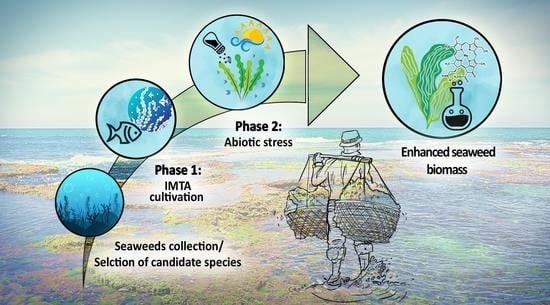
Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds
Abstract
1. Introduction
2. Results
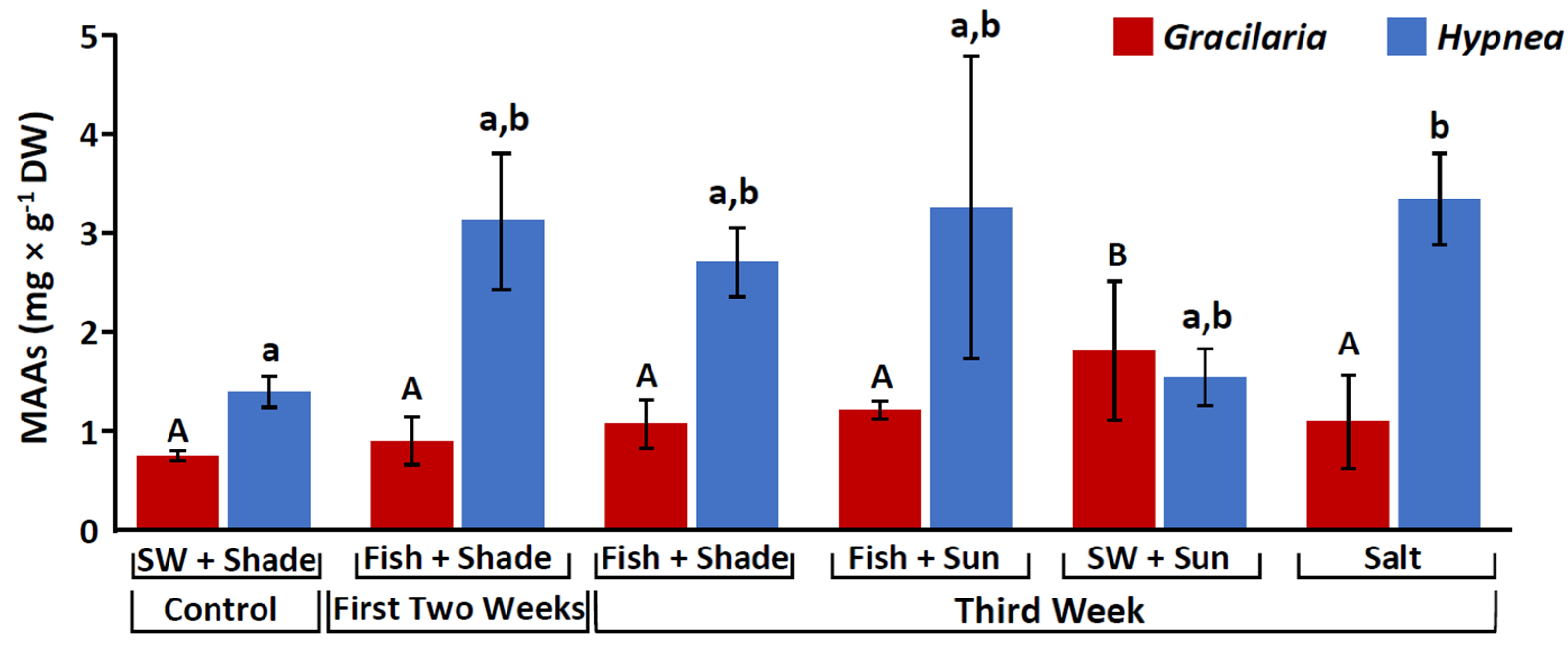
2.1. Mycosporine-like Amino Acids (MAAs)
2.2. Pigment Content (Chlorophylls and Phycobiliproteins)
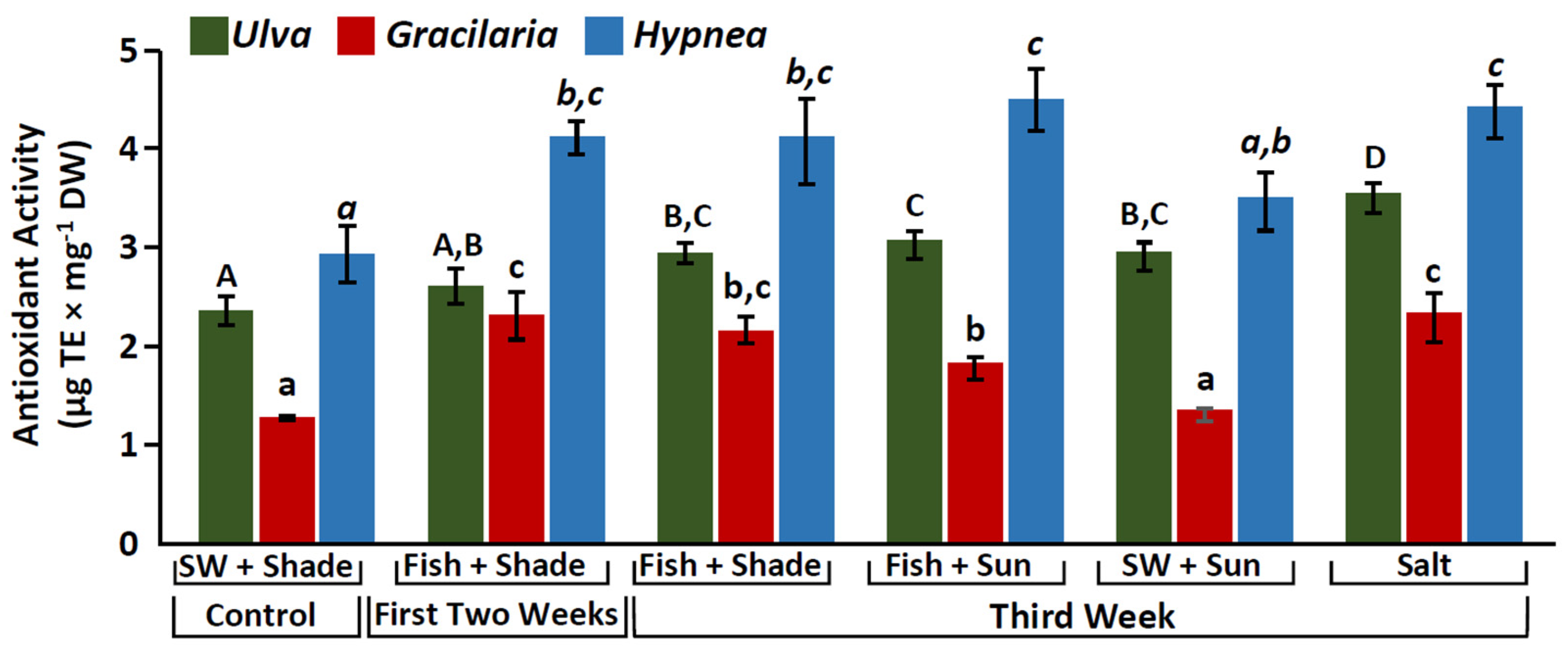
2.3. Antioxidant Activity
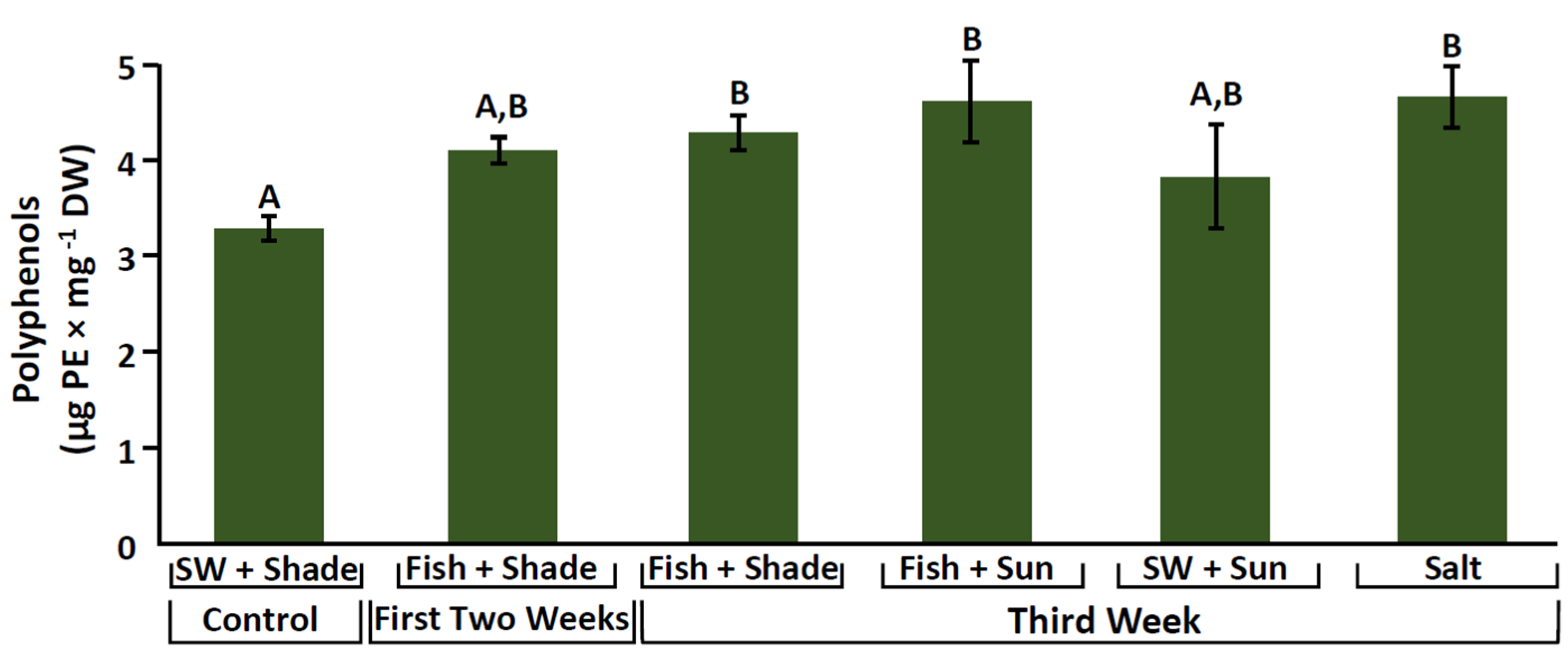
2.4. Phenolic Compounds
2.5. Sun Protection Factor (SPF)
3. Discussion
3.1. Stimulation of Mycosporine-like Amino Acids (MAAs)
3.2. Enhancement of Pigment Content
3.3. Increase in Antioxidant Activity and Phenolic Compounds
3.4. SPF Manipulation
4. Materials and Methods
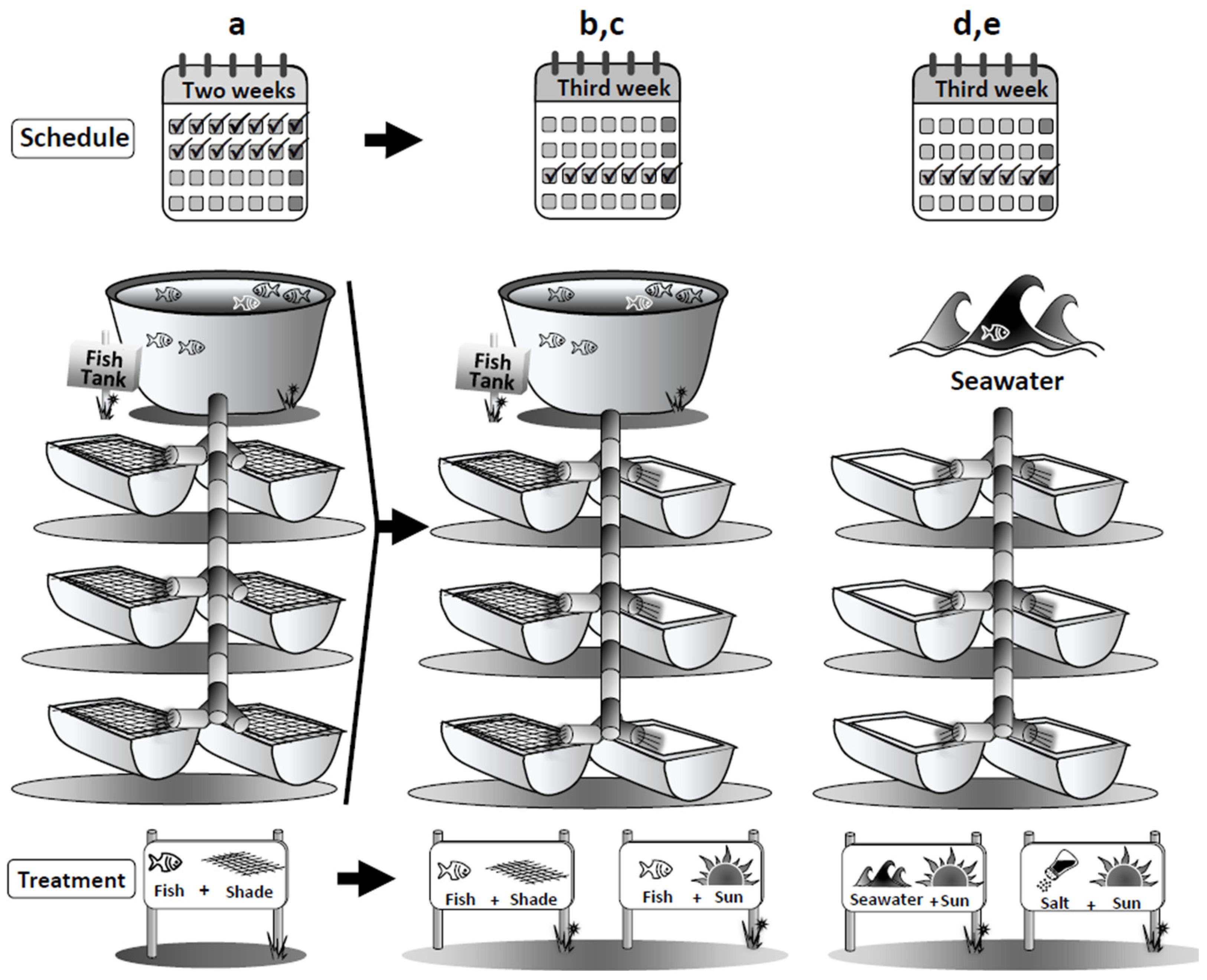
4.1. Integrated Aquaculture System and Experimental Design
4.2. Chemical Composition of Seaweed Tissues: Sample Preparation
4.2.1. Analysis of Mycosporine-like Amino Acids (MAAs)
4.2.2. Pigment Extraction and Evaluation
4.2.3. Determination of Antioxidant Activity
4.2.4. Determination of Phenolic Compounds
4.2.5. Sun Protection Factor (SPF) Evaluation
4.3. Statistical Analysis
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, R.L. Algae: The World’s Most Important “Plants”—An Introduction. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jin, R.; Zhang, X.; Wang, Q.; Wu, J. The Considerable Environmental Benefits of Seaweed Aquaculture in China. Stoch. Environ. Res. Risk Assess. 2019, 33, 1203–1221. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.R.K.; Cronin, H.; Forster, J. Chapter 3—Farming of Seaweeds. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 27–59. ISBN 978-0-12-418697-2. [Google Scholar]
- Smith, S.V. Marine Macrophytes as a Global Carbon Sink. Science 1981, 211, 838–840. [Google Scholar] [CrossRef]
- Lüning, K.; Pang, S. Mass Cultivation of Seaweeds: Current Aspects and Approaches. J. Appl. Phycol. 2003, 15, 115–119. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Landschützer, P.; Gruber, N.; Bakker, D.C.E.; Schuster, U. Recent Variability of the Global Ocean Carbon Sink. Glob. Biogeochem. Cycles 2014, 28, 927–949. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial Role of Macroalgae in Marine Carbon Sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Afflerbach, J.C.; Frazier, M.; Halpern, B.S. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Curr. Biol. 2019, 29, 3087–3093.e3. [Google Scholar] [CrossRef]
- Marinho, G.S.; Holdt, S.L.; Birkeland, M.J.; Angelidaki, I. Commercial Cultivation and Bioremediation Potential of Sugar Kelp, Saccharina latissima, in Danish Waters. J. Appl. Phycol. 2015, 27, 1963–1973. [Google Scholar] [CrossRef]
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient Removal from Chinese Coastal Waters by Large-Scale Seaweed Aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef]
- Racine, P.; Marley, A.; Froehlich, H.E.; Gaines, S.D.; Ladner, I.; MacAdam-Somer, I.; Bradley, D. A Case for Seaweed Aquaculture Inclusion in U.S. Nutrient Pollution Management. Mar. Policy 2021, 129, 104506. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Chopin, T.; Sawhney, M. Seaweeds and Their Mariculture. In Encyclopedia of Ocean Sciences; Academic Press: Cambridge, MA, USA, 2009; pp. 4477–4487. [Google Scholar]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, e537202. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Gnanavel, V.; Roopan, S.M.; Rajeshkumar, S. Aquaculture: An Overview of Chemical Ecology of Seaweeds (Food Species) in Natural Products. Aquaculture 2019, 507, 1–6. [Google Scholar] [CrossRef]
- Chopin, T.; Tacon, A.G.J. Importance of Seaweeds and Extractive Species in Global Aquaculture Production. Rev. Fish. Sci. Aquac. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated Aquaculture: Rationale, Evolution and State of the Art Emphasizing Seaweed Biofiltration in Modern Mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Israel, A.; Abelson, A. A Novel Two-Stage Seaweed Integrated Multi-Trophic Aquaculture. Rev. Aquac. 2019, 11, 246–262. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Segal, Y.; Ben-Valid, S.; Paz, G.; Tsubery, M.N.; Salomon, E.; Abelson, A.; Israel, Á. Enrichment of Nutritional Compounds in Seaweeds via Abiotic Stressors in Integrated Aquaculture. Innov. Food Sci. Emerg. Technol. 2022, 80, 103067. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 978-0-471-62434-9. [Google Scholar]
- Einav, R.; Israel, A. Seaweeds on the Abrasion Platforms of the Intertidal Zone of Eastern Mediterranean Shores. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2007; pp. 193–207. ISBN 978-1-4020-6112-7. [Google Scholar]
- Israel, A.; Golberg, A.; Neori, A. The Seaweed Resources of Israel in the Eastern Mediterranean Sea. Bot. Mar. 2020, 63, 85–95. [Google Scholar] [CrossRef]
- Ferraces-Casais, P.; Lage-Yusty, M.; Rodriguez, A.; Lopez-Hernandez, J. Evaluation of Bioactive Compounds in Fresh Edible Seaweeds. Food Anal. Methods 2011, 5, 828–834. [Google Scholar] [CrossRef]
- Pereira, L.; Neto, J.M. Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-8167-8. [Google Scholar]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress Tolerance and Photoadaptation to Solar Radiation in Rhodymenia pseudopalmata (Rhodophyta) through Mycosporine-like Amino Acids, Phenolic Compounds, and Pigments in an Integrated Multi-Trophic Aquaculture System. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Schneider, G.; Lopez Figueroa, F.; Vega, J.; Chaves, P.; Álvarez-Gómez, F.; Korbee, N.; Bonomi Barufi, J. Photoprotection Properties of Marine Photosynthetic Organisms Grown in High Ultraviolet Exposure Areas: Cosmeceutical Applications. Algal Res. 2020, 49, 101956. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of Sunscreen Compounds in Antarctic Macroalgae by Different Radiation Conditions. Mar. Biol. 2002, 141, 619–627. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising Plant of the Millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; De Souza, M.d.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from Marine Algae of the Genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Häder, D.-P. Photoprotective Compounds from Marine Organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Yang, B.; Lin, X.; Zhou, X.-F.; Yang, X.; Liu, Y. Chemical and Biological Aspects of Marine Cosmeceuticals. In Marine Cosmeceuticals; CRC Press: Boca Raton, FL, USA, 2011; pp. 11–38. ISBN 978-1-4398-6028-1. [Google Scholar]
- Coba, F.; Aguilera, J.; Lopez Figueroa, F.; de Gálvez, M.; Herrera-Ceballos, E. Antioxidant Activity of Mycosporine-like Amino Acids Isolated from Three Red Macroalgae and One Marine Lichen. J. Appl. Phycol. 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Amador-Castro, F.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Robust Natural Ultraviolet Filters from Marine Ecosystems for the Formulation of Environmental Friendlier Bio-Sunscreens. Sci. Total Environ. 2020, 749, 141576. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Álvarez, R.M.C.; Figueroa, F.L. Daily and Seasonal Variations of Optimum Quantum Yield and Phenolic Compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of Ambient Salinity and Copper on Brown Algae: 2. Interactive Effects on Phenolic Pool and Assessment of Metal Binding Capacity of Phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and Challenges for Industrial Production of Seaweed Bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization. Globefish Res. Programme 2018, 124, I. [Google Scholar]
- Palmieri, N.; Forleo, M.B. The Potential of Edible Seaweed within the Western Diet. A Segmentation of Italian Consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Methods in molecular biology; Humana Press Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2683-1. [Google Scholar]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed Aquaculture: Cultivation Technologies, Challenges and Its Ecosystem Services. ALGAE 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Israel, A.A.; Friedlander, M.; Neori, A. Biomass Yield, Photosynthesis and Morphological Expression of Ulva lactuca. Bot. Mar. 1995, 38, 297–302. [Google Scholar] [CrossRef]
- Kräbs, G.; Watanabe, M.; Wlencke, C. A Monochromatic Action Spectrum for the Photoinduction of the UV-Absorbing Mycosporine-like Amino Acid Shinorine in the Red Alga Chondrus crispus. Photochem. Photobiol. 2004, 79, 515–520. [Google Scholar] [CrossRef]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Effect of Light Quality on the Accumulation of Photosynthetic Pigments, Proteins and Mycosporine-like Amino Acids in the Red Alga Porphyra leucosticta (Bangiales, Rhodophyta). J. Photochem. Photobiol. B: Biol. 2005, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Shalev, R.; Ganor, T.; Strimling, S.; Ben-Amotz, A.; Klar, H.; Wax, Y. Seasonal Fluctuations of Growth Rate and Chemical Composition of Gracilaria Cf. Conferta in Outdoor Culture in Israel. In Proceedings of the Twelfth International Seaweed Symposium, Sao Paulo, Brazil, 27 July–1 August 1986; Ragan, M.A., Bird, C.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 501–507. [Google Scholar]
- Israel, A.; Martinez-Goss, M.; Friedlander, M. Effect of Salinity and PH on Growth and Agar Yield of Gracilaria tenuistipitata Var. Liui in Laboratory and Outdoor Cultivation. J. Appl. Phycol. 1999, 11, 543–549. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Korbee, N.; Abdala, R.; Jerez, C.G.; López-de la Torre, M.; Güenaga, L.; Larrubia, M.A.; Gómez-Pinchetti, J.L. Biofiltration of Fishpond Effluents and Accumulation of N-Compounds (Phycobiliproteins and Mycosporine-like Amino Acids) versus C-Compounds (Polysaccharides) in Hydropuntia cornea (Rhodophyta). Mar. Pollut. Bull. 2012, 64, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Marinho, G.S.; Holdt, S.L.; Angelidaki, I. Seasonal Variations in the Amino Acid Profile and Protein Nutritional Value of Saccharina latissima Cultivated in a Commercial IMTA System. J. Appl. Phycol. 2015, 27, 1991–2000. [Google Scholar] [CrossRef]
- Wallner, M.; Lobo, S.; Boccanera, N.; Da Silva, E.M. Biomass, Carrageenan Yield and Reproductive State of Hypnea musciformis (Rhodophyta: Gigartinales) under Natural and Experimental Cultivated Condition. Aquac. Res. 1992, 23, 443–451. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Israel, A.; Neori, A.; Martínez, B.; Malta, E.J.; Put, A.; Inken, S.; Marquardt, R.; Abdala, R.; Korbee, N. Effect of Nutrient Supply on Photosynthesis and Pigmentation to Short-Term Stress (UV Radiation) in Gracilaria conferta (Rhodophyta). Mar. Pollut. Bull. 2010, 60, 1768–1778. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of Mycosporine-Like Amino Acids from Gracilaria vermiculophylla (Rhodophyta) Cultured Through One Year in an Integrated Multi-Trophic Aquaculture (IMTA) System. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Barufi, J.B.; Korbee, N.; Oliveira, M.C.; Figueroa, F.L. Effects of N Supply on the Accumulation of Photosynthetic Pigments and Photoprotectors in Gracilaria tenuistipitata (Rhodophyta) Cultured under UV Radiation. J. Appl. Phycol. 2011, 23, 457–466. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Chapter Four—Salinity and Desiccation Induced Oxidative Stress Acclimation in Seaweeds. In Advances in Botanical Research; Bourgougnon, N., Ed.; Sea Plants; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 91–123. [Google Scholar]
- Peinado, N.K.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W. Ammonium and Uv Radiation Stimulate the Accumulation of Mycosporine-Like Amino Acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina1. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Huovinen, P.; Matos, J.; Pinto, I.S.; Figueroa, F.L. The Role of Ammonium in Photoprotection against High Irradiance in the Red Alga Grateloupia lanceola. Aquat. Bot. 2006, 84, 308–316. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors Controlling the Formation of UV-Absorbing Mycosporine-like Amino Acids in the Marine Red Alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are They Nature’s Sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of Mycosporine-like Amino Acids in Asparagopsis armata Grown in Tanks with Fishpond Effluents of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2008, 39, 692–699. [Google Scholar] [CrossRef]
- Karsten, U.; Franklin, L.A.; Lüning, K.; Wiencke, C. Natural Ultraviolet Radiation and Photosynthetically Active Radiation Induce Formation of Mycosporine-like Amino Acids in the Marine Macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I.; Figueroa, F.L.; Ulloa, N.; Morales, V.; Lovengreen, C. Ultraviolet-Absorbing Mycosporine-like Amino Acids in Red Macroalgae from Chile. Bot. Mar. 2004, 47, 21–29. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of Ammonium Influences Photosynthesis and the Accumulation of Mycosporine-like Amino Acids in Two Porphyra Species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular Photoprotection of Human Keratinocytes in Vitro by the Naturally Occurring Mycosporine-like Amino Acid Palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Mycosporine-like Amino Acids as Osmotic Solutes in a Community of Halophilic Cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240. [Google Scholar] [CrossRef]
- Vale, P. Effects of Light and Salinity Stresses in Production of Mycosporine-Like Amino Acids by Gymnodinium catenatum (Dinophyceae). Photochem. Photobiol. 2015, 91, 1112–1122. [Google Scholar] [CrossRef]
- Ramus, J.; Beale, S.I.; Mauzerall, D.; Howard, K.L. Changes in Photosynthetic Pigment Concentration in Seaweeds as a Function of Water Depth. Mar. Biol. 1976, 37, 223–229. [Google Scholar] [CrossRef]
- Beer, S.; Levy, I. Effects of Photon Fluence Rate and Light Spectrum Composition on Growth, Photosynthesis and Pigment Relations in Gracilaria sp. J. Phycol. 1983, 19, 516–522. [Google Scholar] [CrossRef]
- López-Figueroa, F.; Niell, F.X. Effects of Light Quality on Chlorophyll and Biliprotein Accumulation in Seaweeds. Mar. Biol. 1990, 104, 321–327. [Google Scholar] [CrossRef]
- Bird, K.T.; Habig, C.; DeBusk, T. Nitrogen Allocation and Storage Patterns in Gracilaria tikvahiae (Rhodophyta)1. J. Phycol. 1982, 18, 344–348. [Google Scholar] [CrossRef]
- Tandeau de Marsac, N.; Houmard, J. Adaptation of Cyanobacteria to Environmental Stimuli: New Steps towards Molecular Mechanisms. FEMS Microbiol. Rev. 1993, 10, 119–189. [Google Scholar] [CrossRef]
- Wulff, A.; ke Wängberg, S.-Å.; Sundbäck, K.; Nilsson, C.; Underwood, G.J.C. Effects of UVB Radiation on a Marine Microphytobenthic Community Growing on a Sand-Substratum under Different Nutrient Conditions. Limnol. Oceanogr. 2000, 45, 1144–1152. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive Chlorophyll Composition in the Main Edible Seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Gallo, M.; Baldi, F. Phycoerythrin Productivity and Diversity from Five Red Macroalgae. J. Appl. Phycol. 2018, 30, 2523–2531. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Chapter Eleven—Phycoerythrins: Valuable Proteinic Pigments in Red Seaweeds. In Advances in Botanical Research; Bourgougnon, N., Ed.; Sea Plants; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 321–343. [Google Scholar]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and Utilization of Seaweed Pigments in Food Processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Šubarić, D.; Roje, M.; Babić, J.; Jerković, I.; Jokić, S. Recent Advances on Macroalgal Pigments and Their Biological Activities (2016–2021). Algal Res. 2022, 65, 102748. [Google Scholar] [CrossRef]
- Clark, N.F.; Taylor-Robinson, A.W. COVID-19 Therapy: Could a Chlorophyll Derivative Promote Cellular Accumulation of Zn2+ Ions to Inhibit SARS-CoV-2 RNA Synthesis? Front. Plant Sci. 2020, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.F.; Taylor-Robinson, A.W. COVID-19 Therapy: Could a Copper Derivative of Chlorophyll a Be Used to Treat Lymphopenia Associated With Severe Symptoms of SARS-CoV-2 Infection? Front. Med. 2021, 8, 620175. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.-S.; Hsu, Y.-T.; Hsu, Y.-T.; Wu, T.-M.; Lee, T.-M. Hypersalinity and Hydrogen Peroxide Upregulation of Gene Expression of Antioxidant Enzymes in Ulva Fasciata Against Oxidative Stress. Mar. Biotechnol. 2008, 11, 199. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Gupta, V.; Reddy, C.R.K.; Jha, B. Biochemical Responses of Red Alga Gracilaria corticata (Gracilariales, Rhodophyta) to Salinity Induced Oxidative Stress. J. Exp. Mar. Biol. Ecol. 2010, 391, 27–34. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive Phenolic Compounds from Algae. In Bioactive Compounds from Marine Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 113–129. ISBN 978-1-118-41289-3. [Google Scholar]
- Perini, V.; Bracken, M.E.S. Nitrogen Availability Limits Phosphorus Uptake in an Intertidal Macroalga. Oecologia 2014, 175, 667–676. [Google Scholar] [CrossRef]
- Lesser, M.P.; Cullen, J.J.; Neale, P.J. Carbon Uptake in a Marine Diatom During Acute Exposure to Ultraviolet B Radiation: Relative Importance of Damage and Repair. J. Phycol. 1994, 30, 183–192. [Google Scholar] [CrossRef]
- Schuenhoff, A.; Mata, L.; Santos, R. The Tetrasporophyte of Asparagopsis armata as a Novel Seaweed Biofilter. Aquaculture 2006, 252, 3–11. [Google Scholar] [CrossRef]
- Zubia, M.; Freile-Pelegrín, Y.; Robledo, D. Photosynthesis, Pigment Composition and Antioxidant Defences in the Red Alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta) under Environmental Stress. J. Appl. Phycol. 2014, 26, 2001–2010. [Google Scholar] [CrossRef]
- Lobban, C.S.; Harrison, P.J. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1994; ISBN 978-0-521-40897-4. [Google Scholar]
- Karsten, U. Seaweed Acclimation to Salinity and Desiccation Stress. In Seaweed Biology; Wiencke, C., Bischof, K., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2012; Volume 219, pp. 87–107. ISBN 978-3-642-28450-2. [Google Scholar]
- Dring, M.J. Stress Resistance and Disease Resistance in Seaweeds: The Role of Reactive Oxygen Metabolism. In Advances in Botanical Research; Incorporating Advances in Plant Pathology; Academic Press: Cambridge, MA, USA, 2005; Volume 43, pp. 175–207. [Google Scholar]
- Liu, F.; Pang, S.J. Stress Tolerance and Antioxidant Enzymatic Activities in the Metabolisms of the Reactive Oxygen Species in Two Intertidal Red Algae Grateloupia turuturu and Palmaria palmata. J. Exp. Mar. Biol. Ecol. 2010, 382, 82–87. [Google Scholar] [CrossRef]
- Kirst, G.O. Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Luo, M.B.; Liu, F. Salinity-Induced Oxidative Stress and Regulation of Antioxidant Defense System in the Marine Macroalga Ulva prolifera. J. Exp. Mar. Biol. Ecol. 2011, 409, 223–228. [Google Scholar] [CrossRef]
- Lu, I.-F.; Sung, M.-S.; Lee, T.-M. Salinity Stress and Hydrogen Peroxide Regulation of Antioxidant Defense System in Ulva fasciata. Mar. Biol. 2006, 150, 1–15. [Google Scholar] [CrossRef]
- Jahnke, L.S.; White, A.L. Long-Term Hyposaline and Hypersaline Stresses Produce Distinct Antioxidant Responses in the Marine Alga Dunaliella tertiolecta. J. Plant Physiol. 2003, 160, 1193–1202. [Google Scholar] [CrossRef]
- Alvarez-Gómez, F.; Korbee, N.; Figueroa, F.L.; Alvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Analysis of Antioxidant Capacity and Bioactive Compounds in Marine Macroalgal and Lichenic Extracts Using Different Solvents and Evaluation Methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant Activities and Phenolics Content of Eight Species of Seaweeds from North Borneo. J. Appl. Phycol. 2008, 20, 367. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–Visible Radiation on Growth, Photosynthesis, Pigment Accumulation and UV-Absorbing Compounds in the Red Macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Effects of UV Radiation on Photosynthesis, Antioxidant Capacity and the Accumulation of Bioactive Compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Biondi, D.M.; Amico, V. Antioxidant Activity of Extracts of the Marine Algal Genus Cystoseira in a Micellar Model System. J. Appl. Phycol. 2001, 13, 403–407. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking Beyond the Terrestrial: The Potential of Seaweed Derived Bioactives to Treat Non-Communicable Diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef]
- Ismail, G.A. Biochemical Composition of Some Egyptian Seaweeds with Potent Nutritive and Antioxidant Properties. Food Sci. Technol. 2017, 37, 294–302. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, Antioxidant, and Bactericide Capacities of Phlorotannins from the Brown Macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of in Vivo and in Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hupel, M.; Poupart, N.; Ar Gall, E. Development of a New in Vitro Method to Evaluate the Photoprotective Sunscreen Activity of Plant Extracts against High UV-B Radiation. Talanta 2011, 86, 362–371. [Google Scholar] [CrossRef]
- Malsawmtluangi, C. Determination of Sun Protection Factor (SPF) Number of Some Aqueous Herbal Extracts. J. Appl. Pharm. Sci. 2013, 3, 150–151. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. In Vitro Sun Protection Factor Determination of Herbal Oils Used in Cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Ersalina, E.B.; Abdillah, A.A.; Sulmartiwi, L. Potential of Caulerpa Racemosa Extracts as Sunscreen Creams. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012007. [Google Scholar] [CrossRef]
- Zárate, R.; Portillo, E.; Teixidó, S.; Carvalho, M.A.A.P.d.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
- Chaves-Peña, P.; de la Coba, F.; Figueroa, F.L.; Korbee, N. Quantitative and Qualitative HPLC Analysis of Mycosporine-Like Amino Acids Extracted in Distilled Water for Cosmetical Uses in Four Rhodophyta. Mar. Drugs 2020, 18, 27. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and Structure of Two New Amino Acids, Palythinol and Palythene, from the Zoanthid Palythoa tubercolosa. Tetrahedron Lett. 1978, 19, 4909–4912. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and Structure of a New Amino Acid, Palythine, from the Zoanthid Palythoa Tuberculosa. Tetrahedron Lett. 1978, 19, 2299–2300. [Google Scholar] [CrossRef]
- Tsujino, I.; Yabe, K.; Sekikawa, I. Isolation and Structure of a New Amino Acid, Shinorine, from the Red Alga Chondrus Yendoi Yamada et Mikami. Bot. Mar. 1980, 23, 65–68. [Google Scholar]
- Dunlap, W.C.; Chalker, B.E.; Oliver, J.K. Bathymetric Adaptations of Reef-Building Corals at Davies Reef, Great Barrier Reef, Australia. III. UV-B Absorbing Compounds. J. Exp. Mar. Biol. Ecol. 1986, 104, 239–248. [Google Scholar] [CrossRef]
- Gleason, D.F. Differential Effects of Ultraviolet Radiation on Green and Brown Morphs of the Caribbean Coral Porites astreoides. Limnol. Oceanogr. 1993, 38, 1452–1463. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of Chlorophylls and Carotenoids from Freshwater Algae Using Different Extraction Methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalreu, V. On Tyrosine and Trytophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Mansur, J.D.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Aloanis, A.A.; Karundeng, M.; Paat, V.I.; Tengker, S.M.T.; Siwu, O. Sun Protecting Factor Value of the Ficus benjamina Linn. Fruits Extract. J. Phys. Conf. Ser. 2021, 1968, 012009. [Google Scholar] [CrossRef]
- Kasitowati, R.D.; Wahyudi, A.; Asmara, R.; Aliviyanti, D.; Iranawati, F.; Panjaitan, M.A.P.; Pratiwi, D.C.; Arsad, S. Identification Photoprotective Activity of Marine Seaweed: Eucheuma sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012014. [Google Scholar] [CrossRef]
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–24. ISBN 978-3-540-74181-7. [Google Scholar]
- Borowitzka, M.A. Commercial Production of Microalgae: Ponds, Tanks, Tubes and Fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of Monosaccharides in Marine Macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Ben-Amotz, A. New Mode of Dunaliella Biotechnology: Two-Phase Growth for β-Carotene Production. J. Appl. Phycol. 1995, 7, 65–68. [Google Scholar] [CrossRef]
- Pérez-Legaspi, I.A.; Valadez-Rocha, V.; Ortega-Clemente, L.A.; Jiménez-García, M.I. Microalgal Pigment Induction and Transfer in Aquaculture. Rev. Aquac. 2020, 12, 1323–1343. [Google Scholar] [CrossRef]
- Chopin, T. Integrated Multi-Trophic Aquaculture (IMTA) Will Also Have Its Place When Aquaculture Moves to the Open Ocean. Fish Farmer 2008, 31, 40–41. [Google Scholar]


| Parameter | Ulva rigida | Gracilaria conferta | Hypnea musciformis |
|---|---|---|---|
| Antioxidant activity (µg TE mg−1) | 3.5 ± 0.15 | 2.3 ± 0.25 | 4.5 ± 0.31 |
| Phenolic compounds (µg PE mg−1) | 4.7 ± 0.3 | - | |
| SPF (mg mL−1) | 3.82 ± 0.16 | 1.82 ± 0.1 | 3.12 ± 0.12 |
| Total MAAs (mg g−1) | - | 1.8 ± 0.7 | 3.3 ± 0.5 |
| Palythine (mg g−1) | - | 0.2 ± 0.06 | 0.8 ± 0.16 |
| Asterina-330 (mg g−1) | - | 0.02 ± 0.02 | 0.06 ± 0.01 |
| Palythinol (mg g−1) | - | 1.5 ± 0.6 | 2.05 ± 0.7 |
| Shinorine (mg g−1) | - | 0.3 ± 0.1 | 1 ± 0.2 |
| Porphyra-334 (mg g−1) | - | 0.1 ± 0.02 | 0.05 ± 0.01 |
| Chlorophyll a (mg g−1) | 3.9 ± 0.2 | 0.5 ± 0.1 | 1.2 ± 0.2 |
| Phycoerythrin (mg g−1) | - | 4.1 ± 1.1 | 7.5 ± 1.8 |
| Phycocyanin (mg g−1) | - | 1.6 ± 0.5 | 6.6 ± 1.2 |
| Species | Culture Condition/Treatment | Chlorophyll a (mg g−1 DW) | Chlorophyll b (mg g−1 DW) | Chlorophyll d (mg g−1 DW) | Total Chlorophylls (mg g−1 DW) | Phycoerythrin (mg g−1 DW) | Phycocyanin (mg g−1 DW) |
|---|---|---|---|---|---|---|---|
| Ulva rigida | Control | 0.71 ± 0.05 | 0.36 ± 0.02 | - | 1.06 ± 0.06 | - | - |
| Fish + Shade (initial two weeks) * | 3.89 ± 0.17 | 2.08 ± 0.09 * | - | 5.9 ± 0.24 * | - | - | |
| Fish + shade | 1.66 ± 0.12 | 1.06 ± 0.06 | - | 2.61 ± 0.18 | - | - | |
| Fish + sun | 1.80 ± 0.28 | 1.12 ± 0.18 | - | 2.78 ± 0.43 | - | - | |
| Seawater + sun | 0.31 ± 0.11 | 0.18 ± 0.07 | - | 0.47 ± 0.17 | - | - | |
| High salinity | 0.38 ± 0.04 | 0.23 ± 0.03 | - | 0.58 ± 0.07 | - | - | |
| Gracilaria conferta | Control | 0.12 ± 0.01 | - | 0.002 ± 0.001 | 0.12 ± 0.01 | 0.57 ± 0.05 | 0.31 ± 0.03 |
| Fish + Shade (initial two weeks) * | 0.47 ± 0.09 | - | 0.01 ± 0.002 | 0.49 ± 0.08 * | 4.14 ± 1.16 * | 1.64 ± 0.48 * | |
| Fish + shade | 0.34 ± 0.03 | - | 0.01 ± 0.002 | 0.38 ± 0.04 | 2.5 ± 0.25 | 1.06 ± 0.05 | |
| Fish + sun | 0.38 ± 0.08 | - | 0.005 ± 0.001 | 0.45 ± 0.11 | 2.03 ± 0.68 | 0.95 ± 0.29 | |
| Seawater + sun | 0.14 ± 0.02 | - | 0.004 ± 0.002 | 0.16 ± 0.03 | 0.64 ± 0.08 | 0.35 ± 0.03 | |
| High salinity | 0.09 ± 0.01 | - | 0.01 ± 0.0004 | 0.11 ± 0.01 | 0.36 ± 0.04 | 0.2 ± 0.02 | |
| Hypnea musciformis | Control | 0.34 ± 0.02 | - | 0.01 ± 0.001 | 0.39 ± 0.02 | 1.19 ± 0.08 | 0.6 ± 0.06 |
| Fish + Shade (initial two weeks) * | 1.18 ± 0.19 | - | 0.05 ± 0.01 | 1.32 ± 0.2 * | 7.49 ± 1.77 * | 6.63 ± 1.25 * | |
| Fish + shade | 0.68 ± 0.18 | - | 0.04 ± 0.01 | 0.82 ± 0.21 | 3.88 ± 0.98 | 3.47 ± 1.03 | |
| Fish + sun | 0.62 ± 0.22 | - | 0.03 ± 0.01 | 0.75 ± 0.25 | 2.89 ± 1.12 | 2.5 ± 1.08 | |
| Seawater + sun | 0.31 ± 0.06 | - | 0.01 ± 0.002 | 0.36 ± 0.07 | 1.06 ± 0.32 | 0.97 ± 0.29 | |
| High salinity | 0.35 ± 0.02 | - | 0.01 ± 0.001 | 0.4 ± 0.02 | 1.27 ± 0.18 | 1.28 ± 0.1 |
 ), considerably higher (green,
), considerably higher (green,  ), lower (red,
), lower (red,  ), no major effect (—).
), no major effect (—).
 ), considerably higher (green,
), considerably higher (green,  ), lower (red,
), lower (red,  ), no major effect (—).
), no major effect (—).| Treatment | Control Seawater + Shade | Fish Effluent + Shade | Fish Effluent + Shade | Fish Effluent + Sun | Seawater + Sun Shock | Salt Shock |
|---|---|---|---|---|---|---|
| Time | Initial two weeks | Third week | ||||
| Total MAAs | — |  |  |  | — |  |
| Chlorophylls | — |  |  |  |  |  |
| Phycobiliproteins | — |  |  |  |  |  |
| Antioxidant activity | — |  |  |  | — |  |
| Phenolic compounds | — |  |  |  | — |  |
| SPF | — |  |  |  | — |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. https://doi.org/10.3390/md20120767
Ashkenazi DY, Figueroa FL, Korbee N, García-Sánchez M, Vega J, Ben-Valid S, Paz G, Salomon E, Israel Á, Abelson A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Marine Drugs. 2022; 20(12):767. https://doi.org/10.3390/md20120767
Chicago/Turabian StyleAshkenazi, Doron Yehoshua, Félix L. Figueroa, Nathalie Korbee, Marta García-Sánchez, Julia Vega, Shoshana Ben-Valid, Guy Paz, Eitan Salomon, Álvaro Israel, and Avigdor Abelson. 2022. "Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds" Marine Drugs 20, no. 12: 767. https://doi.org/10.3390/md20120767
APA StyleAshkenazi, D. Y., Figueroa, F. L., Korbee, N., García-Sánchez, M., Vega, J., Ben-Valid, S., Paz, G., Salomon, E., Israel, Á., & Abelson, A. (2022). Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Marine Drugs, 20(12), 767. https://doi.org/10.3390/md20120767