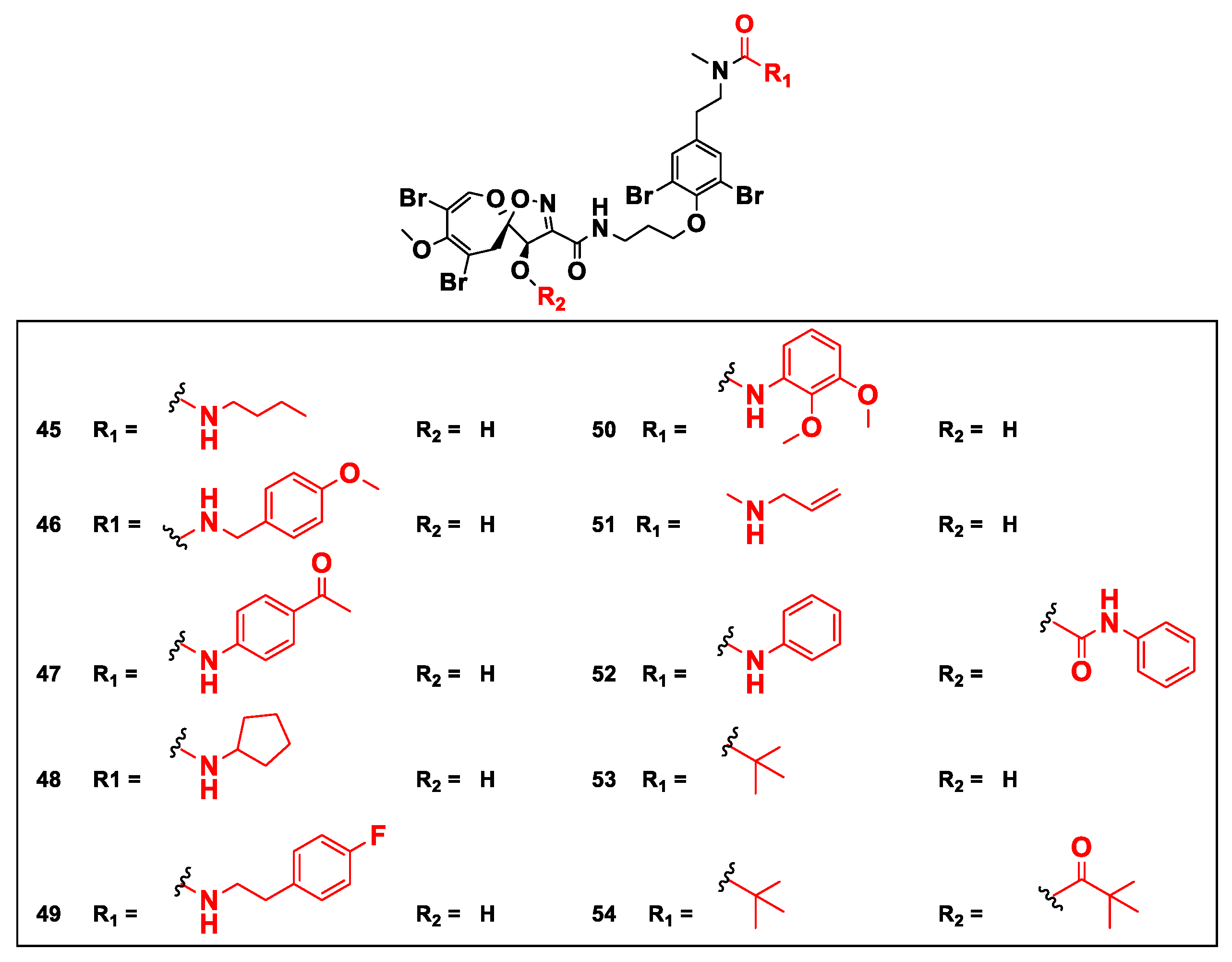
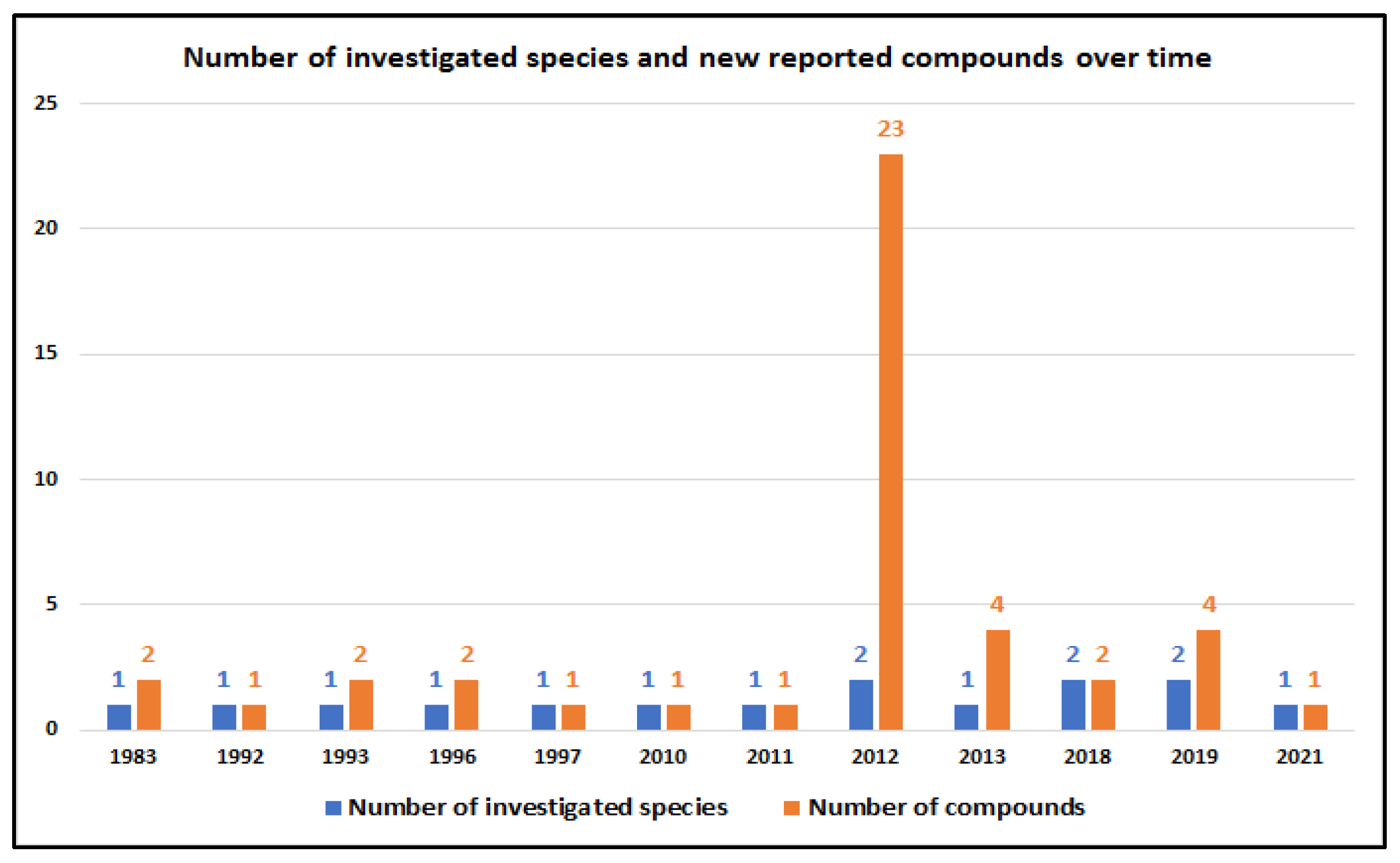
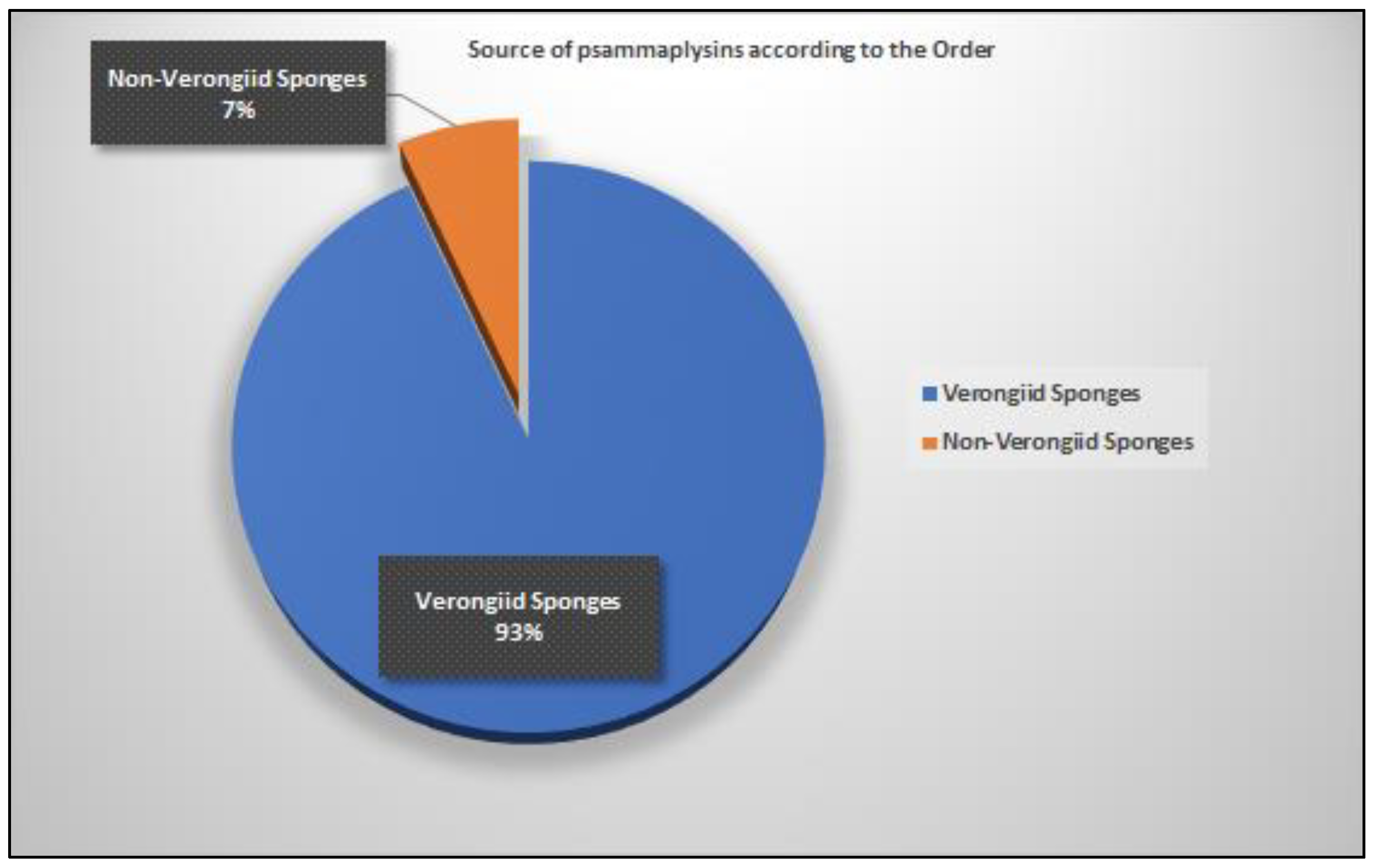
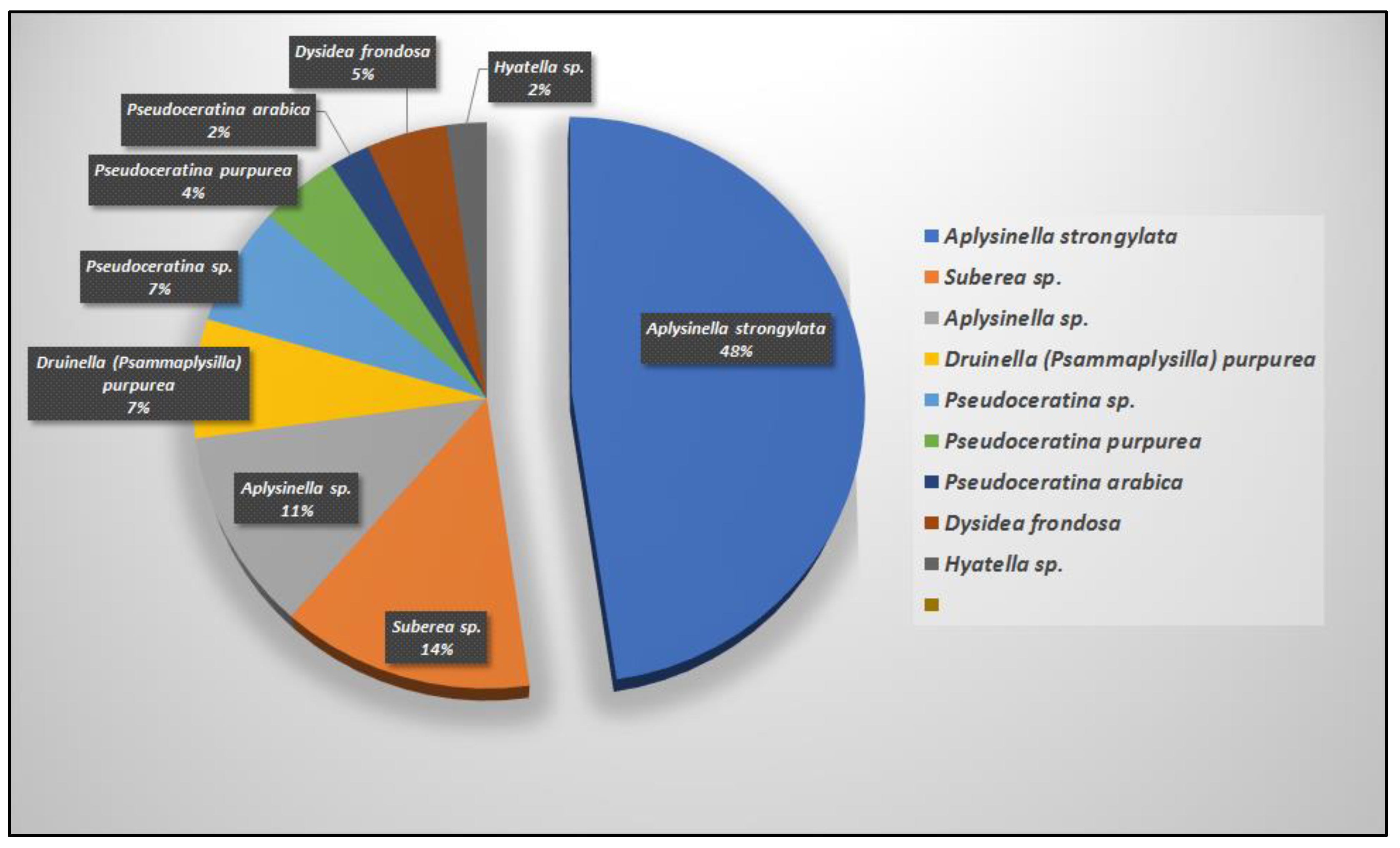
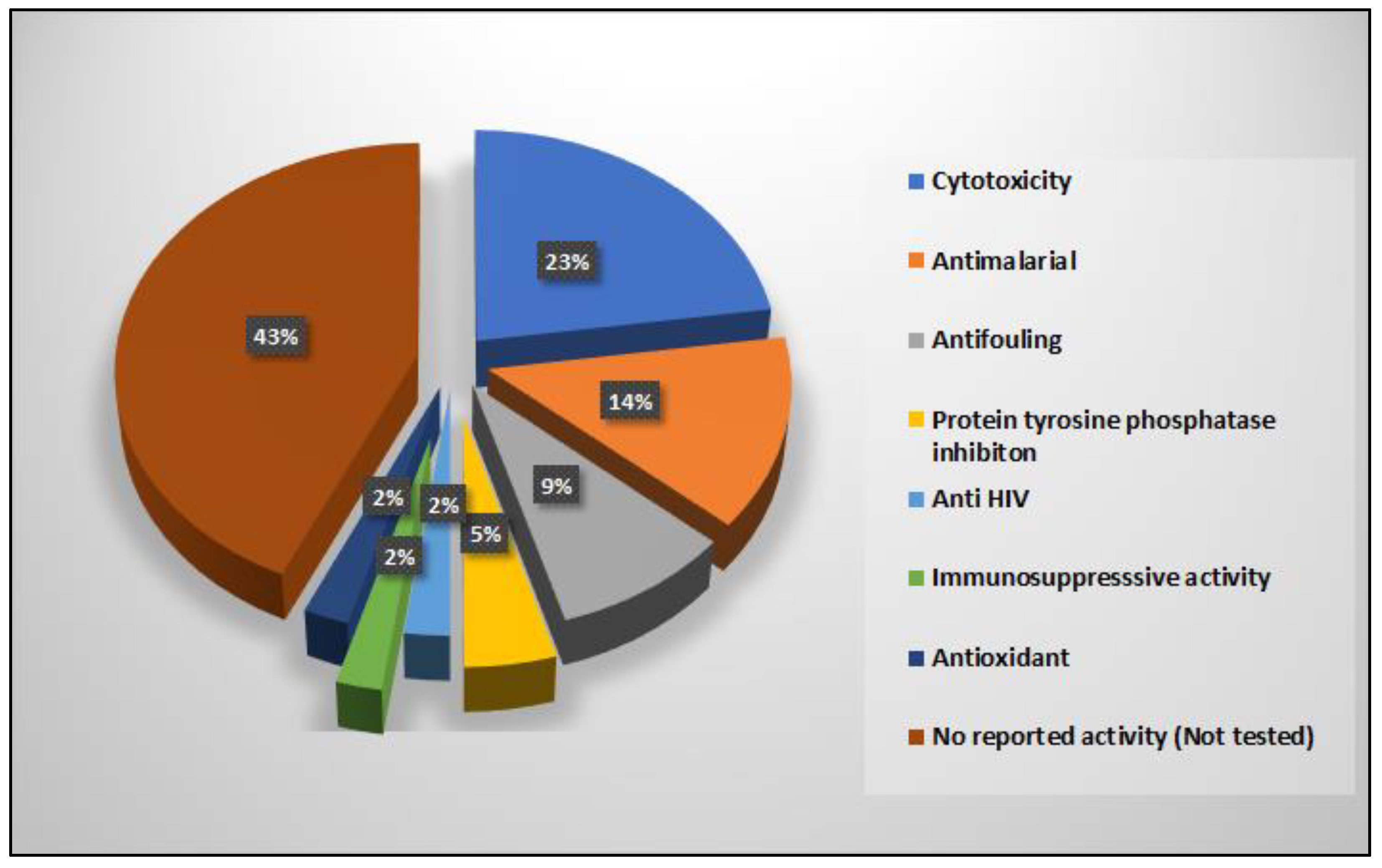
| Compound | Sponge Name | Reference |
|---|---|---|
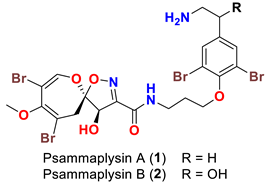 | Psammaplysilla purpurea | [39] |
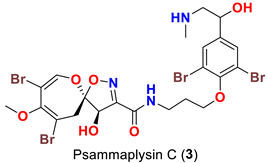 | Psammaplsilla purpurea | [43] |
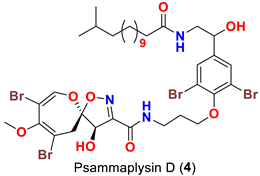 | Aplysinella sp. | [44] |
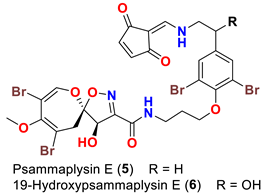 | Aplysinella sp. Aplysinella strongyalata | [44,50] |
 | Pseudoceratina purpurea Suberea sp. | [45,51] |
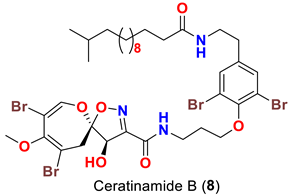 | Pseudoceratina purpurea | [45] |
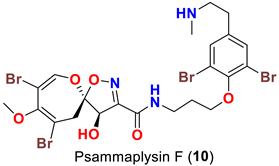 | Aplysinella sp. | [46] |
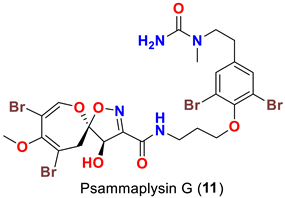 | Hyattella sp. | [47] |
 | Pseudoceratina sp. | [48] |
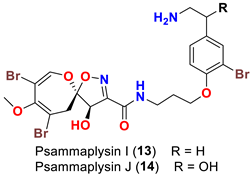 | Suberea sp. | [49] |
 | Aplysinella strongyalata | [50] |
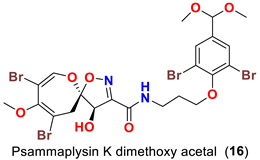 | Aplysinella strongyalata | [50] |
 | Aplysinella strongyalata | [50] |
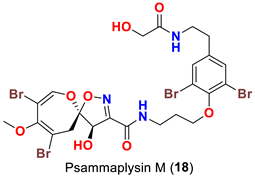 | Aplysinella strongyalata | [50] |
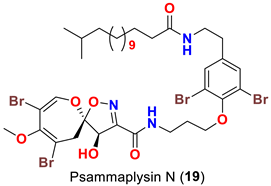 | Aplysinella strongyalata | [50] |
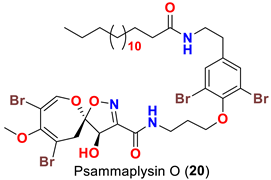 | Aplysinella strongyalata | [50] |
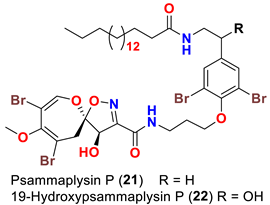 | Aplysinella strongyalata | [50] |
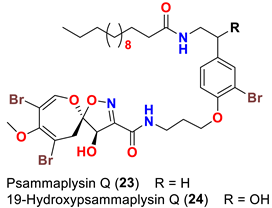 | Aplysinella strongyalata | [50] |
 | Aplysinella strongyalata | [50] |
 | Aplysinella strongyalata | [50] |
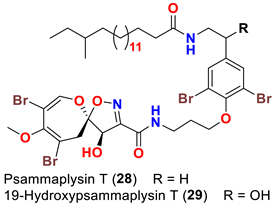 | Aplysinella strongyalata | [50] |
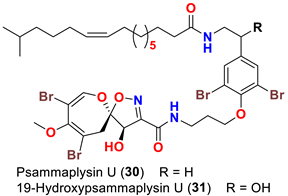 | Aplysinella strongyalata | [50] |
 | Aplysinella strongyalata | [50] |
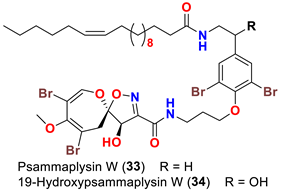 | Aplysinella strongyalata | [50] |
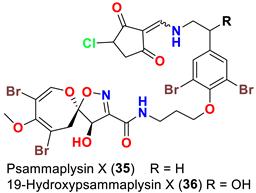 | Suberea sp. | [51] |
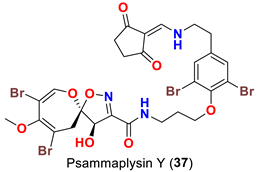 | Suberea sp. | [51] |
 | Aplysinella sp. | [41] |
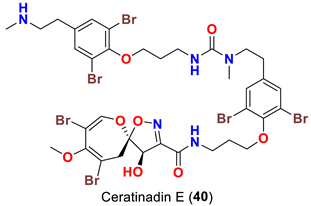 | Pseudoceratina sp. | [52] |
 | Pseudoceratina sp. | [52] |
 | Dysidea frondosa | [53] |
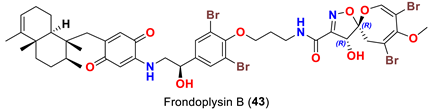 | Dysidea frondosa | [53] |
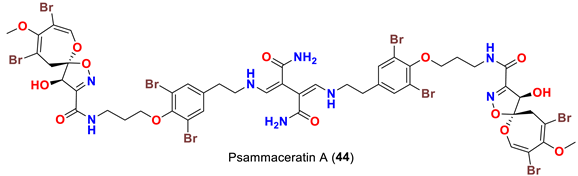 | Pseudoceratina arabica | [54] |
| Compounds | Antimicrobial Activity | Reference |
|---|---|---|
| Psammaplysin A (1) | In vitro activity against gram positive bacteria and E. coli Antibacterial activity against Flavobacterium marinotypicum Inhibits mycothiol-S-conjugate amidase of Mycobacterium tuberculosis with IC50 of 20 µM | [39,45,56] |
| Psammaplysin B (2) | In vitro activity against Gram-positive bacteria and E. coli Inhibits mycothiol-S-conjugate amidase of Mycobacterium tuberculosis with IC50 of 26 µM | [39,56] |
| Psammaplysin F (10) | Inhibits the growth of S. aureus NCTC 6571, S. aureus 1H, E. facecalis NCTC-775, B. cereus NCTC-7464, MRSA MW2 and MRSA USA-30050 with MIC values of 42.8, 42.8, 42.8, 42.8, 40.0, and 80.0 µM | [57] |
| Psammaplysin (12) | Inhibits the growth of S. aureus NCTC 6571, S. aureus 1H, E. facecalis NCTC-775, B. cereus NCTC-7464, MRSA MW2 and MRSA USA-30050 with MIC values of 20.4, 45.0, 81.5, 81.5, 40.0, and 80.0 µM | [57] |
| Compounds | Cytotoxicity | Reference |
|---|---|---|
| Psammaplysin A (1) | - Inhibits HCT-116 with an IC50 of 6 µg/mL - Inhibits HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3 and NCI-H23 with GI50 values 3.9, 6.9, 5.1, 4.3, 3.8, and 12.4 µM, respectively - Inhibits MDA-MB-231 and Hela cells with IC50 values of 2.9 and 8.5 µM respectively - Inhibits HCT116, MDA-MB-231, and Hela cells with IC50 values of 5.1, 3.90, and 8.50 respectively | [41,43,51,54] |
| Psammaplysin B (2) | - Inhibits HCT-116 with IC50 value of 6 µg/mL - Inhibited HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3, and NCI-H23 with GI50 values of 4.0, 2.7, 1.6, 0.53, 2.5, and 3.7 µM, respectively | [43,51] |
| Psammaplysin C (3) | Inhibits HCT-116 with IC50 of 3 µg/mL | [43] |
| Psammaplysin D (4) | - Weak growth inhibition of HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3, and NCI-H23 with GI50 values 24, 25, 27, 21, 26, and 27 µM, respectively | [51] |
| Psammaplysin E (5) | - Inhibited KB and LoVo cells at 5 µg/mL - Inhibited P388 cell with IC50 of 2.1 µg/mL - Inhibited HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3, and NCI-H23 cells with GI50 values 3.8, 1.4, 2.3, 0.51, 2.3, and 3.6 µM, respectively - Antimigratory activity against MDA-MB-231 and Hela cells with IC50 values of 0.29 and 2.1 µM, respectively | [41,44,45,51] |
| Psammaplysin F (10) | Inhibits HEK293 mammalian cell line with IC50 value of 11 μM | [47] |
| Psammaplysin X (35) | Inhibits HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3, and NCI-H23 cells with GI50 values of 3.3, 2.3, 3.3, 1.2, 3.5, and 6.4 µM, respectively | [51] |
| 19-Hydroxypsammaplysin X (36) | Inhibits HCT-15, PC-3, ACHN, MDA-MB-231, NUGC-3, and NCI-H23 cells with GI50 values of 3.5, 2.1, 2.5, 0.8, 4.0, and 3.5 µM, respectively | [51] |
| Psammaplysin Z (38) | Inhibits MDA-MB-231 and Hela cell lines with IC50 values 19.4 and 22.2 µM, respectively | [41] |
| 19-Hydroxypsammaplysin Z (39) | Inhibits MDA-MB-231 and Hela cell lines with IC50 values of 13.2 and 17.6 µM, respectively | [41] |
| Psammaceratin A (44) | Inhibits MDA-MB-231, Hela, and HCT116 cells with IC50 values of 5.25, 9.40, and 3.10 µM, respectively | [54] |
| Compound | Activity | Reference |
|---|---|---|
| 19-Hydroxypsammaplysin E (6) | Inhibits 3D7 chloroquine-sensitive strain of P. falciparum with IC50 of 6.4 μM | [50] |
| Psammaplysin F (10) | - Inhibits 3D7 and Dd2 strains of P. falciparum with IC50 values of 0.87 and 1.4 μM - Inhibits the drug-resistant (K1) and drug-sensitive (FCR3) strains of P. falciparum with IC50 values of 3.77 and 2.45 µg/mL and with selectivity indices of 3.4 and 5.2, respectively | [47,52] |
| Psammaplysin G (11) | Inhibits 98% of Dd2 cell strain of P. falciparum at 40 μM | [47] |
| Psammaplysin H (12) | Inhibits the 3D7 strain of P. falciparum with an IC50 value of 0.41 µM and selective towards the 3D7 strain of P. falciparum with a selectivity index (SI) of >97% | [48] |
| Ceratinadin E (40) | Inhibits K1 and FCR3 strains of P. falciparum, with IC50 values of 1.03 and 0.77 μg/mL, respectively and with selectivity indices (SI) of 15.5 and 20.8, respectively | [52] |
| Ceratinadin F (41) | Inhibits K1 strain of P. falciparum with an IC50 >12.5 μg/mL and selectivity index (SI) value of >4 | [52] |
| Compound | Activity | Reference |
|---|---|---|
| Psammaplysin A (1) | Inhibits metamorphosis and settlement of B. amphitrite with an ED50 0.27 µg/mL | [45] |
| Psammaplysin E (5) | Inhibits metamorphosis and settlement of B. amphitrite with an ED50 4.8 µg/mL | [45] |
| Ceratinamide A (7) | Inhibits metamorphosis and settlement of B. amphitrite with an ED50 0.10 µg/mL Induces metamorphosis on the ascidian Halocynthia roretzi with ED100 of 1.2 µg/mL | [45] |
| Ceratinamide B (8) | Inhibits metamorphosis and settlement of B. amphitrite with an ED50 2.4 µg/mL | [45] |
| Compound | Activity | Reference |
|---|---|---|
| Psammaplysin D (4) | Inhibits 51% of the Haitian RF strain of HIV-I at 0.1 µg/mL | [44] |
| Psammaplysin E (5) | Moderate immunosuppressive activity with a potency of 40, ICW = 8.323-01 for mixed lymphocyte reaction assay | [44] |
| Frondoplysin A (42) | - Inhibits protein tyrosine phosphatase 1B with an IC50 value of 0.39 μM compared to oleanolic acid as a positive control (IC50 3.7 μM) and thiazolidinediones (IC50 5.0 μM) similar to benzofuran and benzothiophene biphenyls (IC50 0.36 μM) - Antioxidant activity in transgenic zebrafish without any cytotoxicity at 64 μM | [53] |
| Frondoplysin B (43) | Inhibits protein tyrosine phosphatase 1B with an IC50 value of 0.65 μM compared to oleanolic acid as a positive control (IC50 3.7 μM) | [53] |
| Psammaplysin F (10) and its urea semisynthetic analogs 45, 51, 53 and 54 | Reduce the mitochondrial membrane potential (MMP) | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, D.T.A.; Shaala, L.A. Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties. Mar. Drugs 2022, 20, 663. https://doi.org/10.3390/md20110663
Youssef DTA, Shaala LA. Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties. Marine Drugs. 2022; 20(11):663. https://doi.org/10.3390/md20110663
Chicago/Turabian StyleYoussef, Diaa T. A., and Lamiaa A. Shaala. 2022. "Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties" Marine Drugs 20, no. 11: 663. https://doi.org/10.3390/md20110663
APA StyleYoussef, D. T. A., & Shaala, L. A. (2022). Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties. Marine Drugs, 20(11), 663. https://doi.org/10.3390/md20110663






