Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice
Abstract
:1. Introduction
2. Results
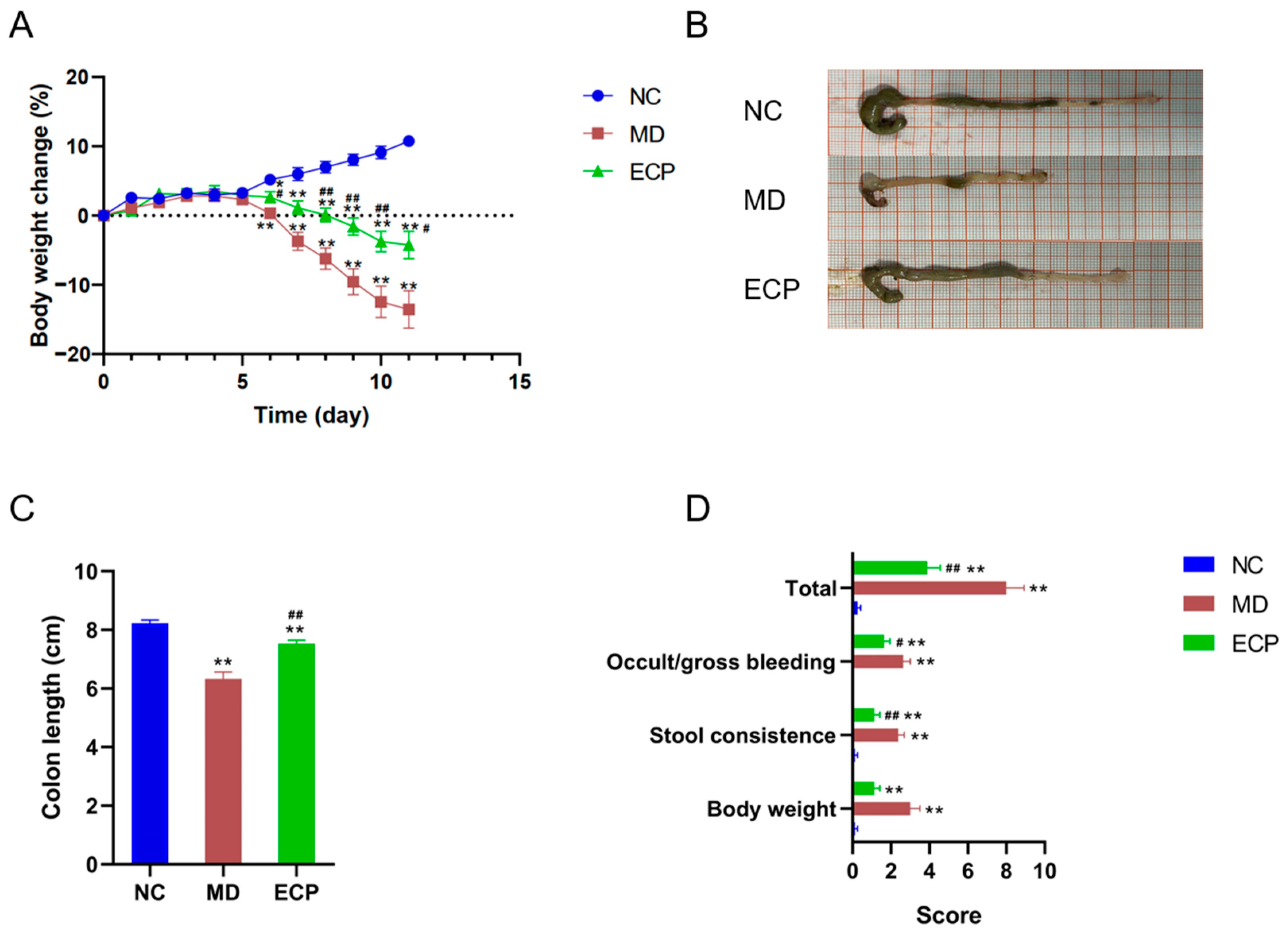
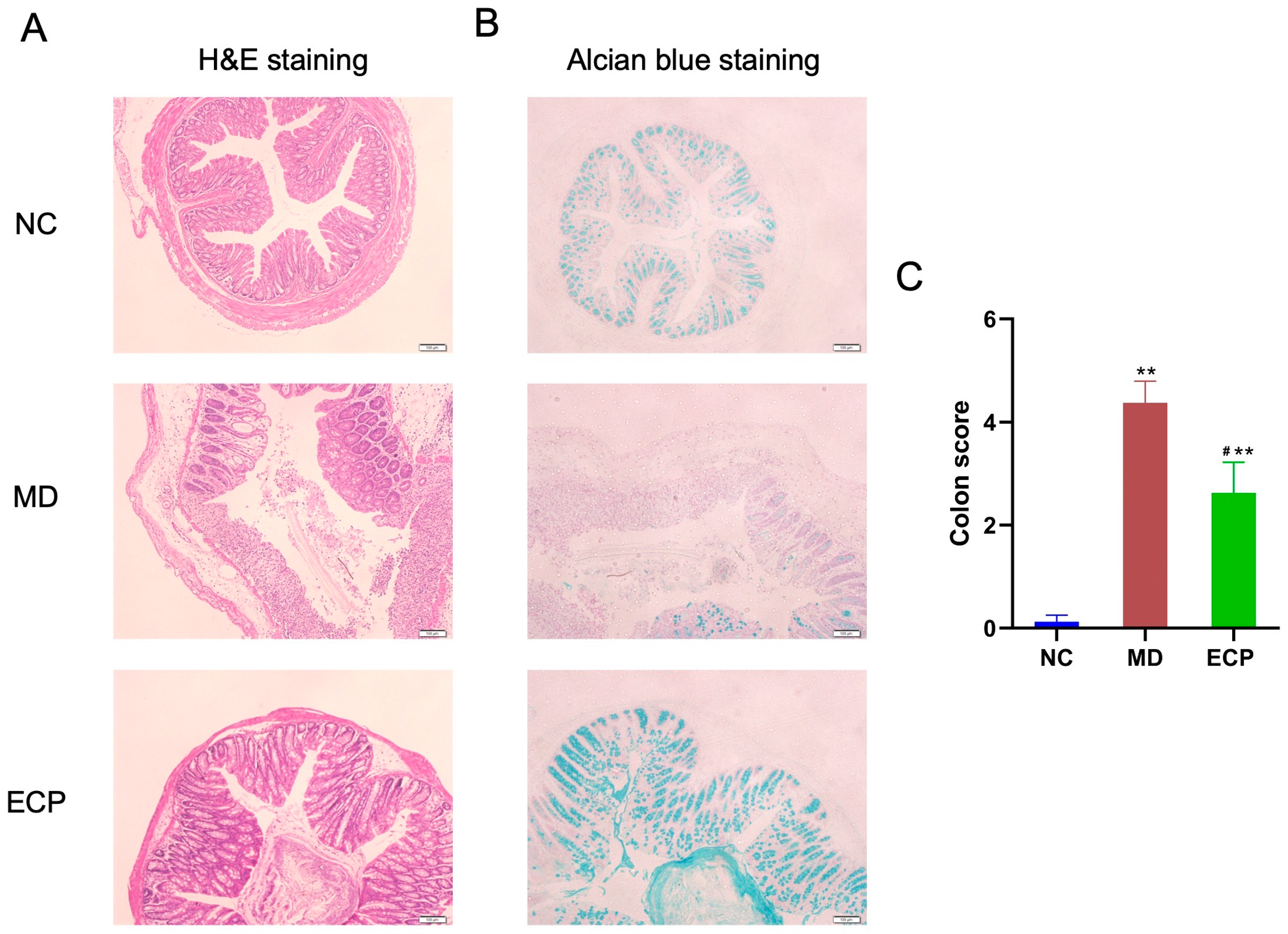
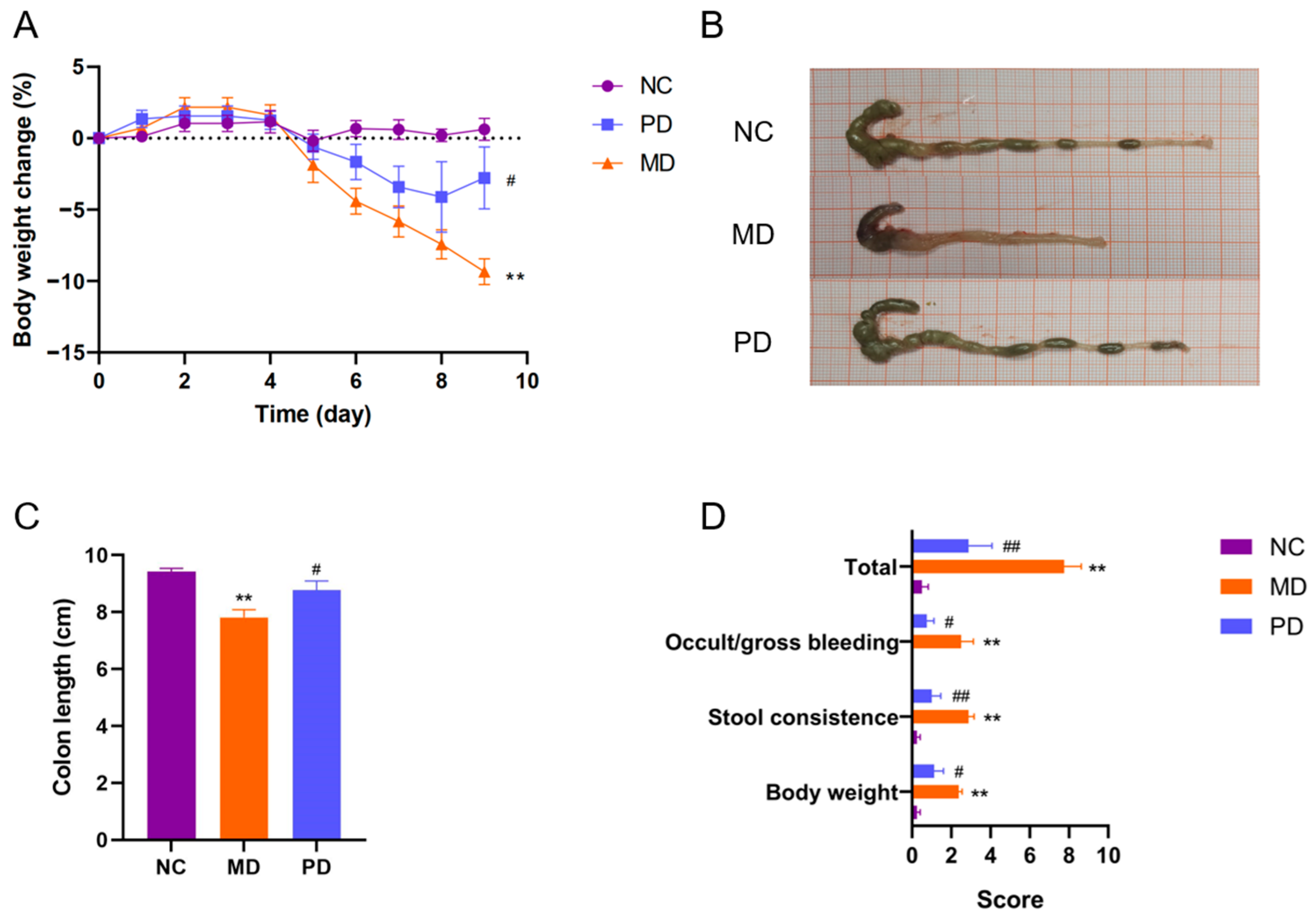
2.1. Dietary ECP Improved Ulcerative Colitis and Ameliorated Mucosal Damage in DSS-Fed Mice
2.2. Dietary ECP Changed the Overall Structure of the Gut Microbiota in DSS-Fed mice
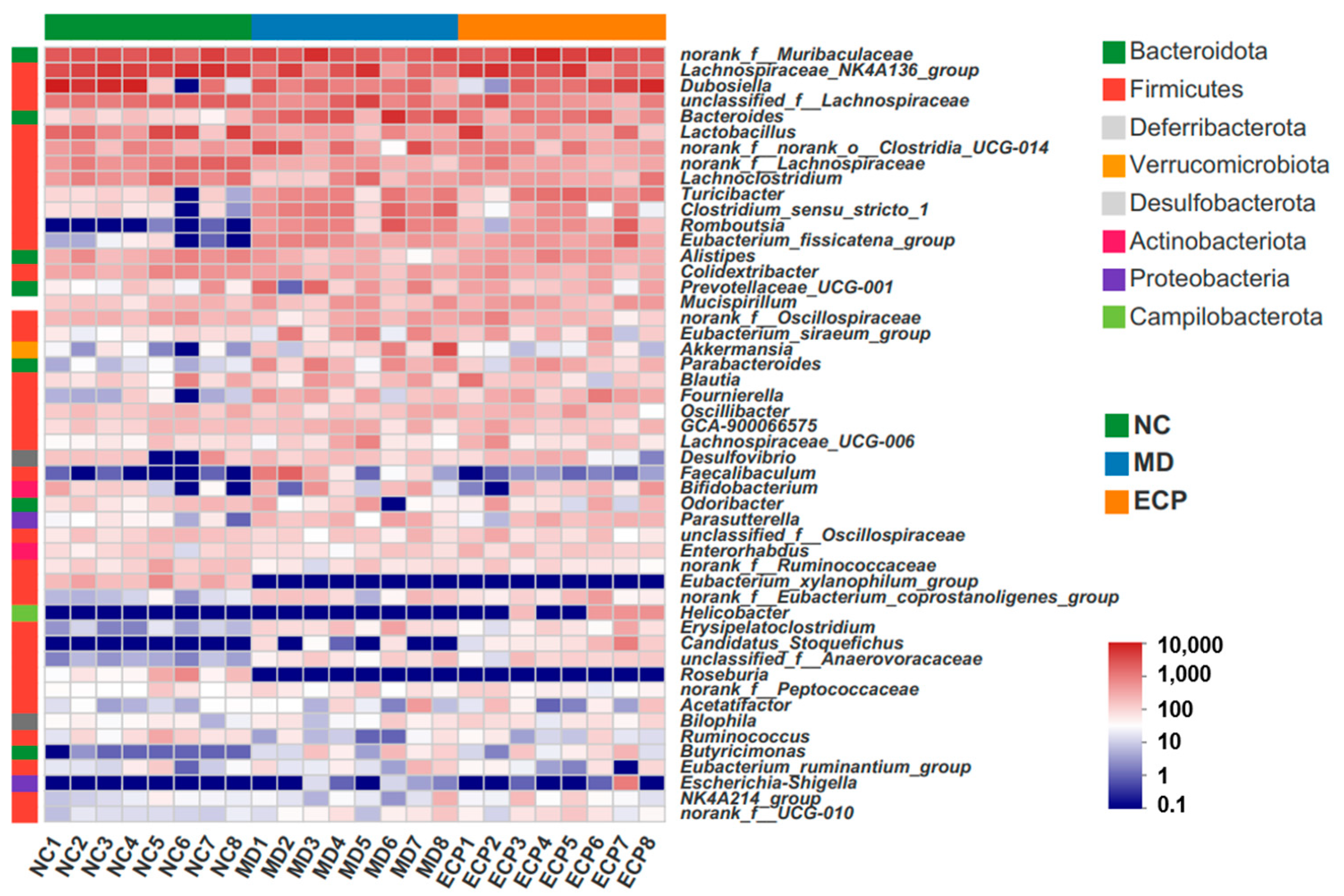
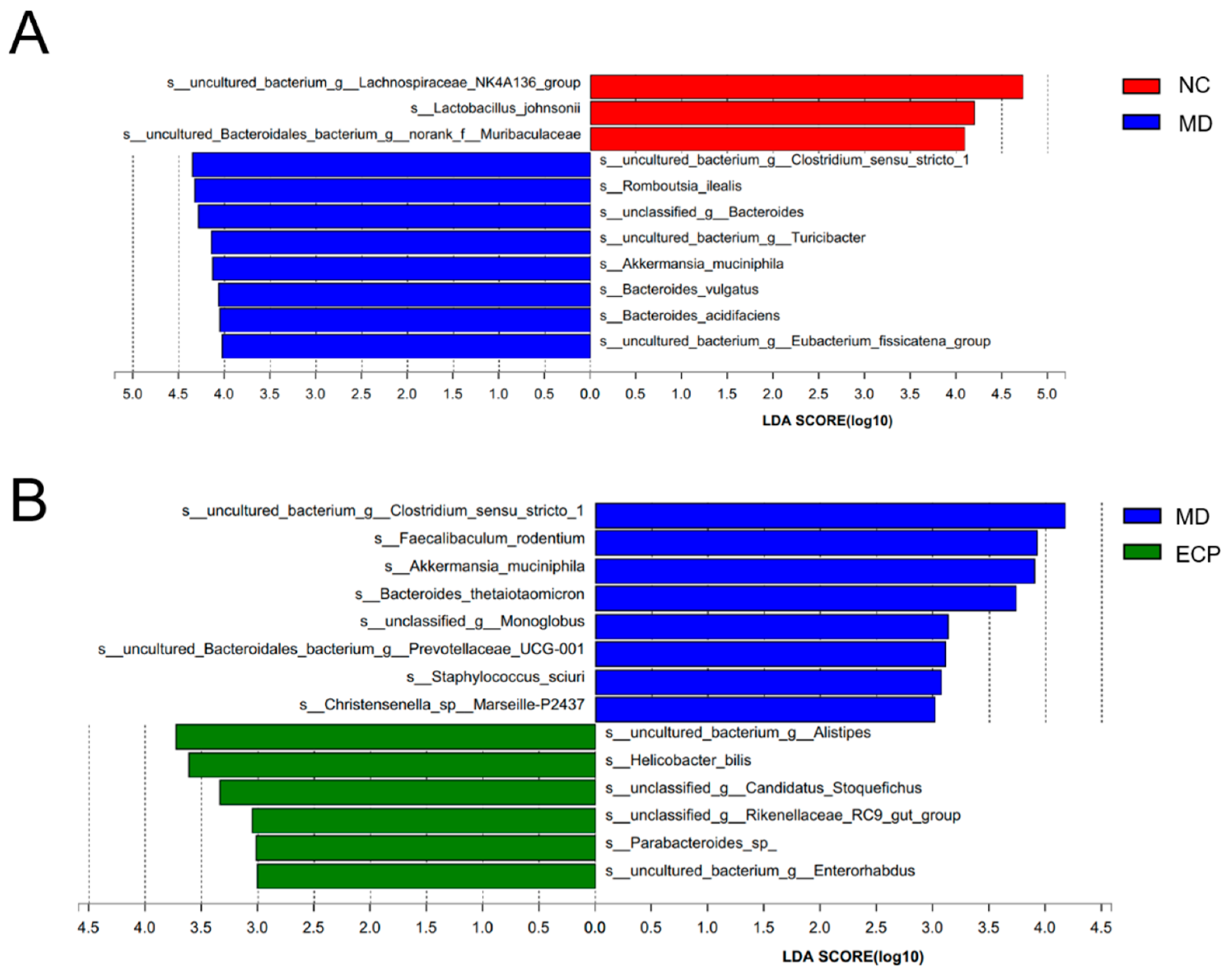
2.3. Dietary ECP Modulated the Composition of the Gut Microbiota and Increased the Abundance of Parabacteroides spp. in DSS-Fed Mice
2.4. ECP Promoted the Growth of P. distasonis F1-28 In Vitro and Increased the Production of Short-Chain Fatty Acids
2.5. P. distasonis F1-28 Alleviated Ulcerative Colitis and Attenuated Mucosal Damage in DSS-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. In Vitro Fermentation of P. distasonis F1-28
4.4. High-Throughput Sequencing and Bioinformatic Analyses
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, K.L.; Yu, B.; Chen, J.; Zhong, S. A comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Y.; Zhou, C.; Zhao, Q.; Zhong, H.; Zhu, X.; Fu, T.; Pan, L.; Shang, Q.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata attenuates obesity and increases the intestinal abundance of butyrate-producing bacterium, Eubacterium xylanophilum, in mice fed a high-fat diet. Polymers 2021, 13, 3286. [Google Scholar] [CrossRef]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Pan, L.; Fu, T.; Cheng, H.; Mi, J.; Shang, Q.; Yu, G. Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans. Carbohydr. Polym. 2022, 278, 118921. [Google Scholar] [CrossRef]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Súkeníková, L.; Desramaut, J.; Boudebbouze, S.; Salomé-Desnoulez, S.; Hrdý, J.; Waligora-Dupriet, A.-J.; Maguin, E.; et al. In vitro characterization of gut microbiota-derived commensal strains: Selection of Parabacteroides distasonis strains alleviating TNBS-induced colitis in mice. Cells 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Gerkins, C.; Oliero, M.; Hajjar, R.; Rendos, H.V.; Fragoso, G.; Calvé, A.; Diop, K.; Routy, B.; Santos, M. The modulation of intestinal inflammation by parabacteroides goldsteinii in dextran sodium sulfate induced colitis in mice. Gut 2022, 71, A60. [Google Scholar]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiratterra, E.; Franco, P.; Porru, E.; Katsanos, K.H.; Christodoulou, D.K.; Roda, G. Role of bile acids in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 266–272. [Google Scholar] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [Green Version]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef]
- Yang, D.; Jacobson, A.; Meerschaert, K.A.; Sifakis, J.J.; Wu, M.; Chen, X.; Yang, T.; Zhou, Y.; Anekal, P.V.; Rucker, R.A.; et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 2022, 185, 4190–4205.e5. [Google Scholar] [CrossRef] [PubMed]
- Tawfiq, R.A.; Nassar, N.N.; Hammam, O.A.; Allam, R.M.; Elmazar, M.M.; Abdallah, D.M.; Attia, Y.M. Obeticholic acid orchestrates the crosstalk between ileal autophagy and tight junctions in non-alcoholic steatohepatitis: Role of TLR4/TGF-β1 axis. Chem. Biol. Interact. 2022, 361, 109953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, D.; Liang, Y.; Zhang, Z.; Guo, J.; Chen, Y.; Yan, Y.; Liu, H.; Lei, L.; Wang, Z.; et al. Licorice-Yuanhua Herbal Pair Induces Ileum Injuries Through Weakening Epithelial and Mucous Barrier Functions: Saponins, Flavonoids, and Di-Terpenes All Involved. Front. Pharmacol. 2020, 11, 869. [Google Scholar] [CrossRef]
- Fu, T.; Pan, L.; Shang, Q.; Yu, G. Fermentation of alginate and its derivatives by different enterotypes of human gut microbiota: Towards personalized nutrition using enterotype-specific dietary fibers. Int. J. Biol. Macromol. 2021, 183, 1649–1659. [Google Scholar] [CrossRef]
- Fu, T.; Zhou, L.; Fu, Z.; Zhang, B.; Li, Q.; Pan, L.; Zhou, C.; Zhao, Q.; Shang, Q.; Yu, G. Enterotype-specific effect of human gut microbiota on the fermentation of marine algae oligosaccharides: A preliminary proof-of-concept in vitro study. Polymers 2022, 14, 770. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Fu, T.; Wang, Y.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Mar. Drugs 2022, 20, 764. https://doi.org/10.3390/md20120764
Ma M, Fu T, Wang Y, Zhang A, Gao P, Shang Q, Yu G. Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Marine Drugs. 2022; 20(12):764. https://doi.org/10.3390/md20120764
Chicago/Turabian StyleMa, Mingfeng, Tianyu Fu, Yamin Wang, Aijun Zhang, Puyue Gao, Qingsen Shang, and Guangli Yu. 2022. "Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice" Marine Drugs 20, no. 12: 764. https://doi.org/10.3390/md20120764
APA StyleMa, M., Fu, T., Wang, Y., Zhang, A., Gao, P., Shang, Q., & Yu, G. (2022). Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Marine Drugs, 20(12), 764. https://doi.org/10.3390/md20120764






