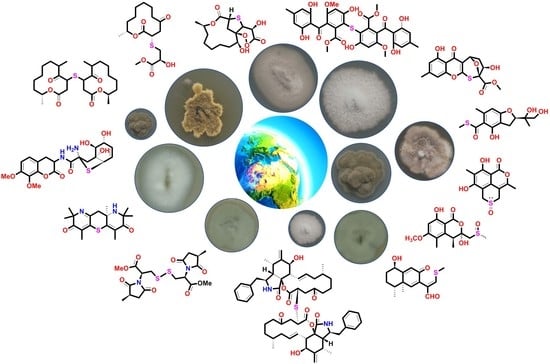
Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities
Abstract
1. Introduction
2. Isolation, Structural Features, and Bioactivities of S-Containing NPs
2.1. Polyketides
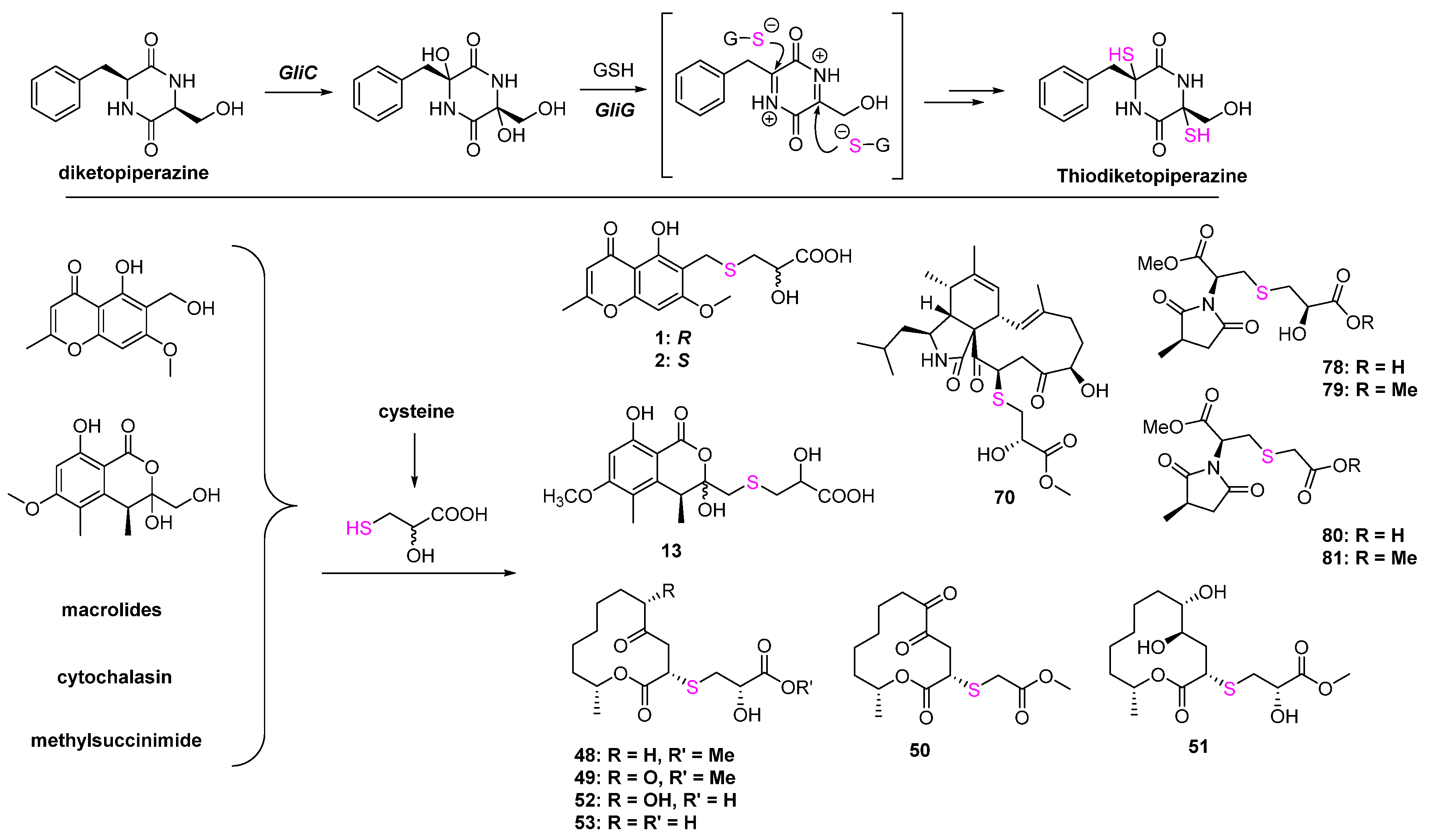
2.1.1. Thioether-Containing Polyketides

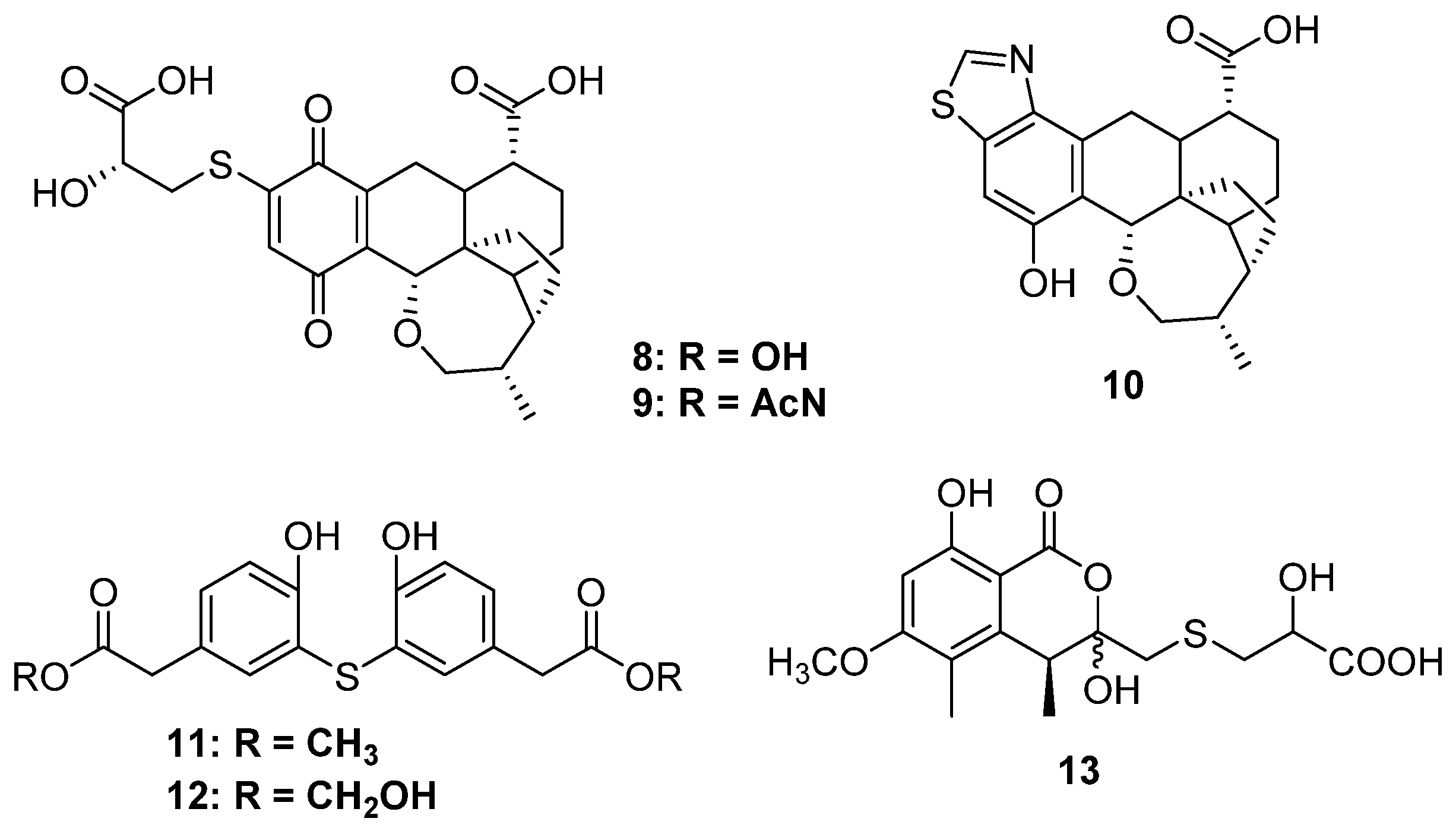
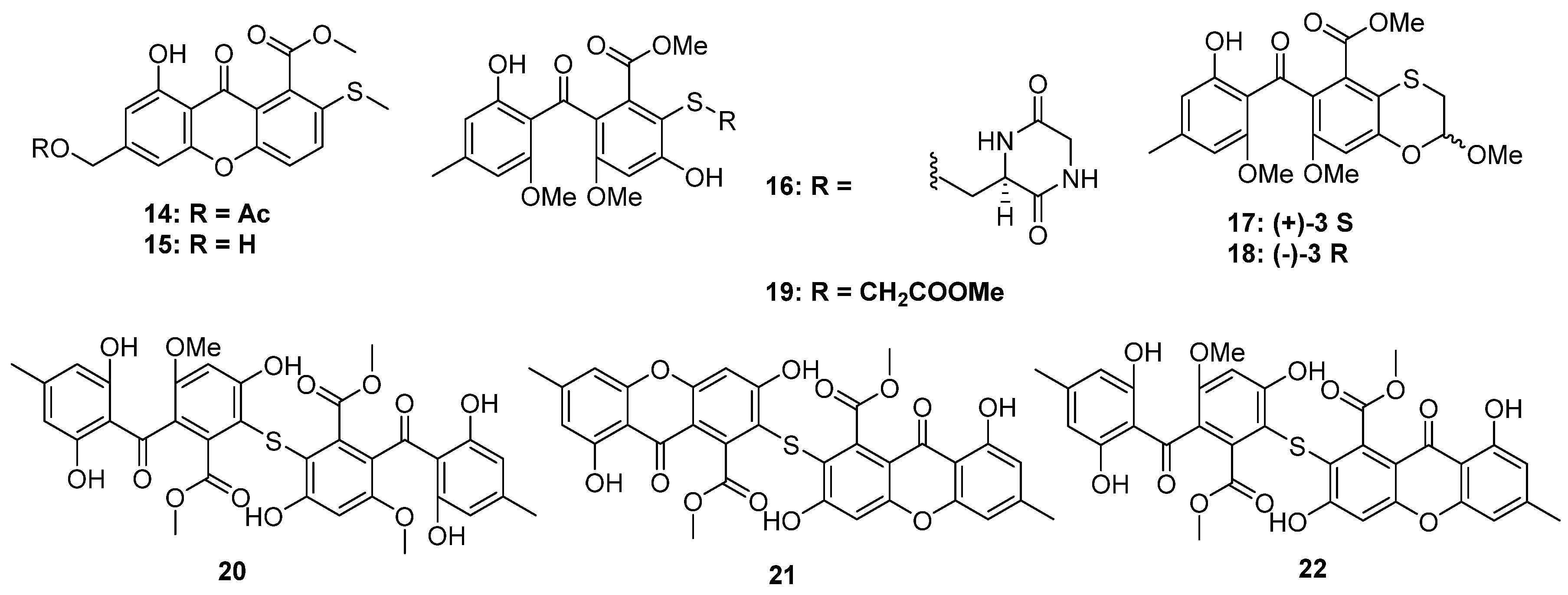
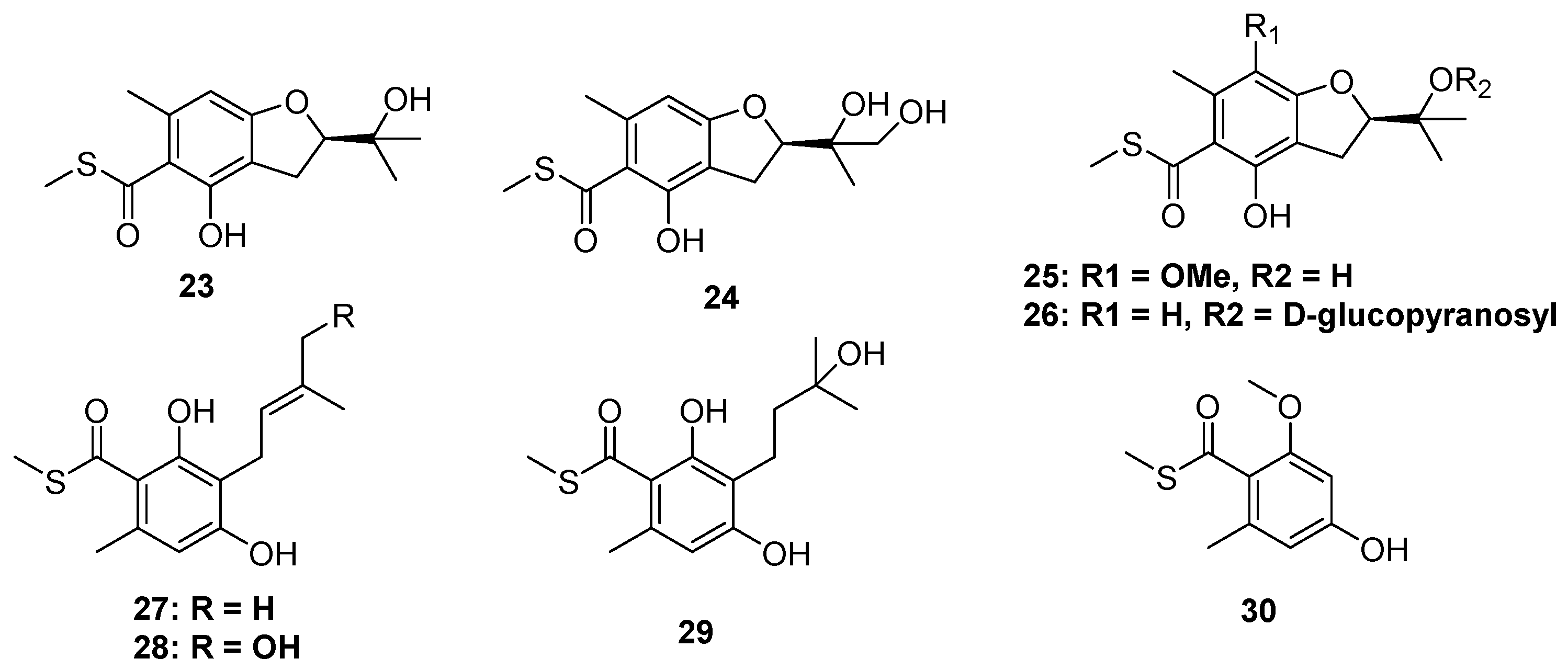
2.1.2. Thioester-Containing Polyketides
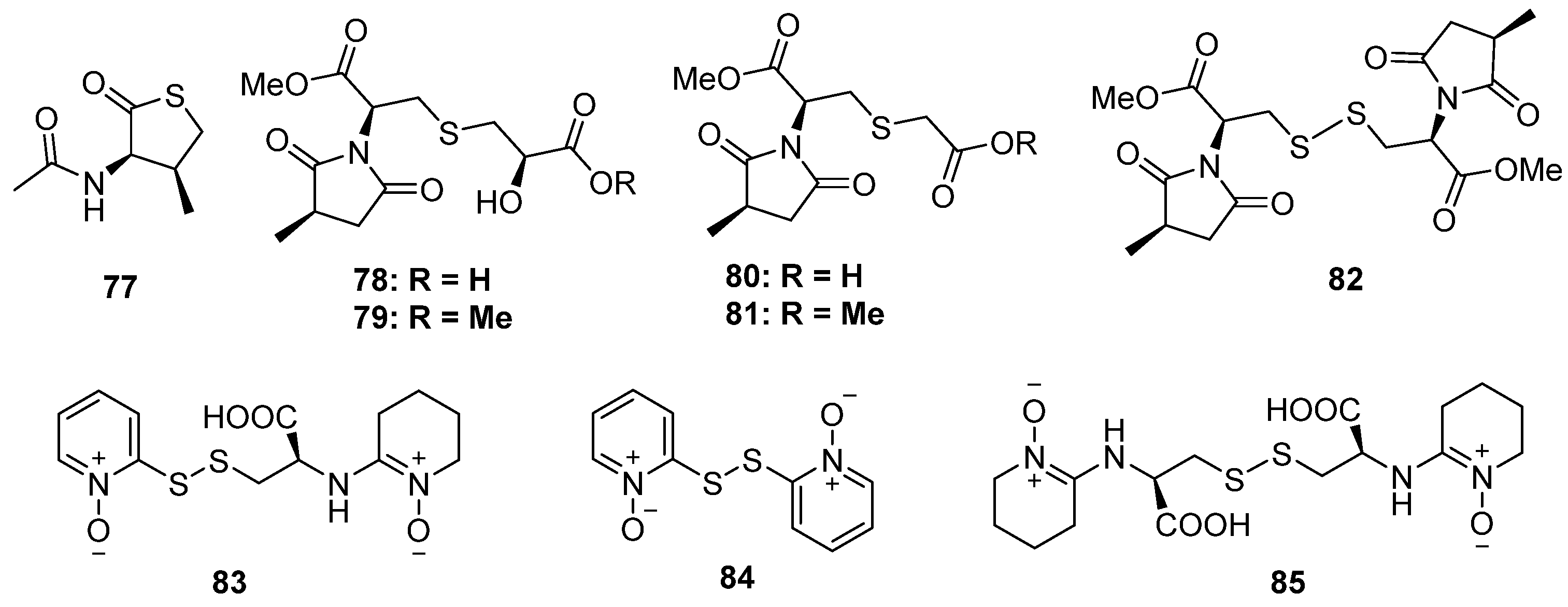
2.1.3. Sulfinyl and Sulfonyl-Containing Polyketides

2.2. Macrolides
2.2.1. Dihydroxyphenylacetic Acid Macrolides
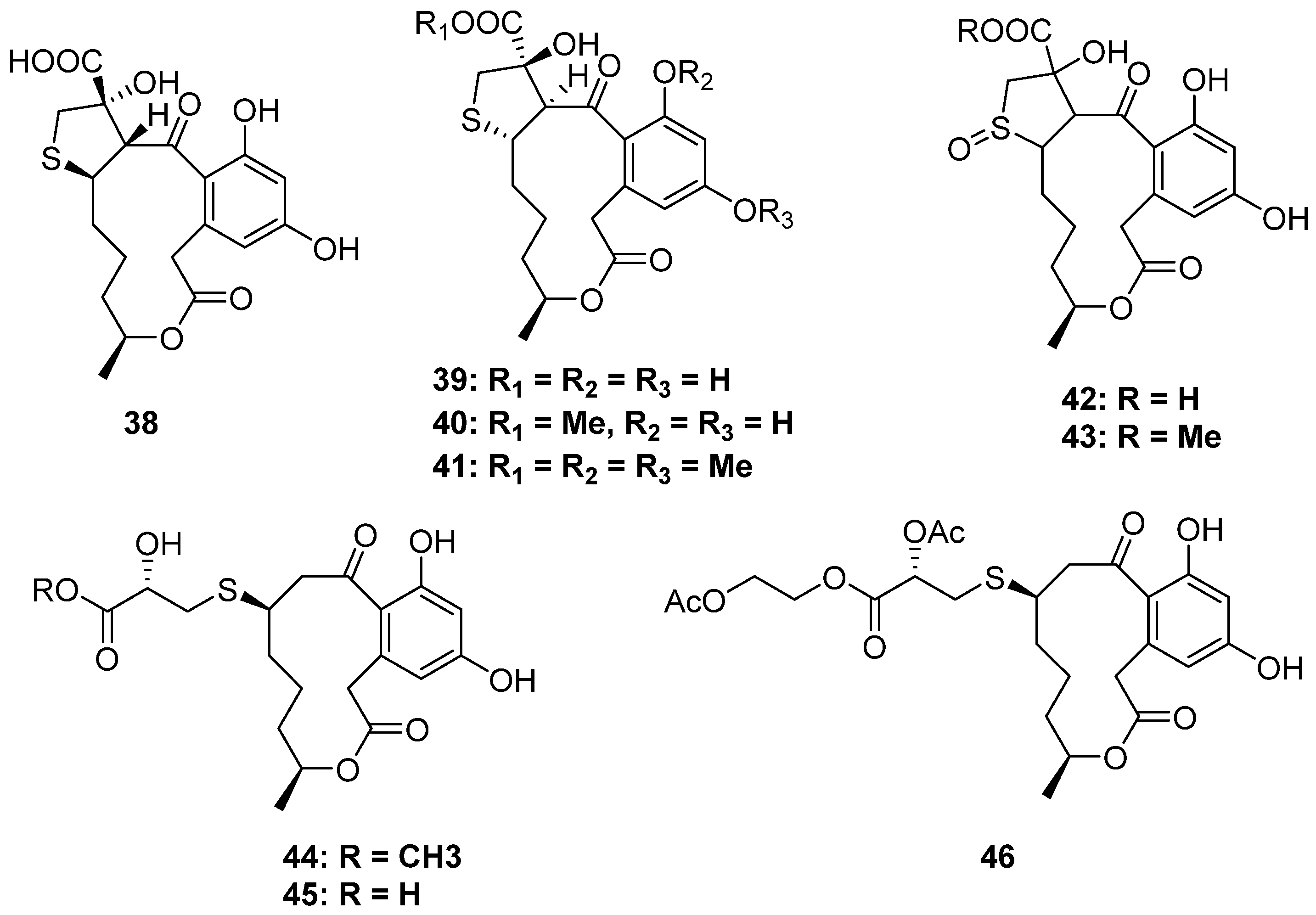
2.2.2. 2-Thioether-Substituted Macrolides
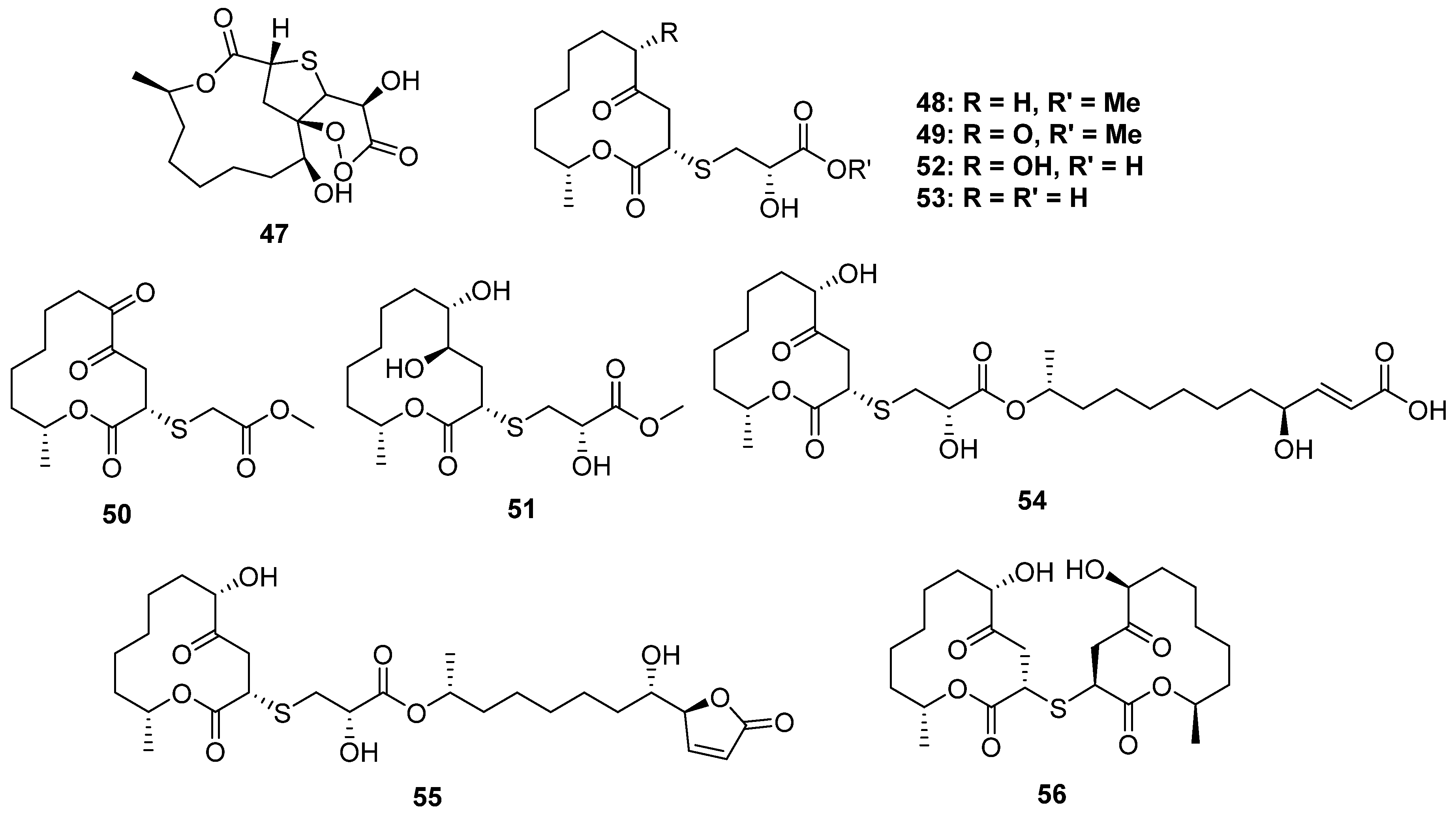

2.2.3. 3-Thioether-Substituted Macrolides
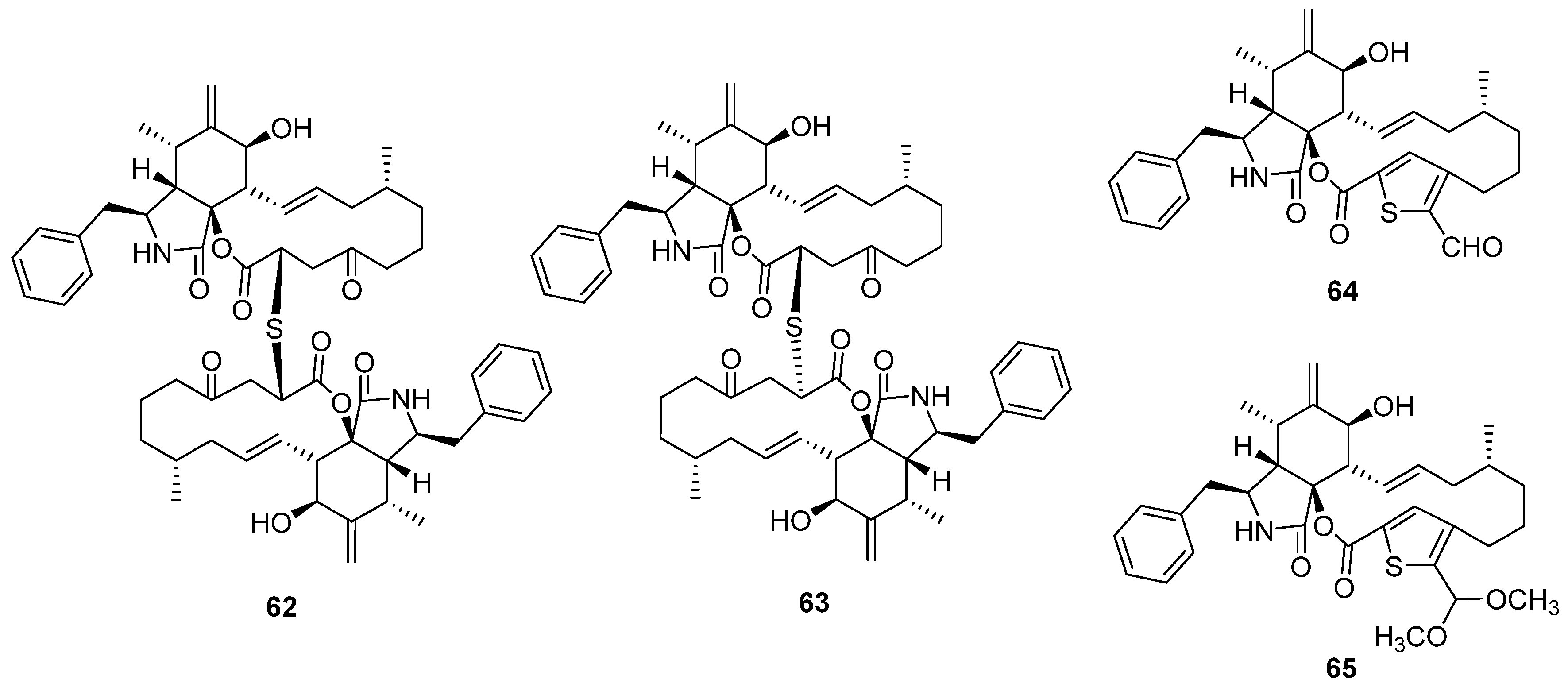
2.3. Cytochalasin
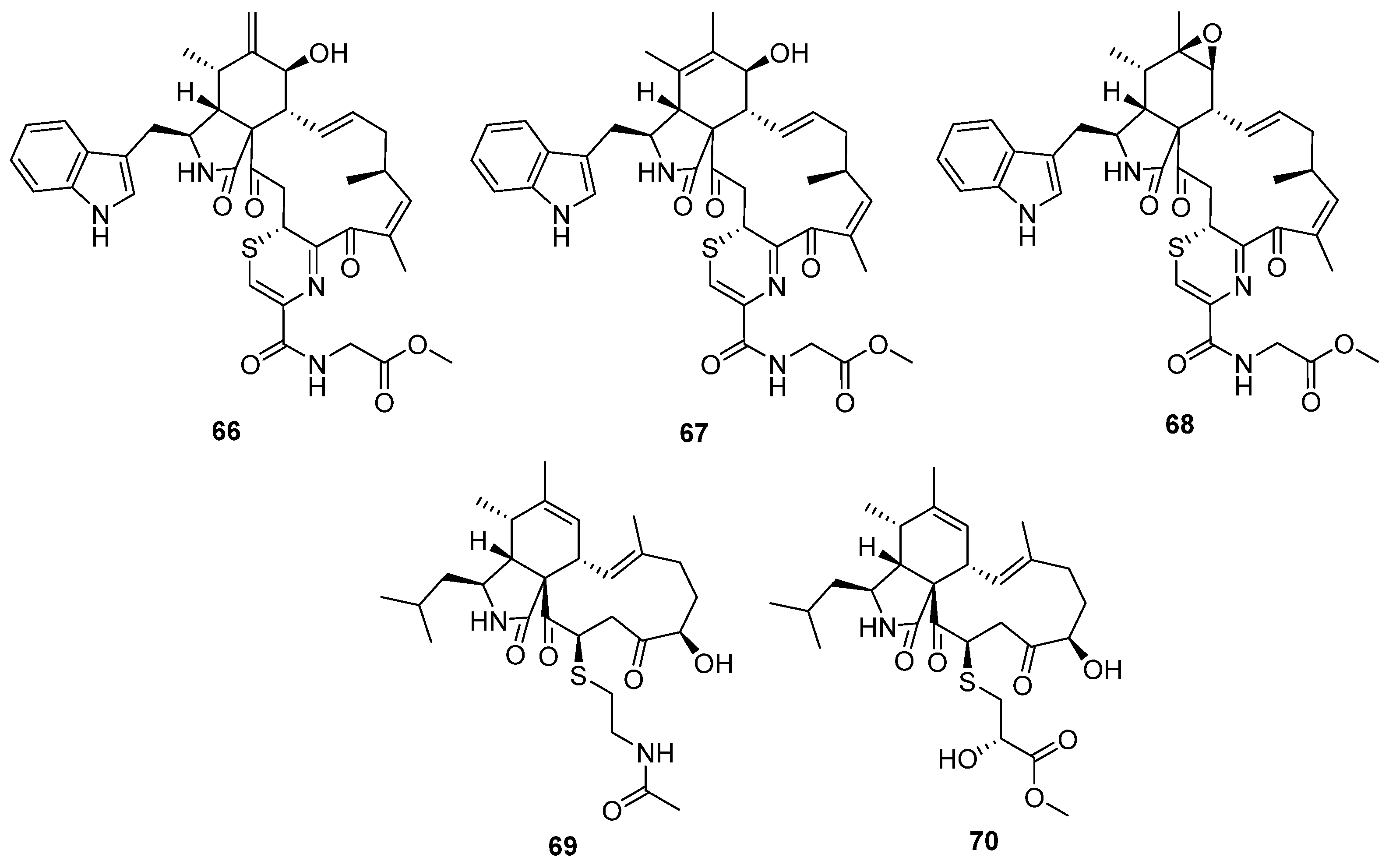
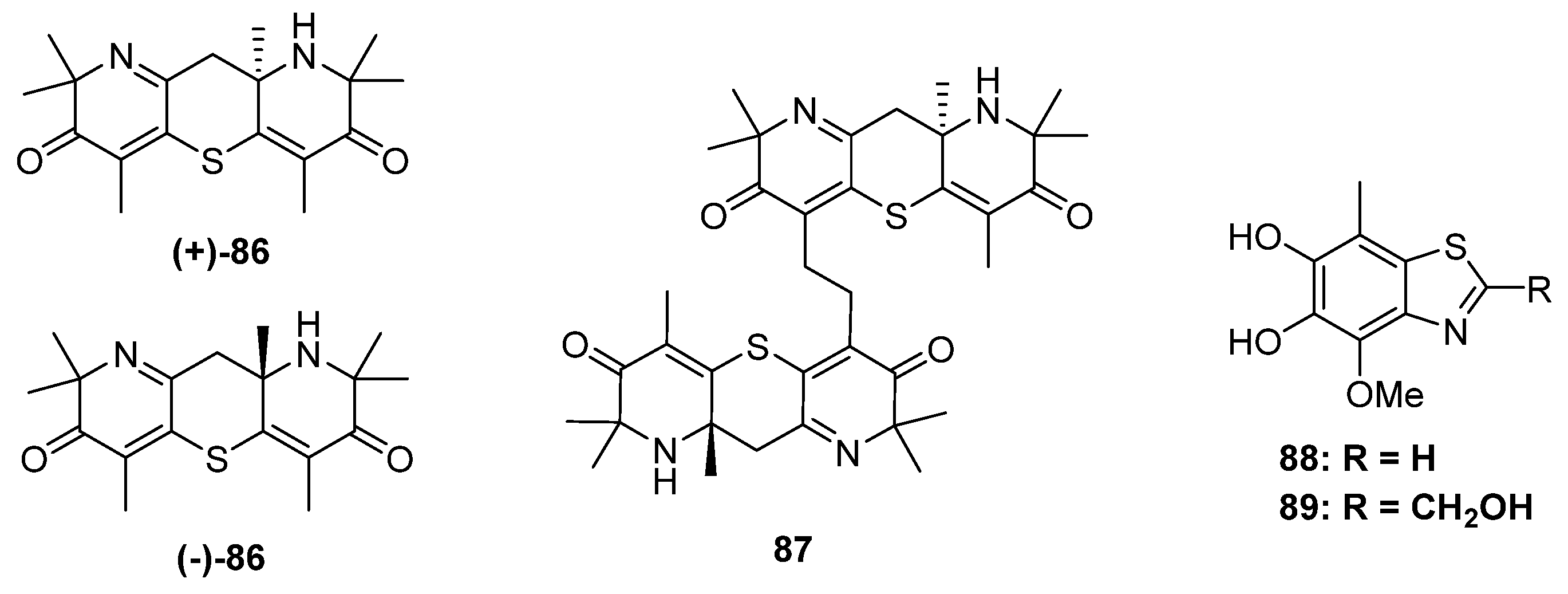
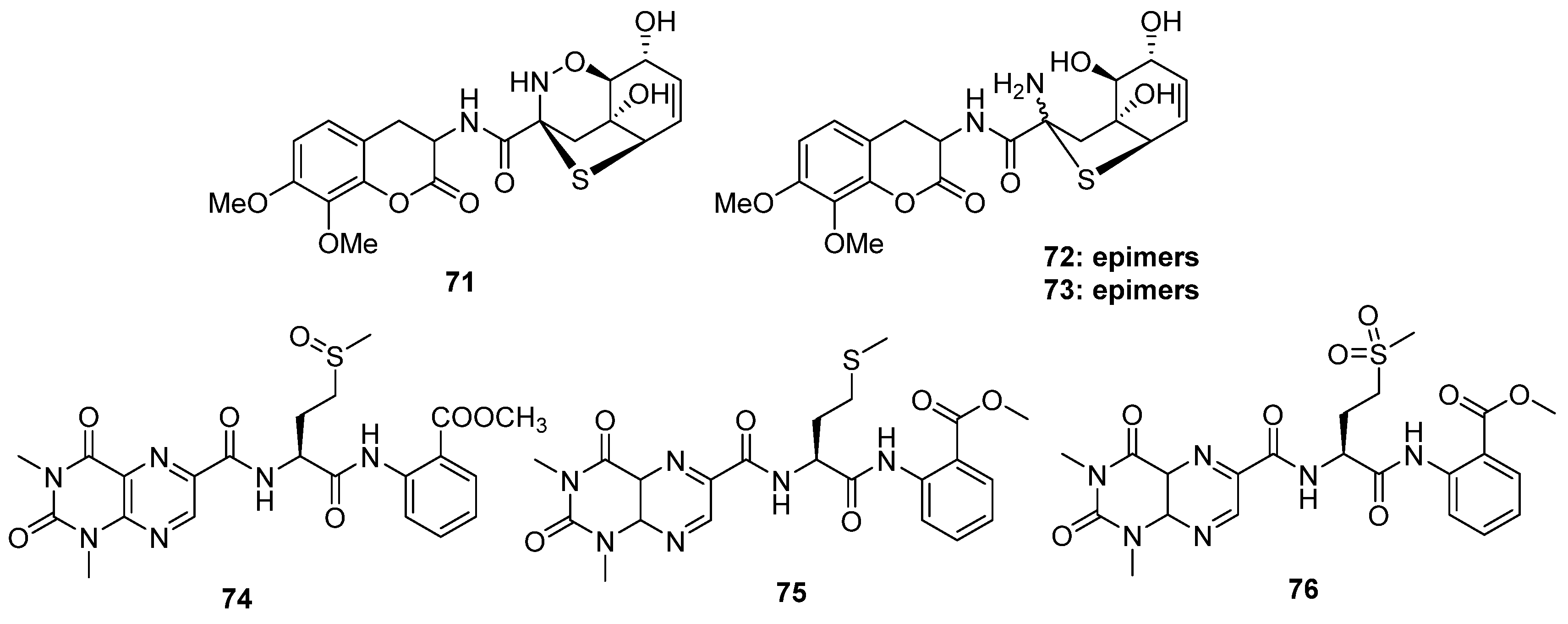
2.4. Alkaloids
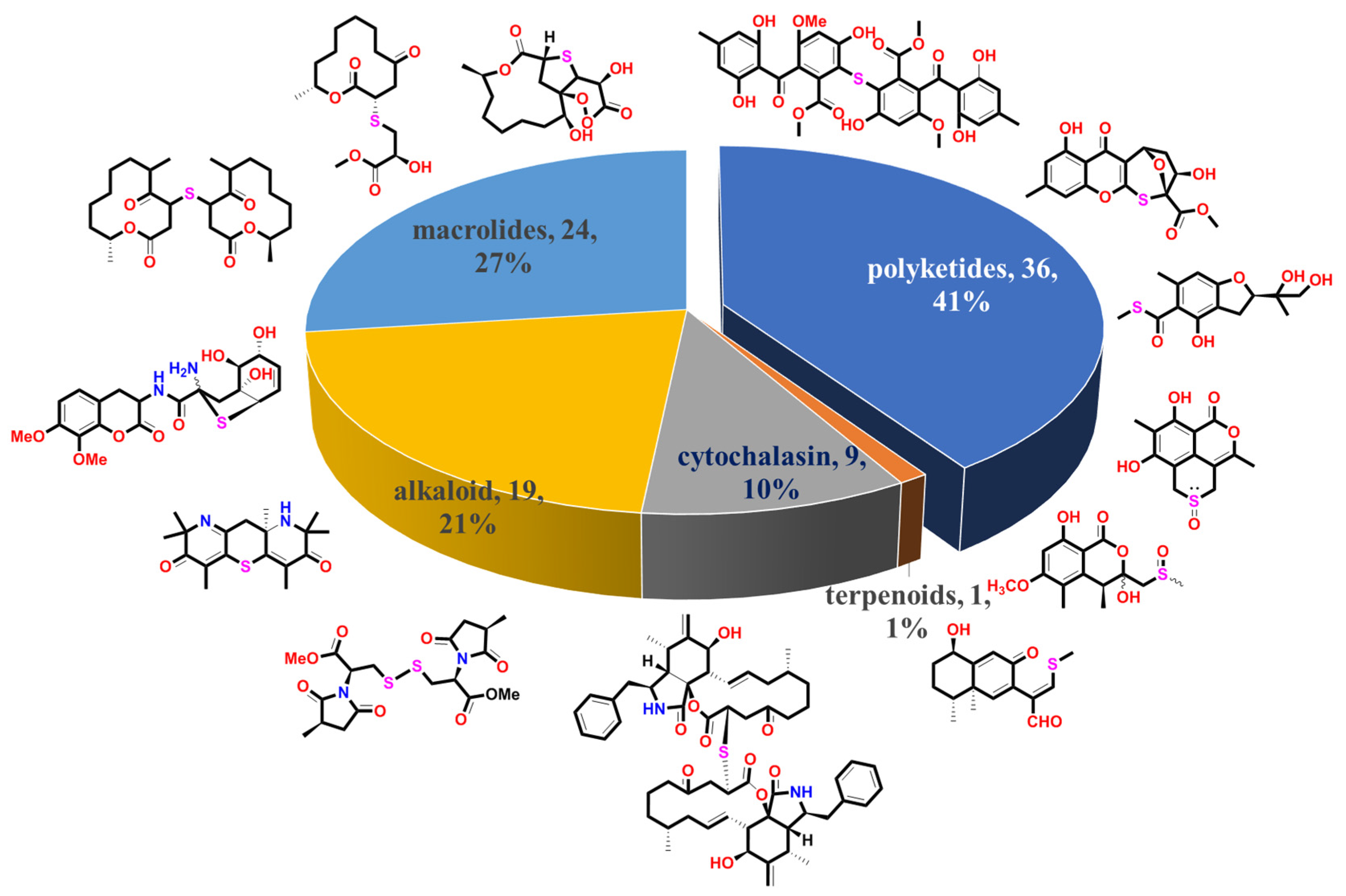
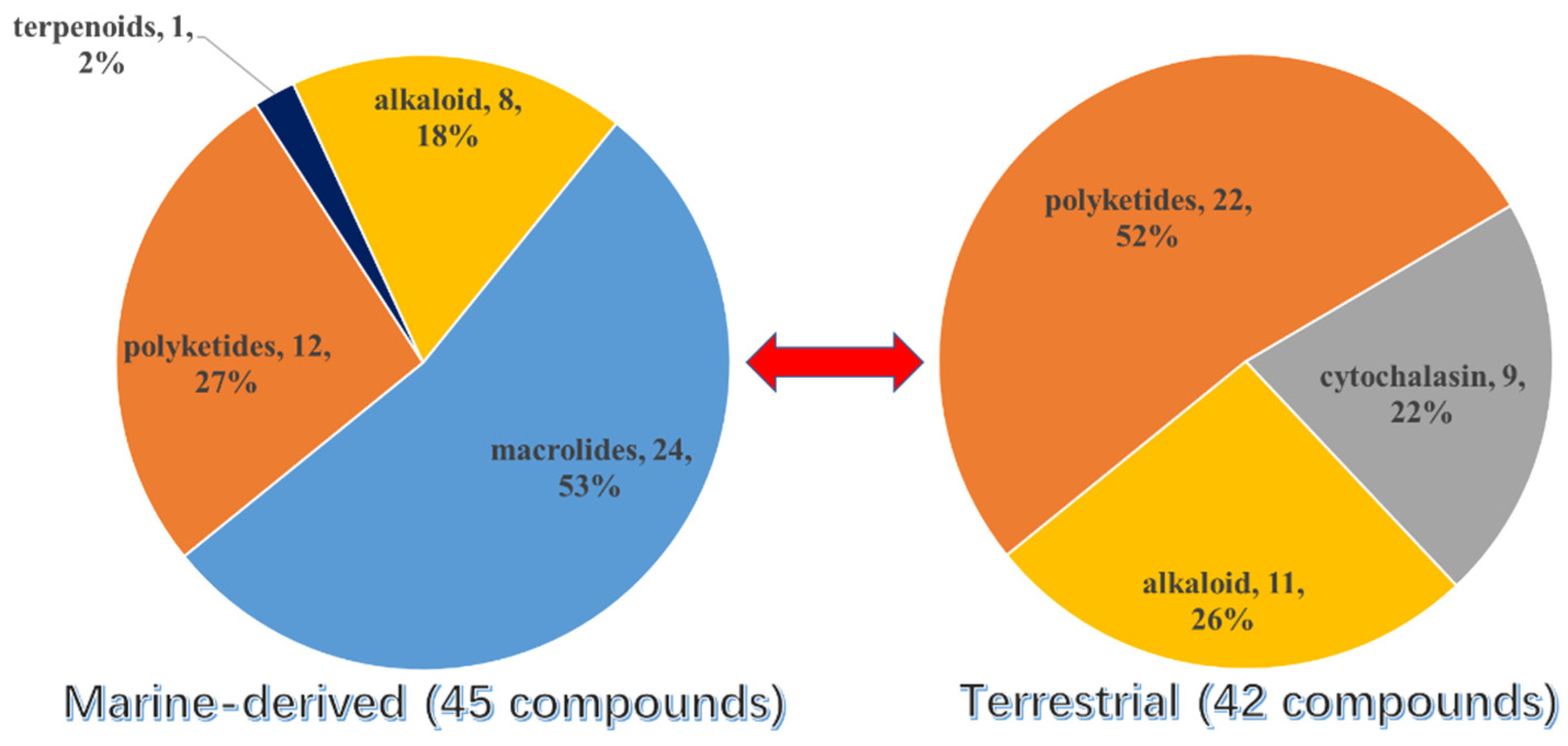
3. Diversity Analysis
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acid: An overview. J. Nutri. 2006, 136, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Milito, A.; Brancaccio, M.; D’Argenio, G.; Castellano, I. Natural sulfur-containing compounds: An alternative therapeutic strategy against liver fibrosis. Cells 2019, 8, 1356. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Zhang, W.; Lu, R.; Bian, J.-S.; Nie, X. Therapeutic potential of sulfur-containing natural products in inflammatory diseases. Pharmacol. Ther. 2020, 216, 107678. [Google Scholar] [CrossRef]
- Pan, C.; Kuranaga, T.; Liu, C.; Lu, S.; Shinzato, N.; Kakeya, H. Thioamycolamides A–E, sulfur-containing cycliclipopeptides produced by the rare Actinomycete Amycolatopsis sp. Org. Lett. 2020, 8, 3014–3017. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Mühling, K.H. Plant-derived sulfur containing natural products produced as a response to biotic and abiotic stresses: A review of their structural diversity and medicinal importance. J. App. Bot. Food Qual. 2019, 92, 204–215. [Google Scholar]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Organosulfur compounds from alliaceae in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 183–193. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačević, D.B. An overview of organosulfur compounds fromAlliumspp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Wei, M.-Y.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Ma, Y.-M.; Liang, X.-A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Huang, X.; Wang, H.; Anjum, K.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Irregularly bridged epipolythiodioxopiperazines and related analogues: Sources, structures, and biological activities. J. Nat. Prod. 2020, 83, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chang, Y.; Che, Q.; Li, D.; Zhang, G.; Zhu, T. Citreobenzofuran D-F and phomenone A-B: Five novel sesquiterpenoids from the mangrove-derived fungus Penicillium sp. HDN13-494. Mar. Drugs 2022, 20, 137. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.-M.; Tang, H.-Y.; Pan, S.-Y.; Zhang, A.-L.; Gao, J.-M. Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv. 2015, 5, 29185–29192. [Google Scholar] [CrossRef]
- Yi, L.; Cui, C.-B.; Li, C.-W.; Peng, J.-X.; Gu, Q.-Q. Chromosulfine, a novel cyclopentachromone sulfide produced by a marine-derived fungus after introduction of neomycin resistance. RSC Adv. 2016, 6, 43975–43979. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, S.; Liu, S.; Guo, L.; Che, Y. The first naturally occurring thiepinols and thienol from an endolichenic fungus Coniochaeta sp. Org. Lett. 2010, 12, 5081–5083. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Niu, S.; Si, Y.; Guo, L.; Jiang, X.; Che, Y. A thiopyranchromenone and other chromone derivatives from an endolichenic fungus, Preussia africana. J. Nat. Prod. 2012, 75, 230–237. [Google Scholar] [CrossRef]
- Sandargo, B.; Thongbai, B.; Stadler, M.; Surup, F. Cysteine-derived pleurotin congeners from the nematode-trapping basidiomycete Hohenbuehelia grisea. J. Nat. Prod. 2018, 81, 285–291. [Google Scholar] [CrossRef]
- Daengrot, C.; Rukachaisirikul, V.; Tansakul, C.; Thongpanchang, T.; Phongpaichit, S.; Bowornwiriyapan, K.; Sakayaroj, J. Eremophilane sesquiterpenes and diphenyl thioethers form the soil fungus Peniciilium copticola PSU-RSPG138. J. Nat. Prod. 2015, 78, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.K.; Pitt, J.I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A.M.; Karuso, P. Banksialactones and banksiamarins: Isochromanones and isocoumarines from an Australian fungus, Aspergillus banksianus. J. Nat. Prod. 2018, 81, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Q.; Zhang, X.; Han, Q.-J.; Li, X.-B.; Li, G.; Li, R.-J.; Jiao, Y.; Zhou, J.-C.; Lou, H.-X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliate S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, W.; Wu, D.; He, W.; He, W.; Zou, M.; Wang, D.; Fu, P.; Wang, L.; Zhu, W. Sulfur-containing phenolic compounds from the cave soil-derived Aspergillus fumigatus GZWMJZ-152. J. Nat. Prod. 2022, 85, 433–440. [Google Scholar] [CrossRef]
- Cai, S.; King, J.B.; Du, L.; Powell, D.R.; Cichewicz, R.H. Bioactive sulfur-containing sulochrin dimers and other metabolites from an Alternaria sp. isolate from a Hawaiian soil sample. J. Nat. Prod. 2014, 77, 2280–2287. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Hu, J.; Gao, X.; Wang, Y.; Liu, Z.; Zhang, W. Thioester-containing benzoate derivatives with α-glucosidase inhibitory activity from the deep-sea-derived fungus Talaromyces indigoticus FS688. Mar. Drugs 2022, 20, 33. [Google Scholar] [CrossRef]
- Pinedo-Rivilla, C.; Collado, I.G.; Aleu, J. Metabolism of antifungal thiochroman-4-ones by Trichoderma viride and Botrytis cinereal. J. Nat. Prod. 2018, 81, 1036–1040. [Google Scholar] [CrossRef]
- Liu, D.-H.; Sun, Y.-Z.; Kurtán, T.; Mándi, A.; Tang, H.; Li, J.; Su, L.; Zhuang, C.-L.; Liu, Z.-Y.; Zhang, W. Osteoclastogenesis regulation metabolites from the coral-associated fungus Pseudallescheria boydii TW-1024-3. J. Nat. Prod. 2019, 82, 1274–1282. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y.; Ikeda, T.; Murakami, K.; One, M.; Nakana, D.; Kinjo, J. Synthesis of diosgenin-ibuprofen derivatives and their activities against insulin-dependent diabetes mellitus. Chem. Pharm. Bull. 2013, 61, 695–699. [Google Scholar] [CrossRef]
- Nohara, T.; Kiyota, Y.; Sakamoto, T.; Manabe, H.; Ono, M.; Ikeda, T.; Fujiwara, Y.; Nakano, D.; Kinjo, J. Garlicnins B1, C1, and D, from the fraction regulating macrophage activation of Allium sativum. Chem. Pharm. Bull. 2012, 60, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Nohara, T.; Fujiwara, Y.; Ikeda, T.; Murakami, K.; Ono, M.; ElAasr, M.; Nakano, D.; Kinjo, J. Two new bicyclic sulfoxides from Welsh onion. J. Nat. Med. 2016, 70, 260–265. [Google Scholar] [CrossRef]
- El-Aasr, M.; Fujiwara, Y.; Takeya, M.; Ikeda, T.; Tsukamoto, S.; Ono, M.; Nakano, D.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; et al. Onionin A from Allium cepa inhibits macrophage activation. J. Nat. Prod. 2010, 73, 1306–1308. [Google Scholar] [CrossRef]
- Meng, D.-H.; Wu, J.; Wang, L.-Y.; Zhao, W.-M. Two new glycosides from Breynia vitis-idaea. J. Asian Nat. Prod. Res. 2010, 12, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Iwanow, A.; Wojtasiewicz, K.; Wróbel, J.T. Sulphoxides of thiobinupharidine thiohemiaminals from Nuphar lutea. Phytochemistry 1986, 25, 2227–2231. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Oda, M.; Asao, Y.; Yoshikawa, M. Potent anti-metastatic activity of dimeric sesquiterpene thioalkaloids from the rhizome of Nuphar pumilum. Bioorganic Med. Chem. Lett. 2003, 13, 4445–4449. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Lu, Z.-H.; Xia, G.-R.; Song, W.-M.; Guo, Z.-Y.; Proksch, P. (+)-/(−)-Prunomarin A and (+)-pestalactone B, three new isocoumarin derivatives from the endophytic fungus Phomopsis prunorum. Tetrahedron Lett. 2021, 75, 153205. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Q.; Liu, S.; Zhang, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Methylsulfonylated polyketides produced by Neosartorya udagawae HDN13-313 via exogenous addition of small molecules. J. Nat. Prod. 2019, 82, 998–1001. [Google Scholar] [CrossRef]
- Passarini, M.R.Z.; Santos, C.; Lima, N.; Berlinck, R.G.S.; Sette, L.D. Filamentous fungi from the Atlantic marine sponge Dragmacidon reticulatum. Arch. Microbiol. 2013, 195, 99–111. [Google Scholar] [CrossRef]
- Meng, L.-H.; Li, X.-M.; Lv, C.-T.; Li, C.-S.; Xu, G.-M.; Huang, C.-G.; Wang, B.-G. Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera racemosa. J. Nat. Prod. 2013, 76, 2145–2149. [Google Scholar] [CrossRef]
- de Castro, M.V.; Ióca, L.P.; Williams, D.E.; Costa, B.Z.; Mizuno, C.M.; Santos, M.F.C.; de Jesus, K.; Ferrerira, É.L.F.; Seleghim, M.H.R.; Sette, L.D.; et al. Condensation of macrocyclic polyketides produced by Penicillium sp. DRF2 with mercaptopyruvate represents a new fungal detoxification pathway. J. Nat. Prod. 2016, 24, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-P.; Song, Y.-P.; Wang, B.-G.; Ji, N.-Y. Sulfurated and iodinated metabolites from the cold-seep fungus Cladosporium cladosporioides 8-1. Tetrahedron Lett. 2022, 93, 153689. [Google Scholar] [CrossRef]
- Zhang, F.-Z.; Li, X.-M.; Yang, S.-Q.; Meng, L.-H.; Wang, B.-G. Thiocladospolides A-D, 12-membered macrolides from the mangrove-derived endophytic fungus Cladosporium cladosporioides MA-299 and structure revision of Pandangolide 3. J. Nat. Prod. 2019, 82, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, H.; Sun, C.; Che, Q.; Zhang, G.; Zhu, T.; Li, D. Thiocladospolides F-J, antibacterial sulfur containing 12-membered macrolides from the mangrove endophytic fungus Cladosporium oxysporum HDN13-314. Phytochemistry 2020, 178, 112462. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Abbanat, D.; Bernan, V.S.; Maiese, W.M.; Greenstein, M.; Jompa, J.; Tahir, A.; Ireland, C.M. Novel polyketides metabolites form a species of marine fungi. J. Nat. Prod. 2000, 63, 142–145. [Google Scholar] [CrossRef]
- Jadulco, R.; Proksch, P.; Wray, V.; Sudarsono; Berg, A.; Grafe, U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J. Nat. Prod. 2001, 64, 527–530. [Google Scholar] [CrossRef]
- Zhang, F.-Z.; Li, X.-M.; Meng, L.-H.; Wang, B.-G. Cladocladosin A, an unusual macrolide with bicyclo 5/9 ring system, and two thiomacrolides from the marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299. Bioorganic Chem. 2020, 101, 103950. [Google Scholar] [CrossRef]
- Peng, X.; Chang, J.; Gao, Y.; Duan, F.; Ruan, H. Thiocytochalasins A–D, four sulfur-containing cytochalasans from an endophytic fungus Phoma multirostrata XJ-2-1. Chin. Chem. Lett. 2022, 10, 4572–4576. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Wang, C.-Y.; Li, X.-M.; Yang, S.-Q.; Li, X.; Wang, B.-G.; Si, S.-Y.; Meng, L.-H. Cytochalasin derivatives from the endozoic Curvularia verruculosa CS-129, a fungus isolated from the deep-sea squat lobster Shinkaia crosnieri living in the cold seep environment. J. Nat. Prod. 2021, 84, 3122–3130. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, F.; Bie, Q.; Dai, C.; Chen, C.; Tong, Q.; Liu, J.; Wang, J.; Zhou, Y.; Zhu, H.; et al. Cytochathiazines A–D, three merocytochalasans with a 2H-1,4-thiazine functionality from coculture of Chaetomium globosum and Aspergillus flavipes. Org. Lett. 2018, 20, 6817–6821. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Al Anbari, W.H.; Zhou, Q.; Zhou, P.; Zhang, M.; Zeng, F.; Chen, C.; Tong, Q.; Wang, J.; et al. Cysteine residue containing merocytochalasans and 17,18-seco-aspochalasins from Aspergillus micronesinsis. J. Nat. Prod. 2019, 82, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. The antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. infuenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar]
- Newton, G.G.F.; Abraham, E.P. Isolation of cephalosporin C, a penicillin-like antibiotic containing d-α-aminoadipic acid. Bochem. J. 1956, 62, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazine A–E: Novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.W.; Mordhorst, T.F.; Lee, C.; Jensen, P.R.; Jensen, P.R.; Fenical, W.; Kock, M. Penilumamide, a novel lumazine peptide isolated from the marine-derived fungus, Penicillium sp. CNL-338. Org. Biomol. Chem. 2010, 8, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, C.-L.; Fu, X.-M.; Kong, C.-J.; She, Z.-G.; Wang, C.-Y. Lumazine peptides Penilumamides B–D and the cyclic pentapeptide asperpeptide A from a gorgonian-derived Aspergillus sp. fungus. J. Nat. Prod. 2014, 77, 1601–1606. [Google Scholar] [CrossRef]
- Han, X.; Li, P.; Luo, X.; Qiao, X.; Tang, X.; Li, G. Two new compounds from the marine sponge derived fungus Penicillium chrysogenum. Nat. Prod. Res. 2019, 20, 19–24. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, C.; Huang, J.; Zhang, J.; Liu, D.; Huang, J.; Proksch, P.; Lin, W. Violaceimides A–E, sulfur-containing metabolites from a sponge-associated fungus Aspergillus violaceus. Tetrahedron Lett. 2018, 59, 3157–3160. [Google Scholar] [CrossRef]
- Nicholas, G.M.; Blunt, J.W.; Munro, M.H.G. Cortamidine oxide, a novel disulfide metabolite from the New Zealand basidiomycete (mushroom) Cortinarius species. J. Nat. Prod. 2001, 64, 341–344. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.-X.; Li, Z.-H.; He, J.; Huang, R.; Zheng, Y.-S.; Li, L.-Q.; Wang, X.; Feng, T.; Liu, J.-K. Xylaridines C and D, unusual thiopyranodipyridine alkaloids from the fungus Xylaria longipes. Org. Lett. 2019, 21, 6145–6148. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Zhang, Q.; Liu, W. Differences in plp-dependent cysteinyl processing lead to diverse S-functionalization of lincosamide antibiotics. J. Am. Chem. Soc. 2016, 138, 6348–6351. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, R.; Lin, C.I.; Sasaki, E.; Liu, H.W. Characterization of enzymes catalyzing transformations of cysteine S-conjugated intermediates in the lincosamide biosynthetic pathway. Chem. Bio. Chem. 2016, 17, 1606–1611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia, I.; Vior, N.M.; Braña, A.F.; Gonzá lez-Sabin, J.; Rohr, J.; Moris, F.; Méndez, C.; Salas, J.A. Elucidating the biosynthetic pathway for the polyketide-nonribosomal peptide collismycin A: Mechanism for formation of the 2,2′-Bipyridyl Ring. Chem. Biol. 2012, 19, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Pang, B.; Zhang, Z.; Chen, M.; Wu, Z.; Zhao, Q.; Zhang, Q.; Wang, Y.; Liu, Y.; Liu, W. Caerulomycins and collismycins share a common paradigm for 2,2’-Bipyridine biosynthesis via an unusual hybrid polyketide-peptide assembly logic. J. Am. Chem. Soc. 2012, 134, 9038–9041. [Google Scholar] [CrossRef]
- Podust, L.M.; Sherman, D.H. Diversity of P450 enzymes in the biosynthesis of natural products. Nat. Prod. Rep. 2012, 29, 1251–1266. [Google Scholar] [CrossRef]
- Fluhe, L.; Knappe, T.A.; Gattner, M.J.; Schafer, A.; Burghaus, O.; Linne, U.; Marahiel, M.A. The radical sam enzyme alba catalyzes thioether bond formation in subtilosin A. Nat. Chem. Biol. 2012, 8, 350–357. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Tang, K.; Liu, W.; He, X.; Huang, X.; Deng, Z. Identification and analysis of the biosynthetic gene cluster encoding the thiopeptide antibiotic cyclothiazomycin in Streptomyces Hygroscopicus 10–22. Appl. Environ. Microbiol. 2010, 76, 2335–2344. [Google Scholar] [CrossRef]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic carbon-sulfur bond formation in natural product biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, M.; Wang, S.; Huang, H.; Zhang, W. Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Mar. Drugs 2022, 20, 765. https://doi.org/10.3390/md20120765
Liu Z, Li M, Wang S, Huang H, Zhang W. Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Marine Drugs. 2022; 20(12):765. https://doi.org/10.3390/md20120765
Chicago/Turabian StyleLiu, Zhaoming, Mingqiong Li, Shuo Wang, Huibin Huang, and Weimin Zhang. 2022. "Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities" Marine Drugs 20, no. 12: 765. https://doi.org/10.3390/md20120765
APA StyleLiu, Z., Li, M., Wang, S., Huang, H., & Zhang, W. (2022). Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Marine Drugs, 20(12), 765. https://doi.org/10.3390/md20120765