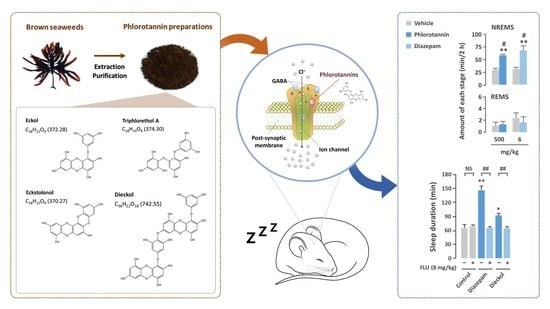
Marine Polyphenol Phlorotannins as a Natural Sleep Aid for Treatment of Insomnia: A Review of Sedative–Hypnotic Effects and Mechanism of Action
Abstract
:1. Introduction
2. Extraction and Purification of Phlorotannins
3. Safety and Toxicity of Phlorotannins
3.1. In Vitro
3.2. In Vivo
3.3. Clinical Human Studies
3.4. The Regulation of Phlorotannins as Human Supplements
4. Sedative–Hypnotic Effects of Phlorotannins in Animal Models
4.1. Phlorotannin Preparations
4.2. Individual Phlorotannin Compounds
5. Sleep-Promoting Effects of Phlorotannins in Clinical Trials
6. Action Mechanism of Phlorotannins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.H.; Jeon, Y.J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory Activity of Brown Algal Phlorotannins against Hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; He, X.; Pei, S.; Li, D. Effects of Brown Seaweed Polyphenols, a Class of Phlorotannins, on Metabolic Disorders: Via Regulation of Fat Function. Food Funct. 2021, 12, 2378–2388. [Google Scholar] [CrossRef]
- Cho, S.; Yoon, M.; Pae, A.N.; Jin, Y.H.; Cho, N.C.; Takata, Y.; Urade, Y.; Kim, S.; Kim, J.S.; Yang, H.; et al. Marine Polyphenol Phlorotannins Promote Non-Rapid Eye Movement Sleep in Mice via the Benzodiazepine Site of the GABAA Receptor. Psychopharmacology 2014, 231, 2825–2837. [Google Scholar] [CrossRef] [PubMed]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Jeong, G.J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A Brown Algal Phlorotannin with Biological Potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef]
- Kwon, S.; Yoon, M.; Lee, J.; Moon, K.D.; Kim, D.; Kim, S.B.; Cho, S. A Standardized Phlorotannin Supplement Attenuates Caffeine-Induced Sleep Disruption in Mice. Nutrients 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia Cava (Phaeophyceae): Biological Activities and Potential Health Benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.R.; Yoon, E.; Kim, O.Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia Cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Drugs 2018, 17, 7. [Google Scholar] [CrossRef]
- Kang, M.C.; Wijesinghe, W.A.J.P.; Lee, S.H.; Kang, S.M.; Ko, S.C.; Yang, X.; Kang, N.; Jeon, B.T.; Kim, J.; Lee, D.H.; et al. Dieckol Isolated from Brown Seaweed Ecklonia Cava Attenuates Type II Diabetes in Db/Db Mouse Model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical Components and Its Antioxidant Properties in Vitro: An Edible Marine Brown Alga, Ecklonia Cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.K.; Yu, J.M.; Kim, S.T.; Kim, G.H.; Park, D.W.; Lee, D.I.; Joo, S.S. An Aβ42 Uptake and Degradation via Rg3 Requires an Activation of Caveolin, Clathrin and Aβ-Degrading Enzymes in Microglia. Eur. J. Pharmacol. 2015, 758, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Li, X.F.; Jin, L.F.; Zhao, Y.; Zhu, G.J.; Shen, W.Z. Dieckol Inhibits Non-Small–Cell Lung Cancer Cell Proliferation and Migration by Regulating the PI3K/AKT Signaling Pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-Rich Fraction from Ishige Foliacea Brown Seaweed Prevents the Scopolamine-Induced Memory Impairment via Regulation of ERK-CREB-BDNF Pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Baek, N.I.; Kim, D.; Kang, S.M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the Edible Brown Seaweed Ecklonia Cava Kjellman Induce Sleep via Positive Allosteric Modulation of Gamma-Aminobutyric Acid Type A-Benzodiazepine Receptor: A Novel Neurological Activity of Seaweed Polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef]
- Kwon, S.; Jung, J.H.; Cho, S.; Moon, K.D.; Lee, J. Dieckol Is a Natural Positive Allosteric Modulator of GABAA-Benzodiazepine Receptors and Enhances Inhibitory Synaptic Activity in Cultured Neurons. Nutr. Neurosci. 2021, 24, 835–842. [Google Scholar] [CrossRef]
- Yoon, M.; Kim, J.S.; Seo, S.; Lee, K.; Um, M.Y.; Lee, J.; Jung, J.; Cho, S. Dieckol, a Major Marine Polyphenol, Enhances Non-Rapid Eye Movement Sleep in Mice via the GABAA-Benzodiazepine Receptor. Front. Pharmacol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- McCarthy, B.; O’Neill, G.; Abu-Ghannam, N. Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges. Mar. Drugs 2022, 20, 493. [Google Scholar] [CrossRef]
- Hu, Z.; Oh, S.; Ha, T.W.; Hong, J.T.; Oh, K.W. Sleep-Aids Derived from Natural Products. Biomol. Ther. 2018, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, D.N. New and Emerging Pharmacotherapeutic Approaches for Insomnia. Int. Rev. Psychiatry 2014, 26, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Drake, C. Evolution of Insomnia: Current Status and Future Direction. Sleep Med. 2004, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Shimizu, M. Natural sleep aids and polyphenols as treatments for insomnia. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–151. [Google Scholar]
- Fang, X.S.; Hao, J.F.; Zhou, H.Y.; Zhu, L.X.; Wang, J.H.; Song, F.Q. Pharmacological studies on the sedative-hypnotic effect of Semen ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010, 17, 75–80. [Google Scholar] [CrossRef]
- Yoon, M.; Kim, J.S.; Um, M.Y.; Yang, H.; Kim, J.; Kim, Y.T.; Lee, C.; Kim, S.B.; Kwon, S.; Cho, S. Extraction Optimization for Phlorotannin Recovery from the Edible Brown Seaweed Ecklonia Cava. J. Aquat. Food Prod. Technol. 2017, 26, 801–810. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of Polyphenolics from Plant Material for Functional Foods—Engineering and Technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Sridhar, A.; Vaishampayan, V.; Senthil Kumar, P.; Ponnuchamy, M.; Kapoor, A. Extraction Techniques in Food Industry: Insights into Process Parameters and Their Optimization. Food Chem. Toxicol. 2022, 166, 113207. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Huang, Z.; Bi, R.; Musil, S.; Pétursdóttir, Á.H.; Luo, B.; Zhao, P.; Tan, X.; Jia, Y. Arsenic Species and Their Health Risks in Edible Seaweeds Collected along the Chinese Coastline. Sci. Total Environ. 2022, 847, 157429. [Google Scholar] [CrossRef] [PubMed]
- Šlejkovec, Z.; Kápolna, E.; Ipolyi, I.; van Elteren, J.T. Arsenosugars and Other Arsenic Compounds in Littoral Zone Algae from the Adriatic Sea. Chemosphere 2006, 63, 1098–1105. [Google Scholar] [PubMed]
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, P. An Insight of Environmental Contamination of Arsenic on Animal Health. Emerg. Contam. 2017, 3, 17–22. [Google Scholar]
- Chen, C.J.; Wang, S.L.; Chiou, J.M.; Tseng, C.H.; Chiou, H.Y.; Hsueh, Y.M.; Chen, S.Y.; Wu, M.M.; Lai, M.S. Arsenic and Diabetes and Hypertension in Human Populations: A Review. Toxicol. Appl. Pharmacol. 2007, 222, 298–304. [Google Scholar] [CrossRef]
- Singh, A.P.; Goel, R.K.; Kaur, T. Mechanisms Pertaining to Arsenic Toxicity. Toxicol. Int. 2011, 18, 87–93. [Google Scholar]
- Engel, R.R.; Hopenhayn-Rich, C.; Receveur, O.; Smith, A.H. Vascular Effects of Chronic Arsenic Exposure: A Review. Epidemiol. Rev. 1994, 16, 184–209. [Google Scholar]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.J.; Cho, S. Enrichment and Purification of Marine Polyphenol Phlorotannins Using Macroporous Adsorption Resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef]
- Pal, P.; Sen, M.; Manna, A.; Pal, J.; Pal, P.; Roy, S.; Roy, P. Contamination of Groundwater by Arsenic: A Review of Occurrence, Causes, Impacts, Remedies and Membrane-Based Purification. J. Integr. Environ. Sci. 2009, 6, 295–316. [Google Scholar]
- Jiang, Z.; Wang, Y. Stepwise Elution by High-Speed Counter-Current Chromatography Combined with a Modified Macroporous Resin to Isolate and Purify Antioxidant Phenolics from Discarded Jackfruit (Artocarpusheterophyllus Lam.) Peels. Anal Methods 2020, 12, 4674–4681. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, Y.; Lin, Z.; Han, X.; Liao, W. Macroporous Resin Purification and Characterization of Flavonoids from Platycladus Orientalis (L.) Franco and Their Effects on Macrophage Inflammatory Response. Food Funct. 2017, 8, 86–95. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K.; Li, Y.; Li, Y.X. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia Stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular Activities and Docking Studies of Eckol Isolated from Ecklonia Cava (Laminariales, Phaeophyceae) as Potential Tyrosinase Inhibitor. Algae 2015, 30, 163–170. [Google Scholar]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Sameera Madushan Fernando, P.D.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia Cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia Cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Ko, S.C.; Cha, S.H.; Heo, S.J.; Lee, S.H.; Kang, S.M.; Jeon, Y.J. Protective Effect of Ecklonia Cava on UVB-Induced Oxidative Stress: In Vitro and in Vivo Zebrafish Model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Le, Q.T.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Inhibitory Effects of Polyphenols Isolated from Marine Alga Ecklonia Cava on Histamine Release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia Cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid.-Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, Y.; Shi, X.; Kim, S.-K. Inhibition of the expression on MMP-2, 9 and morphological changes via human fibrosarcoma cell line by 6,6′-bieckol from marine alga Ecklonia cava. BMB Rep. 2010, 43, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory Effect of Phlorotannins Isolated from Ecklonia Cava on Mushroom Tyrosinase Activity and Melanin Formation in Mouse B16F10 Melanoma Cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In Vitro and Cellular Antioxidant Activities of Seaweed Extracts Prepared from Five Brown Seaweeds Harvested in Spring from the West Coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Quéguineur, B.; Goya, L.; Ramos, S.; Martín, M.A.; Mateos, R.; Guiry, M.D.; Bravo, L. Effect of Phlorotannin-Rich Extracts of Ascophyllum Nodosum and Himanthalia Elongata (Phaeophyceae) on Cellular Oxidative Markers in Human HepG2 Cells. J. Appl. Phycol. 2013, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Fernando, P.D.S.M.; Cho, S.J.; Ahn, M.J.; Hyun, J.W. Diphlorethohydroxycarmalol Attenuates Fine Particulate Matter-Induced Subcellular Skin Dysfunction. Mar. Drugs 2019, 17, 95. [Google Scholar] [CrossRef] [Green Version]
- Kang, J., II; Kim, S.C.; Kim, M.K.; Boo, H.J.; Jeon, Y.J.; Koh, Y.S.; Yoo, E.S.; Kang, S.M.; Kang, H.K. Effect of Dieckol, a Component of Ecklonia Cava, on the Promotion of Hair Growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal Effect of Phlorotannins from the Brown Alga Ecklonia Kurome on Red Tide Microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Kang, M.C.; Cha, S.H.; Wijesinghe, W.A.J.P.; Kang, S.M.; Lee, S.H.; Kim, E.A.; Song, C.B.; Jeon, Y.J. Protective Effect of Marine Algae Phlorotannins against AAPH-Induced Oxidative Stress in Zebrafish Embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Choi, H.S.; Jeon, H.J.; Lee, O.H.; Lee, B.Y. Dieckol, a Major Phlorotannin in Ecklonia Cava, Suppresses Lipid Accumulation in the Adipocytes of High-Fat Diet-Fed Zebrafish and Mice: Inhibition of Early Adipogenesis via Cell-Cycle Arrest and AMPKα Activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef]
- Hwang, H.; Terada, M.; Shin, H.-C. Single Dose Oral Toxicity and 4-Weeks Repeated Oral Toxicity Studies of Ecklonia Cava Extract. Seikatsu Eisei 2008, 52, 282–289. [Google Scholar]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant Activity in Vitro and in Vivo of Two Fucus Vesiculosus Extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Yang, H.; Yoon, M.; Kim, J.; Cho, S. Acute Oral Toxicity of Phlorotannins in Beagle Dogs. Kor. J. Fish Aquat. Sci. 2014, 47, 356–362. [Google Scholar]
- Shin, H.C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-Week Oral Supplementation of Ecklonia Cava Polyphenols on Anthropometric and Blood Lipid Parameters in Overweight Korean Individuals: A Double-Blind Randomized Clinical Trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (Poly)Phenol-Rich Extract from the Brown Algae Ascophyllum Nodosum on DNA Damage and Antioxidant Activity in an Overweight or Obese Population: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, M.E.; Couture, P.; Lamarche, B. A Randomised Crossover Placebo-Controlled Trial Investigating the Effect of Brown Seaweed (Ascophyllum Nodosum and Fucus Vesiculosus) on Postchallenge Plasma Glucose and Insulin Levels in Men and Women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Kim, J.Y.; Han, J.K.; Kim, J.; Yang, H.; Yoon, M.; Kim, J.; Kang, S.W.; Cho, S. Phlorotannin Supplement Decreases Wake after Sleep Onset in Adults with Self-Reported Sleep Disturbance: A Randomized, Controlled, Double-Blind Clinical and Polysomnographic Study. Phytother. Res. 2018, 32, 698–704. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Ecklonia Cava Phlorotannins as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e05003. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus Vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration (FDA). Recent Updates to the Notifications for New Dietary Ingredients. 2022. Available online: https://www.fda.gov/food/new-dietary-ingredients-ndi-notification-process/submitted-75-day-premarket-notifications-new-dietary-ingredients (accessed on 21 October 2022).
- Ministry of Food and Drug Safety (MFDS). Functional Ingredients for Health Functional Foods That Help Improve Sleep Quality. 2022. Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/searchHomeHFDetail.do?prdlstReportLedgNo=2015021000031576 (accessed on 21 October 2022).
- Cho, S.; Han, D.; Kim, S.B.; Yoon, M.; Yang, H.; Jin, Y.H.; Jo, J.; Yong, H.; Lee, S.H.; Jeon, Y.J.; et al. Depressive Effects on the Central Nervous System and Underlying Mechanism of the Enzymatic Extract and Its Phlorotannin-Rich Fraction from Ecklonia Cava Edible Brown Seaweed. Biosci. Biotechnol. Biochem. 2012, 76, 163–168. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Yoon, M.; Kim, J.; Kim, D.; Kim, J.; Kim, S.B. Arousal Inhibitory Effect of Phlorotannins on Caffeine in Pentobarbital-Induced Mice. Fish Aquatic. Sci. 2014, 17, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Askari, V.R.; Rahimi, V.B.; Ghorbani, A.; Rakhshandeh, H. Hypnotic Effect of Ocimum Basilicum on Pentobarbital-Induced Sleep in Mice. Iran Red. Crescent Med. J. 2016, 18, e24261. [Google Scholar] [CrossRef] [Green Version]
- Rakhshandeh, H.; Heidari, A.; Pourbagher-Shahri, A.M.; Rashidi, R.; Forouzanfar, F. Hypnotic Effect of A. Absinthium Hydroalcoholic Extract in Pentobarbital-Treated Mice. Neurol. Res. Int. 2021, 2021, 5521019. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Tajani, A.S.; Hosseini, A.; Rakhshandeh, H. Evaluation of the Sleep-Prolonging Effect of Lagenaria Vulgaris and Cucurbita Pepo Extracts on Pentobarbital-Induced Sleep and Possible Mechanisms of Action. Medicina 2018, 54, 55. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Han, D.; Kim, J.; Yoon, M.; Yang, H.; Kim, J. Potential claims and evaluation methods for sleep-promoting effects of foods. Food Sci. Ind. 2013, 46, 8–22. [Google Scholar]
- Yoon, M.; Kim, J.S.; Jo, J.; Han, D.; Cho, S. Sleep-Promoting Effect of Ecklonia Cava: Ethanol Extract Promotes Non-Rapid Eye Movement Sleep in C57BL/6N Mice. Fish Aquatic. Sci. 2014, 17, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Masaki, M.; Aritake, K.; Tanaka, H.; Shoyama, Y.; Huang, Z.L.; Urade, Y. Crocin Promotes Non-Rapid Eye Movement Sleep in Mice. Mol. Nutr. Food Res. 2012, 56, 304–308. [Google Scholar] [CrossRef]
- Huang, Z.L.; Qu, W.M.; Eguchi, N.; Chen, J.F.; Schwarzschild, M.A.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine A2A, but Not A1, Receptors Mediate the Arousal Effect of Caffeine. Nat. Neurosci. 2005, 8, 858–859. [Google Scholar] [CrossRef]
- Revel, F.G.; Gottowik, J.; Gatti, S.; Wettstein, J.G.; Moreau, J.L. Rodent Models of Insomnia: A Review of Experimental Procedures That Induce Sleep Disturbances. Neurosci. Biobehav. Rev. 2009, 33, 874–899. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Ahn, G. Marine Algal Flavonoids and Phlorotannins; an Intriguing Frontier of Biofunctional Secondary Metabolites. Crit. Rev. Biotechnol. 2022, 42, 23–45. [Google Scholar] [CrossRef]
- Yoon, M.; Cho, S. Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Mar. Drugs 2018, 16, 139. [Google Scholar] [CrossRef]
- Lee, S.M.; Jeong, H.H.; Lee, J.C.; Park, M.Y.; Kim, S.C. A Clinical Case Study on the Effects of Acupuncture Therapy and Ecklonia Cava Extract on Sleep Disturbances in ALS Patients. J. Acupunct. Res. 2013, 30, 247–252. [Google Scholar] [CrossRef]
- Borja, N.L.; Daniel, K.L. Ramelteon for the treatment of insomnia. Clin. Ther. 2006, 28, 1540–1555. [Google Scholar] [CrossRef]
- Ebert, B.; Wafford, K.A.; Deacon, S. Treating Insomnia: Current and Investigational Pharmacological Approaches. Pharmacol. Ther. 2006, 112, 612–629. [Google Scholar] [CrossRef]
- Trevor, A.J.; Way, W.L. Sedative-Hypnotic Drugs. In Basic and Clinical Pharmacology, 12th ed.; Katzung, B.G., Ed.; McGraw-Hill Medical: New York, NY, USA, 2007; pp. 373–388. ISBN 978-0-07-176402-5. [Google Scholar]
- Erman, M.K. Therapeutic options in the treatment of insomnia. J. Clin. Psychiatr. 2005, 66, 18–23. [Google Scholar]
- Brogden, R.N.; Goa, K.L. Flumazenil. A Reappraisal of Its Pharmacological Properties and Therapeutic Efficacy as a Benzodiazepine Antagonist. Drugs 1991, 42, 1061–1089. [Google Scholar] [CrossRef]
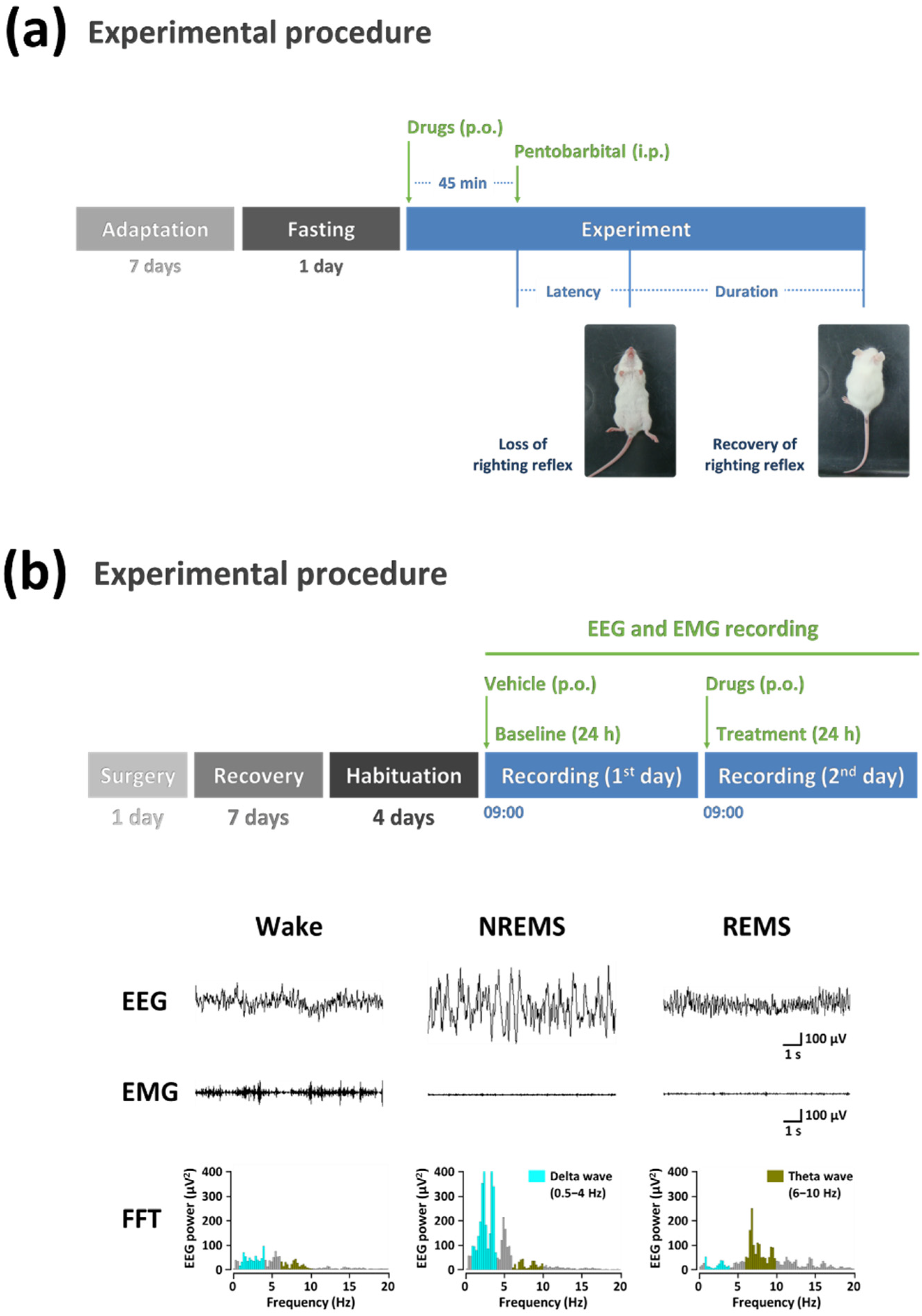


| Methods | Pentobarbital-Induced Sleep Test | Polygraphic Recordings |
|---|---|---|
| Animal | ICR mice or SD rats | C57BL/6N mice or SD rats |
| Measurements | Righting reflex | EEG and EMG |
| Evaluation markers | Sleep latency, sleep duration, and sleep onset | Sleep latency, amount of NREMS and REMS, delta activity, sleep–wake episodes |
| Advantages | Short assay time, possible to screen many samples | Assessment of both sleep quantity and quality |
| Disadvantages | Impossible to evaluate sleep quality | Long assay time, high cost |
| Compound | Methods (Dose) and Activities |
|---|---|
| Eckol | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] |
| Eckstolonol | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] Polygraphic recordings (50 mg/kg) NREMS ↑, latency ↓ Delta activity − [4] |
| Dieckol | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] Polygraphic recordings (150 mg/kg) NREMS ↑, latency ↓ Delta activity − [19] |
| Triphlorethol A | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] Polygraphic recordings (50 mg/kg) NREMS ↑, latency ↓ Delta activity − [83] |
| Fucodiphlorethol G | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] |
| 6,6′-Bieckol | Pentobarbital-induced sleep test (50 mg/kg) duration ↑ [4] |
| Samples | Binding Affinity to the BZD Binding Site (IC50) | Functional Assay for the GABAA Receptors |
|---|---|---|
| Preparations from Ecklonia cava | ||
| Enzymatic extract | 1.409 mg/mL [72] | - |
| Methanol extract | 0.392 mg/mL [17] | - |
| Ethanol extract (EE) | 0.127 mg/mL [17] | - |
| Ethyl acetate fraction from EE | 0.019 mg/mL [17] | - |
| Butanol fraction from EE | 0.103 mg/mL [17] | - |
| Hexane fraction from EE | 0.141 mg/mL [17] | - |
| Purified phlorotannin supplement | 0.012 mg/mL [4] | Positive allosteric activation to the GABAA receptors [18] |
| Individual phlorotannin compounds | ||
| Eckstolonol | 2.422 μM [17] | Positive allosteric activation to the GABAA receptors [4] |
| Eckol | 1.739 μM [17] | - |
| Triphlorethol-A | 7.180 μM [17] | - |
| Dieckol | 4.991 μM [17] | Positive allosteric activation to the GABAA receptors [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, D.; Um, M.Y.; Yoon, M.; Choi, J.-S.; Choi, Y.H.; Cho, S. Marine Polyphenol Phlorotannins as a Natural Sleep Aid for Treatment of Insomnia: A Review of Sedative–Hypnotic Effects and Mechanism of Action. Mar. Drugs 2022, 20, 774. https://doi.org/10.3390/md20120774
Kim S, Kim D, Um MY, Yoon M, Choi J-S, Choi YH, Cho S. Marine Polyphenol Phlorotannins as a Natural Sleep Aid for Treatment of Insomnia: A Review of Sedative–Hypnotic Effects and Mechanism of Action. Marine Drugs. 2022; 20(12):774. https://doi.org/10.3390/md20120774
Chicago/Turabian StyleKim, Seonghui, Duhyeon Kim, Min Young Um, Minseok Yoon, Jae-Suk Choi, Yung Hyun Choi, and Suengmok Cho. 2022. "Marine Polyphenol Phlorotannins as a Natural Sleep Aid for Treatment of Insomnia: A Review of Sedative–Hypnotic Effects and Mechanism of Action" Marine Drugs 20, no. 12: 774. https://doi.org/10.3390/md20120774
APA StyleKim, S., Kim, D., Um, M. Y., Yoon, M., Choi, J.-S., Choi, Y. H., & Cho, S. (2022). Marine Polyphenol Phlorotannins as a Natural Sleep Aid for Treatment of Insomnia: A Review of Sedative–Hypnotic Effects and Mechanism of Action. Marine Drugs, 20(12), 774. https://doi.org/10.3390/md20120774