Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications
Abstract
:1. Introduction
2. Sponges
2.1. Biomaterials
2.2. Bioactive Molecules
3. Cnidarians
3.1. Biomaterials
3.2. Bioactive Molecules
4. Molluscs
4.1. Biomaterials
4.2. Bioactive Molecules
5. Echinoderms
5.1. Biomaterials
| Holoturoidea | Sea cucumbers (and other echinoderms) | Proteins/neutral carbohydrates | Mutable collagenous tissue (MCT) components | Mutable collagenous tissue/ECM components | design of an MCT-inspired synthetic material | [166] |
| Holoturoidea | Sea cucumbers (and other echinoderms) | Proteins/neutral carbohydrates | Mutable collagenous tissue (MCT) components | dermis/ECM components | design of an MCT-inspired stimuli-responsive synthetic nanocomposite | [156,157] |
| Holoturoidea | Sea cucumbers | Proteins/neutral carbohydrates | Mutable collagenous tissue (MCT) components | dermis/ECM components | design of mechanically tunable synthetic biomaterials | [167] |
| Holoturoidea | Sea cucumbers | Proteins/neutral carbohydrates | Mutable collagenous tissue (MCT) components | dermis/ECM components | biomimetic design of artificial polymer nanocomposites | [168] |
| Holoturoidea | Holothuria forskal, H. leucospilota, B. subrubra, P. graeffei | Proteins/neutral carbohydrates | Proteins rich in small side amino acid | Cuvier tubule | bioadhesives | [165] |
| Holoturoidea | Holothuria tubulosa | Proteins | Collagen | dermis/ECM components | membranes for guided tissue regeneration | [149,152] |
| Asteroidea | Pisaster giganteous | Bioceramics | High-magnesium calcite | ossicles (skeletal microstructure) | scaffold for mammalian cell culture | [161] |
| Asteroidea | Asterias rubens | Proteins/glycosylated proteins | Glycosylated proteins | tube feet | bioadhesives | [169,170] |
| Asteroidea | Echinaster sepositus | Proteins | Collagen | dermis/ECM components | membranes for guided tissue regeneration | [149] |
| Asteroidea | Asterias rubens | Proteins | Sea star footprint protein 1 (Sfp1) | tube feet | bioadhesives | [165,171] |
| Echinoidea | Heart urchins | Bioceramics | High-magnesium calcite | ossicles | production of bioceramic nanopowder | [172] |
| Echinoidea | Sea urchins | Bioceramics | High-magnesium calcite | ossicles (skeletal microstructure) | production of structured hydroxyapatite material | [173] |
| Echinoidea | Sea urchin | Bioceramics | High-magnesium calcite | spine | bio-inspired design of super-resistant concrete materials | [164] |
| Echinoidea | Tripneustes gratilla | Bioceramics | High-magnesium calcite | ossicles (skeletal microstructure) | production of magnesium substituted β-tricalcium phosphate for bone graft materials | [162] |
| Echinoidea | Paracentrotus lividus | ECM components | Collagen | peristomial membrane/ECM components | membranes/scaffolds for tissue regeneration | [149,152] |
| Echinoidea | Paracentrotus lividus | ECM components | Mutable collagenous tissue (MCT) components | peristomial membrane/ECM components | decellularized membranes for invertebrate cell culture | [174] |
| Echinoidea | Paracentrotus lividus | Proteins | tube feet | bioadhesives | [175] | |
| Ophiuroidea | Brittle stars | Bioceramics | High-magnesium calcite | dorsal arm plates (microstructure) | brittle-star-inspired micro-lens | [176] |
5.2. Bioactive Molecules
6. Tunicates
6.1. Biomaterials
6.2. Bioactive Molecules
7. Omics Approach to Discover New Marine Natural Products
7.1. Metabolomics
7.1.1. NMR and MS Approaches
7.1.2. Compounds Databases
8. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about Clinically Approved and Preclinically Investigated Marine Natural Products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Le, H.M.; Newman, D.J.; Glaser, K.B.; Mayer, A.M. The Marine Pharmacology and Pharmaceuticals Pipeline in 2020. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Pereira, F. Have Marine Natural Product Drug Discovery Efforts Been Productive and How Can We Improve Their Efficiency? Expert Opin. Drug Discov. 2019, 14, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral Lead Compounds from Marine Sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [Green Version]
- Schöffski, P.; Dumez, H.; Wolter, P.; Stefan, C.; Wozniak, A.; Jimeno, J.; Van Oosterom, A.T. Clinical Impact of Trabectedin (Ecteinascidin-743) in Advanced/Metastatic Soft Tissue Sarcoma. Expert Opin. Pharmacother. 2008, 9, 1609–1618. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Ballarin, L.; Rinkevich, B.; Bartscherer, K.; Burzynski, A.; Cambier, S.; Cammarata, M.; Domart-Coulon, I.; Drobne, D.; Encinas, J.; Frank, U.; et al. Maristem—Stem Cells of Marine/Aquatic Invertebrates: From Basic Research to Innovative Applications. Sustainability 2018, 10, 526. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhang, Y. Marine Invertebrate Cell Culture: A Decade of Development. J Oceanogr. 2014, 70, 405–414. [Google Scholar] [CrossRef]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in Modern Marine Biomaterials Research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef]
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern Scaffolding Strategies Based on Naturally Pre-Fabricated 3D Biomaterials of Poriferan Origin. Appl. Phys. A Mater. Sci. Processing 2020, 126, 382. [Google Scholar] [CrossRef]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Biomaterial for Topical Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Hwang, B.H.; Yang, Y.J.; Kim, B.J.; Choi, B.H.; Jung, G.Y.; Cha, H.J. Rapidly Light-Activated Surgical Protein Glue Inspired by Mussel Adhesion and Insect Structural Crosslinking. Biomaterials 2015, 67, 11–19. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; Voogd, N.J.D.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Barros, A.A.; Aroso, I.M.A.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Marine Sponges: A New Source of Bioactive Ceramics for Tissue Engineering and Regenerative Medicine Applications. J. Tissue Eng. Regen. Med. 2013, 7 (Supp. 1), 6–52. Available online: http://onlinelibrary.wiley.com/doi/10.1002/term.1822/pdf (accessed on 13 February 2022).
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. In Vitro Bioactivity Studies of Ceramic Structures Isolated from Marine Sponges. Biomed. Mater. 2016, 11, 045004. [Google Scholar] [CrossRef]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Surface Modification of Silica-Based Marine Sponge Bioceramics Induce Hydroxyapatite Formation. Cryst. Growth Des. 2014, 14, 4545–4552. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, E.; Dunne, N.; Walker, G.; Maggs, C.; Wilcox, R.; Buchanan, F. Hydroxyapatite Bone Substitutes Developed via Replication of Natural Marine Sponges. J. Mater. Sci. Mater. Med. 2010, 21, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Wang, X.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Ushijima, H.; Schröder, H.C.; Müller, W.E.G. Osteogenic Potential of Biosilica on Human Osteoblast-Like (SaOS-2) Cells. Calcif Tissue Int. 2010, 87, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Wang, X.H.; Wiens, M.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Müller, W.E.G. Silicate Modulates the Cross-Talk between Osteoblasts (SaOS-2) and Osteoclasts (RAW 264.7 Cells): Inhibition of Osteoclast Growth and Differentiation. J. Cell. Biochem. 2012, 113, 3197–3206. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Draenert, F.G.; Albert, O.; Schröder, H.C.; Mailänder, V.; Mitov, G.; Müller, W.E.G. Bioactive and Biodegradable Silica Biomaterial for Bone Regeneration. Bone 2014, 67, 292–304. [Google Scholar] [CrossRef]
- Clarke, S.A.; Choi, S.Y.; McKechnie, M.; Burke, G.; Dunne, N.; Walker, G.; Cunningham, E.; Buchanan, F. Osteogenic Cell Response to 3-D Hydroxyapatite Scaffolds Developed via Replication of Natural Marine Sponges. J. Mater. Sci. Mater. Med. 2016, 27, 22. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [Green Version]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally Drug-Loaded Chitin: Isolation and Applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [Green Version]
- Mutsenko, V.V.; Rogulska, O.Y.; Petrenko, Y.A.; Ehrlich, H.; Mazur, S.P.; Volkova, N.A.; Petrenko, A.Y. Cryosensitivity of Mesenchymal Stromal Cells Cryopreserved Within Marine Sponge Ianthella Basta Skeleton-Based Carriers. Probl. Cryobiol. Cryomedicine 2016, 26, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Żółtowska-Aksamitowska, S.; Tsurkan, M.V.; Lim, S.-C.; Meissner, H.; Tabachnick, K.; Shaala, L.A.; Youssef, D.T.A.; Ivanenko, V.N.; Petrenko, I.; Wysokowski, M.; et al. The Demosponge Pseudoceratina purpurea as a New Source of Fibrous Chitin. Int. J. Biol. Macromol. 2018, 112, 1021–1028. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Shaala, L.A.; Youssef, D.T.A.; Elhady, S.S.; Tsurkan, M.V.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.N.; et al. First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.; Shaala, L.A.; Youssef, D.T.A.; Zoltowska-Aksamitowska, S.; Tsurkan, M.; Galli, R.; Meissner, H.; Wysokowski, M.; Petrenko, I.; Tabachnick, K.R.; et al. Discovery of Chitin in Skeletons of Non-Verongiid Red Sea Demosponges. PLoS ONE 2018, 13, e0195803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.A.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New Source of 3D Chitin Scaffolds: The Red Sea Demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D Chitinous Scaffolds Derived from Cultivated Marine Demosponge Aplysina aerophoba for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells. Int. J. Biol. Macromol. 2017, 104B, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel Chitin Scaffolds Derived from Marine Sponge Ianthella basta for Tissue Engineering Approaches Based on Human Mesenchymal Stromal Cells: Biocompatibility and Cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine Biomaterials: Biomimetic and Pharmacological Potential of Cultivated Aplysina aerophoba Marine Demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, O.Y.; Mutsenko, V.V.; Revenko, E.B.; Petrenko, Y.A.; Ehrlich, H.; Petrenko, A.Y. Culture and Differentiation of Human Adipose Tissue Mesenchymal Stromal Cells within Carriers Based on Sea Sponge Chitin Skeletons. Probl. Cryobiol. Cryomedicine 2013, 23, 267–270. [Google Scholar]
- Moreira da Silva, J.C.R.; Carlos, G.S.D.; de Prata, M.B.; Henriques da Silva, T.J.Q.L.; Marques, A.M.P.; Gonçalves dos Reis, R.L.; Cerqueira, M.T. Marine-Sponge Type IV Collagen Membranes Its Production and Biomedical Applications Thereof. International Application Published Under The Patent Cooperation Treaty; International Publication Number: WO/2015/186118. 2015. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2015186118 (accessed on 13 February 2022).
- Nandi, S.K.; Kundu, B.; Mahato, A.; Thakur, N.L.; Joardar, S.N.; Mandal, B.B. In Vitro and in Vivo Evaluation of the Marine Sponge Skeleton as a Bone Mimicking Biomaterial. Integr. Biol. 2015, 7, 250–262. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In Vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallela, R.; Venkatesan, J.; Janapala, V.; Kim, S. Biophysicochemical Evaluation of Chitosan-Hydroxyapatite-Marine Sponge Collagen Composite for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2011, 100, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Parisi, J.R.; Fernandes, K.R.; Aparecida do Vale, G.C.; de França Santana, A.; de Almeida Cruz, M.; Fortulan, C.A.; Zanotto, E.D.; Peitl, O.; Granito, R.N.; Rennó, A.C.M. Marine Spongin Incorporation into Biosilicate® for Tissue Engineering Applications: An in Vivo Study. J. Biomater. Appl. 2020, 35, 205–214. [Google Scholar] [CrossRef]
- Ehrlich, H.; Luczak, M.; Ziganshin, R.; Mikšík, I.; Wysokowski, M.; Simon, P.; Baranowska-Bosiacka, I.; Kupnicka, P.; Ereskovsky, A.; Galli, R.; et al. Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges. Adv. Sci. 2022, 2105059. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Rothenberger, M.; Boreiko, A.; Tremel, W.; Reiber, A.; Schröder, H.C. Formation of Siliceous Spicules in the Marine Demosponge Suberites domuncula. Cell Tissue Res. 2005, 321, 285–297. [Google Scholar] [CrossRef]
- Wang, X.; Schröder, H.C.; Grebenjuk, V.; Diehl-Seifert, B.; Mailänder, V.; Steffen, R.; Schloßmacher, U.; Müller, W.E.G. The Marine Sponge-Derived Inorganic Polymers, Biosilica and Polyphosphate, as Morphogenetically Active Matrices/Scaffolds for the Differentiation of Human Multipotent Stromal Cells: Potential Application in 3D Printing and Distraction Osteogenesis. Mar. Drugs 2014, 12, 1131–1147. [Google Scholar] [CrossRef] [Green Version]
- Fidler, A.L.; Darris, C.E.; Chetyrkin, S.V.; Pedchenko, V.K.; Boudko, S.P.; Brown, K.L.; Gray Jerome, W.; Hudson, J.K.; Rokas, A.; Hudson, B.G. Collagen IV and Basement Membrane at the Evolutionary Dawn of Metazoan Tissues. eLife 2017, 6, e24176. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Ouazana, R.; Cloning, G.R.; Garrone, R. Cloning and Sequencing of a Porifera Partial CDNA Coding for a Short-Chain Collagen. Eur. J. Biochem. 1990, 190, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine Sponge Collagen: Isolation, Characterization and Effects on the Skin Parameters Surface-PH, Moisture and Sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and Characterization of Marine Sponge Collagen Nanoparticles and Employment for the Transdermal Delivery of 17β-Estradiol-Hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Müller, W.E.G.; Kreuter, J. Microparticles Derived from Marine Sponge Collagen (SCMPs): Preparation, Characterization and Suitability for Dermal Delivery of All-Trans Retinol. Eur. J. Pharm. Biopharm. 2002, 54, 125–133. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; Pée, K.-H. Van Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella Basta: Analytical and Biochemical Investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-Dimensional Chitin-Based Scaffolds from Verongida Sponges (Demospongiae: Porifera). Part II: Biomimetic Potential and Applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the Study of Marine Products. 32. The Nucleosides of Sponges. 1. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Walwick, E.R.; Roberts, W.K.; Dekker, C.A. Cyclisation during the Phosphorylation of Uridine and Cytidine by Polyphosphoric Acid—a New Route to the O-2,2′-Cyclonucleosides. Proc. Chem. Soc. Lond. 1959, 3, 84. [Google Scholar]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Cruz, P.G.; Martínez Leal, J.F.; Daranas, A.H.; Pérez, M.; Cuevas, C. On the Mechanism of Action of Dragmacidins I and J, Two New Representatives of a New Class of Protein Phosphatase 1 and 2A Inhibitors. ACS Omega 2018, 3, 3760–3767. [Google Scholar] [CrossRef]
- De Souza, R.T.M.P.; Freire, V.F.; Gubiani, J.R.; Ferreira, R.O.; Trivella, D.B.B.; Moraes, F.C.; Paradas, W.C.; Salgado, L.T.; Pereira, R.C.; Amado Filho, G.M.; et al. Bromopyrrole Alkaloid Inhibitors of the Proteasome Isolated from a Dictyonella Sp. Marine Sponge Collected at the Amazon River Mouth. J. Nat. Prod. 2018, 81, 2296–2300. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Montano, N.; Amador, L.A.; Rodríguez, A.D.; Golovko, M.Y.; Golovko, S.A.; Reguera, R.M.; Álvarez-Velilla, R.; Balaña-Fouce, R. Novel Very Long-Chain α-Methoxylated Δ5,9 Fatty Acids from the Sponge Asteropus niger Are Effective Inhibitors of Topoisomerases IB. Lipids 2016, 51, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Tianero, M.D.; Balaich, J.N.; Donia, M.S. Localized Production of Defence Chemicals by Intracellular Symbionts of Haliclona Sponges. Nat. Microbiol. 2019, 4, 1149–1159. [Google Scholar] [CrossRef]
- Schofield, M.M.; Jain, S.; Porat, D.; Dick, G.J.; Sherman, D.H. Identification and Analysis of the Bacterial Endosymbiont Specialized for Production of the Chemotherapeutic Natural Product ET-743. Env. Microbiol. 2015, 17, 3964–3975. [Google Scholar] [CrossRef] [Green Version]
- Teta, R.; Della Sala, G.; Esposito, G.; Via, C.W.; Mazzoccoli, C.; Piccoli, C.; Bertin, M.J.; Costantino, V.; Mangoni, A. A Joint Molecular Networking Study of a Smenospongia Sponge and a Cyanobacterial Bloom Revealed New Antiproliferative Chlorinated Polyketides. Org. Chem. Front. 2019, 6, 1762–1774. [Google Scholar] [CrossRef]
- Cantrell, T.P.; Freeman, C.J.; Paul, V.J.; Agarwal, V.; Garg, N. Mass Spectrometry-Based Integration and Expansion of the Chemical Diversity Harbored Within a Marine Sponge. J. Am. Soc. Mass. Spectr. 2019, 30, 1373–1384. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Aplysinopsin, a New Tryptophan Derivative from a Sponge. Tetrahedron. Lett. 1977, 18, 61–64. [Google Scholar] [CrossRef]
- Lewellyn, K.; Zjawiony, J.K. Aplysinopsins as promising marine natural product drug leads: Recent developments. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Grand Challenges in Biology and Biotechnology; Springer International Publishing: Cham, Switzerland, 2018; pp. 191–215. ISBN 978-3-319-69075-9. [Google Scholar]
- Ehrlich, H.; Etnoyer, P.; Litvinov, S.D.; Olennikova, M.M.; Domaschke, H.; Hanke, T.; Born, R.; Meissner, H.; Worch, H. Biomaterial Structure in Deep-Sea Bamboo Coral (Anthozoa: Gorgonacea: Isididae): Perspectives for the Development of Bone Implants and Templates for Tissue Engineering. Mater. Werkst. 2006, 37, 552–557. [Google Scholar] [CrossRef]
- Boller, M.L.; Swain, T.D.; Lasker, H.R. Skeletal Morphology and Material Properties of a Fragmenting Gorgonian Coral. Mar. Ecol. Prog. Ser. 2002, 228, 131–141. [Google Scholar] [CrossRef]
- Day, A.G.E.; Francis, W.R.; Fu, K.; Pieper, I.L.; Guy, O.; Xia, Z. Osteogenic Potential of Human Umbilical Cord Mesenchymal Stem Cells on Coralline Hydroxyapatite/Calcium Carbonate Microparticles. Stem Cells Int. 2018, 2018, e4258613. [Google Scholar] [CrossRef]
- Guillemin, G.; Meunier, A.; Dallant, P.; Christel, P.; Pouliquen, J.C.; Sedel, L. Comparison of Coral Resorption and Bone Apposition with Two Natural Corals of Different Porosities. J. Biomed. Mater. Res. 1989, 23, 765–779. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Natural Bioceramics: From Coral to Bone and Beyond. Curr. Opin. Solid State Mater. Sci. 2003, 7, 283–288. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Lemoine, M.; Clarke, D.; Gonzalez Vazquez, A.; O’Brien, F.J. The Incorporation of Marine Coral Microparticles into Collagen-Based Scaffolds Promotes Osteogenesis of Human Mesenchymal Stromal Cells via Calcium Ion Signalling. Mar. Drugs 2020, 18, 74. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H. Living bone implants of bamboo corals origin. In Marine Biological Materials of Invertebrate Origin; Ehrlich, H., Ed.; Biologically-Inspired Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 127–131. ISBN 978-3-319-92483-0. [Google Scholar]
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and Identification of Chitin in the Black Coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.A.; Daly, N.L.; Wilson, D.T. Coral Venom Toxins. Front. Ecol. Evol. 2019, 7, 320. [Google Scholar] [CrossRef] [Green Version]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and Its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Carvalho, D.N.; López-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine Collagen-Chitosan-Fucoidan Cryogels as Cell-Laden Biocomposites Envisaging Tissue Engineering. Biomed. Mater. 2020, 15, 055030. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gonçalves, C.; Oliveira, J.M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Innovative Methodology for Marine Collagen–Chitosan–Fucoidan Hydrogels Production, Tailoring Rheological Properties towards Biomedical Application. Green Chem. 2021, 23, 7016–7029. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish Collagen Scaffolds for Cartilage Tissue Engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Rojko, N.; Dalla Serra, M.; Maček, P.; Anderluh, G. Pore Formation by Actinoporins, Cytolysins from Sea Anemones. Biochim. Biophys. Acta 2016, 1858, 446–456. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [Green Version]
- Sweet, M.J.; Croquer, A.; Bythell, J.C. Bacterial Assemblages Differ between Compartments within the Coral Holobiont. Coral Reefs 2011, 30, 39–52. [Google Scholar] [CrossRef]
- Cooper, E.L.; Hirabayashi, K.; Strychar, K.B.; Sammarco, P.W. Corals and Their Potential Applications to Integrative Medicine. Evid. Based Complementary Altern. Med. 2014, 2014, e184959. [Google Scholar] [CrossRef]
- Rocha, J.; Calado, R.; Leal, M. Marine bioactive compounds from cnidarians. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 823–849. ISBN 978-3-642-53971-8. [Google Scholar]
- Tammam, M.A.; Rárová, L.; Kvasnicová, M.; Gonzalez, G.; Emam, A.M.; Mahdy, A.; Strnad, M.; Ioannou, E.; Roussis, V. Bioactive Steroids from the Red Sea Soft Coral Sinularia polydactyla. Mar. Drugs 2020, 18, 632. [Google Scholar] [CrossRef]
- Guillen, P.O.; Jaramillo, K.B.; Genta-Jouve, G.; Thomas, O.P. Marine Natural Products from Zoantharians: Bioactivity, Biosynthesis, Systematics, and Ecological Roles. Nat. Prod. Rep. 2020, 37, 515–540. [Google Scholar] [CrossRef]
- Sunagawa, S.; DeSalvo, M.K.; Voolstra, C.R.; Reyes-Bermudez, A.; Medina, M. Identification and Gene Expression Analysis of a Taxonomically Restricted Cysteine-Rich Protein Family in Reef-Building Corals. PLoS ONE 2009, 4, e4865. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Dickschat, J.S.; Guo, Y.-W. Diving into the World of Marine 2,11-Cyclized Cembranoids: A Summary of New Compounds and Their Biological Activities. Nat. Prod. Rep. 2020, 37, 1367–1383. [Google Scholar] [CrossRef]
- Ne’eman, I.; Fishelson, L.; Kashman, Y. Sarcophine—A New Toxin from the Soft Coral Sarcophyton glaucum (Alcyonaria). Toxicon 1974, 12, 593–594, IN5–IN6. [Google Scholar] [CrossRef]
- Gerhart, D.J.; Rittschof, D.; Mayo, S.W. Chemical Ecology and the Search for Marine Antifoulants: Studies of a Predator-Prey Symbiosis. J. Chem. Ecol. 1988, 14, 1905–1917. [Google Scholar] [CrossRef]
- Shapo, J.L.; Moeller, P.D.; Galloway, S.B. Antimicrobial Activity in the Common Seawhip, Leptogorgia virgulata (Cnidaria: Gorgonaceae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 65–73. [Google Scholar] [CrossRef]
- Clare, A.S.; Rittschof, D.; Gerhart, D.J.; Hooper, I.R.; Bonaventura, J. Antisettlement and Narcotic Action of Analogues of Diterpene Marine Natural Product Antifoulants from Octocorals. Mar. Biotechnol. 1999, 1, 427–436. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; Heaton, A.; Scheuer, P.J.; Li, M.K.; Clardy, J.; Schulte, G.K.; Finer-Moore, J. Structures and Possible Functions of Epoxypukalide and Pukalide: Diterpenes Associated with Eggs of Sinularian Soft Corals (Cnidaria, Anthozoa, Octocorallia, Alcyonacea, Alcyoniidae). J. Chem. Ecol. 1989, 15, 1177–1191. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural Cholinesterase Inhibitors from Marine Organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Urda, C.; Fernández, R.; Pérez, M.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Protoxenicins A and B, Cytotoxic Long-Chain Acylated Xenicanes from the Soft Coral Protodendron repens. J. Nat. Prod. 2017, 80, 713–719. [Google Scholar] [CrossRef]
- Liao, Q.; Feng, Y.; Yang, B.; Lee, S.M.-Y. Cnidarian Peptide Neurotoxins: A New Source of Various Ion Channel Modulators or Blockers against Central Nervous Systems Disease. Drug Discov. Today 2019, 24, 189–197. [Google Scholar] [CrossRef]
- Coll, J.C. The Chemistry and Chemical Ecology of Octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Tomono, Y.; Hirota, H.; Fusetani, N. Isogosterones A∓D, Antifouling 13,17-Secosteroids from an Octocoral Dendronephthya sp. J. Org. Chem. 1999, 64, 2272–2275. [Google Scholar] [CrossRef]
- Qi, S.-H.; Gao, C.-H.; Qian, P.-Y.; Zhang, S. Steroids from the South China Sea Gorgonian Subergorgia suberosa. Nat. Prod. Commun. 2010, 5, 1934578X1000500206. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.-H.; Ma, X. Antifouling Compounds from Marine Invertebrates. Mar. Drugs 2017, 15, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pénez, N.; Culioli, G.; Pérez, T.; Briand, J.-F.; Thomas, O.P.; Blache, Y. Antifouling Properties of Simple Indole and Purine Alkaloids from the Mediterranean Gorgonian Paramuricea clavata. J. Nat. Prod. 2011, 74, 2304–2308. [Google Scholar] [CrossRef]
- Tello, E.; Castellanos, L.; Arévalo-Ferro, C.; Duque, C. Disruption in Quorum-Sensing Systems and Bacterial Biofilm Inhibition by Cembranoid Diterpenes Isolated from the Octocoral Eunicea knighti. J. Nat. Prod. 2012, 75, 1637–1642. [Google Scholar] [CrossRef]
- Brusca, R.C.; Moore, W. ; Shuster, Stephen, M. Invertebrates; Sinauer Associates Inc.: Sunderland, MA USA, 2016; ISBN 978-1-60535-375-3. [Google Scholar]
- Plazzi, F.; Passamonti, M. Towards a Molecular Phylogeny of Mollusks: Bivalves’ Early Evolution as Revealed by Mitochondrial Genes. Mol. Phylogenetics Evol. 2010, 57, 641–657. [Google Scholar] [CrossRef]
- Clancy, S.K.; Sodano, A.; Cunningham, D.J.; Huang, S.S.; Zalicki, P.J.; Shin, S.; Ahn, B.K. Marine Bioinspired Underwater Contact Adhesion. Biomacromolecules 2016, 17, 1869–1874. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent Approaches in Designing Bioadhesive Materials Inspired by Mussel Adhesive Protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef]
- Cha, H.J.; Hwang, D.S.; Lim, S. Development of Bioadhesives from Marine Mussels. Biotechnol. J. 2008, 3, 631–638. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, J.M.; Jung, H.S. Marine Structural Biomaterials in Medical Biomimicry. Tissue Eng. Part B Rev. 2015, 21, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macha, I.J.; Ben-Nissan, B. Marine Skeletons: Towards Hard Tissue Repair and Regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yamauchi, K.; Kurokawa, M.; Asakura, T. Design of Silk-like Biomaterials Inspired by Mussel-Adhesive Protein. Tissue Eng. 2007, 13, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Gim, Y.; Kang, D.G.; Kim, Y.K.; Cha, H.J. Recombinant Mussel Adhesive Protein Mgfp-5 as Cell Adhesion Biomaterial. J. Biotechnol. 2007, 127, 727–735. [Google Scholar] [CrossRef]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. J. Am. Chem. Soc. 2013, 135, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.Y.; Choi, B.H.; Jung, D.; Hwang, B.H.; Cha, H.J. Natural Healing-Inspired Collagen-Targeting Surgical Protein Glue for Accelerated Scarless Skin Regeneration. Biomaterials 2017, 134, 154–165. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.E.; Koh, E.K.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Hong, J.T.; Hwang, D.Y. Selenium-Loaded Cellulose Film Derived from Styela clava Tunic Accelerates the Healing Process of Cutaneous Wounds in Streptozotocin-Induced Diabetic Sprague–Dawley Rats. J. Dermatol. Treat. 2018, 29, 606–616. [Google Scholar] [CrossRef]
- Choi, B.-H.; Cheong, H.; Jo, Y.K.; Bahn, S.Y.; Seo, J.H.; Cha, H.J. Highly Purified Mussel Adhesive Protein to Secure Biosafety for in Vivo Applications. Microb. Cell Factories 2014, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.; Merritt, D.J.; Dixon, N.E. Synthesis and Properties of Crosslinked Recombinant Pro-Resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Jeschke, M.G. Combined Gene and Stem Cell Therapy for Cutaneous Wound Healing. Mol. Pharm. 2011, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Hausser, H.; Beavan, L.; Kresse, H. Decorin-Type I Collagen Interaction. Presence of Separate Core Protein-Binding Domains. J. Biol. Chem. 1995, 270, 8877–8883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.; Martinović, R.; Joksimović, D.; Petrenko, I.; Schiaparelli, S.; Wysokowski, M.; Tsurkan, D.; Stelling, A.L.; Springer, A.; Gelinsky, M.; et al. Conchixes: Organic Scaffolds Which Resemble the Size and Shapes of Mollusks Shells, Their Isolation and Potential Multifunctional Applications. Appl. Phys. A 2020, 126, 562. [Google Scholar] [CrossRef]
- Wägele, H.; Klussmann-Kolb, A. Opisthobranchia (Mollusca, Gastropoda)—More than Just Slimy Slugs. Shell Reduction and Its Implications on Defence and Foraging. Front. Zool. 2005, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Ciavatta, M.L.; Lefranc, F.; Carbone, M.; Mollo, E.; Gavagnin, M.; Betancourt, T.; Dasari, R.; Kornienko, A.; Kiss, R. Marine Mollusk-Derived Agents with Antiproliferative Activity as Promising Anticancer Agents to Overcome Chemotherapy Resistance. Med. Res. Rev. 2017, 37, 702–801. [Google Scholar] [CrossRef]
- Cimino, G.; Spinella, A.; Sodano, G. Potential Alarm Pheromones from the Mediterranean Opisthobranch Scaphander Lignarius. Tetrahedron Lett. 1989, 30, 5003–5004. [Google Scholar] [CrossRef]
- Della Sala, G.; Cutignano, A.; Fontana, A.; Spinella, A.; Calabrese, G.; Coll, A.D.; d’Ippolito, G.; Monica, C.D.; Cimino, G. Towards the Biosynthesis of the Aromatic Products of the Mediterranean Mollusc Scaphander lignarius: Isolation and Synthesis of Analogues of Lignarenones. Tetrahedron 2007, 63, 7256–7263. [Google Scholar] [CrossRef]
- Cutignano, A.; Avila, C.; Rosica, A.; Romano, G.; Laratta, B.; Domenech-Coll, A.; Cimino, G.; Mollo, E.; Fontana, A. Biosynthesis and Cellular Localization of Functional Polyketides in the Gastropod Mollusc Scaphander lignarius. ChemBioChem 2012, 13, 1759–1766. [Google Scholar] [CrossRef]
- Cutignano, A.; Avila, C.; Domenech-Coll, A.; d’Ippolito, G.; Cimino, G.; Fontana, A. First Biosynthetic Evidence on the Phenyl-Containing Polyketides of the Marine Mollusc Scaphander lignarius. Org. Lett. 2008, 10, 2963–2966. [Google Scholar] [CrossRef]
- Llorach-Pares, L.; Rodriguez-Urgelles, E.; Nonell-Canals, A.; Alberch, J.; Avila, C.; Sanchez-Martinez, M.; Giralt, A. Meridianins and Lignarenone B as Potential GSK3β Inhibitors and Inductors of Structural Neuronal Plasticity. Biomolecules 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, A.; Manzo, E.; Ciavatta, M.L.; Cutignano, A.; Gavagnin, M.; Cimino, G. Biosynthetic studies through feeding experiments in marine organisms. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, O., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 895–946. ISBN 978-90-481-3834-0. [Google Scholar]
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A Bacterial Source for Mollusk Pyrone Polyketides. Chem. Biol. 2013, 20, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. 2.19-Natural products of therapeutic importance. In Comprehensive Natural Products II; Liu, H.-W. (Ben), Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 623–650. ISBN 978-0-08-045382-8. [Google Scholar]
- Olivera, B.M. ω-Conotoxin MVIIA: From Marine Snail Venom to Analgesic Drug. In Drugs From The Sea; Fusetani, N., Ed.; Karger: Basel, Switzerland, 2000; pp. 74–85. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.; Kaas, Q.; Jones, A.; Alewood, P.F.; Lewis, R.J. Deep Venomics Reveals the Mechanism for Expanded Peptide Diversity in Cone Snail Venom. Mol. Cell. Proteom. 2013, 12, 312–329. [Google Scholar] [CrossRef] [Green Version]
- Barghi, N.; Concepcion, G.P.; Olivera, B.M.; Lluisma, A.O. High Conopeptide Diversity in Conus tribblei Revealed through Analysis of Venom Duct Transcriptome Using Two High-Throughput Sequencing Platforms. Mar. Biotechnol. 2015, 17, 81–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.; Yang, L.; Xu, S.; Wang, C. Various Conotoxin Diversifications Revealed by a Venomic Study of Conus flavidus. Mol. Cell. Proteom. 2014, 13, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.D.; Norton, R.S. Conotoxin Gene Superfamilies. Mar. Drugs 2014, 12, 6058–6101. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Li, C.; Dong, S.; Wu, Y.; Zhangsun, D.; Luo, S. Discovery Methodology of Novel Conotoxins from Conus Species. Mar. Drugs 2018, 16, 417. [Google Scholar] [CrossRef] [Green Version]
- Bjørn-Yoshimoto, W.E.; Ramiro, I.B.L.; Yandell, M.; McIntosh, J.M.; Olivera, B.M.; Ellgaard, L.; Safavi-Hemami, H. Curses or Cures: A Review of the Numerous Benefits Versus the Biosecurity Concerns of Conotoxin Research. Biomedicines 2020, 8, 235. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid Glycosides from Marine Organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef]
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and Productive Source of Biopolymer Inspiration: Marine Collagens. Biomacromolecules 2021, 22, 1815–1834. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-Derived Collagen Biomaterials from Echinoderm Connective Tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, K.L.; Holmes, D.F. Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkie, I.C.; Sugni, M.; Gupta, H.S.; Carnevali, M.D.C.; Elphick, M.R. The Mutable Collagenous Tissue of Echinoderms: From Biology to Biomedical Applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Benedetto, C.D.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus Lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Melotti, L.; Martinello, T.; Perazzi, A.; Iacopetti, I.; Ferrario, C.; Sugni, M.; Sacchetto, R.; Patruno, M. A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep. Animals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Biomimetic Mechanically Adaptive Nanocomposites. Prog. Polym. Sci. 2010, 35, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-Responsive Polymer Nanocomposites Inspired by the Sea Cucumber Dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards Biocompatible Intracortical Microelectrodes for Neural Interfacing Applications. J. Neural. Eng. 2015, 12, 011001. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Mirantsev, G.V.; Rozhnov, S.V. General Features of Echinoderm Skeleton Formation. Paleontol. J. 2014, 48, 1532–1539. [Google Scholar] [CrossRef]
- Weber, J.N.; White, E.W.; Lebiedzik, J. New Porous Biomaterials by Replication of Echinoderm Skeletal Microstructures. Nature 1971, 233, 337–339. [Google Scholar] [CrossRef]
- Fontaine, A.R.; Hall, B.D. Biocompatibility of Echinoderm Skeleton with Mammalian Cells in Vitro: Preliminary Evidence. J. Biomed. Mater. Res. 1981, 15, 61–71. [Google Scholar] [CrossRef]
- Martina, M.; Subramanyam, G.; Weaver, J.C.; Hutmacher, D.W.; Morse, D.E.; Valiyaveettil, S. Developing Macroporous Bicontinuous Materials as Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 5609–5616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Huang, H.-Y.; Lin, H.-M.; Hsu, F.-Y.; Su, Y.-P.; Hwang, D.-F. Biomaterial Availability of Magnesium Substituted Beta-Tricalcium Phosphate Converted from Sea Urchin Tripneustes gratilla Shell. J. Ceram. Soc. Jpn. 2015, 123, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Gómez Vázquez, N.S.; Luque Morales, P.A.; Gomez Gutierrez, C.M.; Nava Olivas, O.D.J.; Villarreal Sánchez, R.C.; Vilchis Nestor, A.R.; Chinchillas Chinchillas, M.D.J. Hydroxyapatite Biosynthesis Obtained from Sea Urchin Spines (Strongylocentrotus purpuratus): Effect of Synthesis Temperature. Processes 2020, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Picker, A.; Nicoleau, L.; Burghard, Z.; Bill, J.; Zlotnikov, I.; Labbez, C.; Nonat, A.; Cölfen, H. Mesocrystalline Calcium Silicate Hydrate: A Bioinspired Route toward Elastic Concrete Materials. Sci. Adv. 2017, 3, e1701216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flammang, P.; Santos, R.; Haesaerts, D. Echinoderm Adhesive Secretions: From Experimental Characterization to Biotechnological Applications. Prog. Mol. Subcell. Biol. 2005, 39, 201–220. [Google Scholar]
- Trotter, J.A.; Tipper, J.; Lyons-Levy, G.; Chino, K.; Heuer, A.H.; Liu, Z.; Mrksich, M.; Hodneland, C.; Dillmore, W.S.; Koob, T.J.; et al. Towards a Fibrous Composite with Dynamically Controlled Stiffness: Lessons from Echinoderms. Biochem. Soc. Trans. 2000, 28, 357–362. [Google Scholar] [CrossRef]
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar Stiffening of Echinoderm Mutable Collagenous Tissue Demonstrated at the Nanoscale. PNAS 2016, 113, E6362–E6371. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z. Biomimetic Principles and Design of Advanced Engineering Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-92625-3. [Google Scholar]
- Hennebert, E.; Leroy, B.; Wattiez, R.; Ladurner, P. An Integrated Transcriptomic and Proteomic Analysis of Sea Star Epidermal Secretions Identifies Proteins Involved in Defense and Adhesion. J. Proteom. 2015, 128, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Hennebert, E.; Wattiez, R.; Flammang, P. Characterisation of the Carbohydrate Fraction of the Temporary Adhesive Secreted by the Tube Feet of the Sea Star Asterias Rubens. Mar. Biotechnol. 2011, 13, 484–495. [Google Scholar] [CrossRef]
- Hennebert, E.; Wattiez, R.; Demeuldre, M.; Ladurner, P.; Hwang, D.S.; Waite, J.H.; Flammang, P. Sea Star Tenacity Mediated by a Protein That Fragments, Then Aggregates. PNAS 2014, 111, 6317–6322. [Google Scholar] [CrossRef] [Green Version]
- Ağaoğullari, D.; Kel, D.; Gökçe, H.; Duman, I.; Öveçoğlu, M.L.; Akarsubaşi, A.T.; Bılgıç, D.; Oktar, F.N. Bioceramic Production from Sea Urchins. Acta Phys. Pol. A 2012, 121, 23–25. [Google Scholar] [CrossRef]
- Mancilla-Sanchez, E.; Gómez-Gutiérrez, C.M.; Guerra-Rivas, G.; Soto-Robles, C.A.; Vilchis-Nestor, A.R.; Vargas, E.; Luque, P.A. Obtaining Hydroxyapatite from the Exoskeleton and Spines of the Purple Sea Urchin Strongylocentrotus purpuratus. Int. J. Appl. Ceram. Technol. 2019, 16, 438–443. [Google Scholar] [CrossRef]
- Di Benedetto, C. Progenitor cells and regenerative potential in echinoderms: An in vivo and in vitro approach. In Progenitor Cells and Regenerative Potential in Echinoderms; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2011; ISBN 978-3-8443-1105-1. [Google Scholar]
- Viana, A.S.; Santos, R. Nanoscale Characterization of the Temporary Adhesive of the Sea Urchin Paracentrotus lividus. Beilstein J. Nanotechnol. 2018, 9, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.L.; Weiner, S. (Eds.) Learning from nature how to design new implantable biomaterials: From biomineralization fundamentals to biomimetic materials and processing routes. In Proceedings of the NATO Advanced Study Institute, Held in Alvor, Algarve, Portugal, 13–24 October 2003; NATO Science Series II: Mathematics, Physics and Chemistry. Springer: Dordercht, The Netherlands, 2005. ISBN 978-1-4020-2644-7. [Google Scholar]
- Lefevre, M.; Flammang, P.; Aranko, A.S.; Linder, M.B.; Scheibel, T.; Humenik, M.; Leclercq, M.; Surin, M.; Tafforeau, L.; Wattiez, R.; et al. Sea Star-Inspired Recombinant Adhesive Proteins Self-Assemble and Adsorb on Surfaces in Aqueous Environments to Form Cytocompatible Coatings. Acta Biomater. 2020, 112, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Ghosh, J.; Buckley, K.M.; Clow, L.A.; Dheilly, N.M.; Haug, T.; Henson, J.H.; Li, C.; Lun, C.M.; Majeske, A.J.; et al. Echinoderm Immunity. Adv. Exp. Med. Biol. 2010, 708, 260–301. [Google Scholar] [CrossRef]
- Stabili, L.; Pagliara, P.; Roch, P. Antibacterial Activity in the Coelomocytes of the Sea Urchin Paracentrotus lividus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 113, 639–644. [Google Scholar] [CrossRef]
- Haug, T.; Kjuul, A.K.; Styrvold, O.B.; Sandsdalen, E.; Olsen, Ø.M.; Stensvåg, K. Antibacterial Activity in Strongylocentrotus Droebachiensis (Echinoidea), Cucumaria Frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J. Invertebr. Pathol. 2002, 81, 94–102. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Styrvold, O.B.; Jørgensen, T.Ø.; Stensvåg, K. Strongylocins, Novel Antimicrobial Peptides from the Green Sea Urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2008, 32, 1430–1440. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Moe, M.K.; Styrvold, O.B.; Stensvåg, K. Centrocins: Isolation and Characterization of Novel Dimeric Antimicrobial Peptides from the Green Sea Urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2010, 34, 959–968. [Google Scholar] [CrossRef]
- Li, C.; Blencke, H.-M.; Haug, T.; Jørgensen, Ø.; Stensvåg, K. Expression of Antimicrobial Peptides in Coelomocytes and Embryos of the Green Sea Urchin (Strongylocentrotus droebachiensis). Dev. Comp. Immunol. 2014, 43, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Haug, T.; Stensvåg, K. Antimicrobial Peptides in Echinoderms. ISJ-Invert. Surviv. J. 2010, 7, 132–140. [Google Scholar]
- Solstad, R.G.; Li, C.; Isaksson, J.; Johansen, J.; Svenson, J.; Stensvåg, K.; Haug, T. Novel Antimicrobial Peptides EeCentrocins 1, 2 and EeStrongylocin 2 from the Edible Sea Urchin Echinus esculentus Have 6-Br-Trp Post-Translational Modifications. PLoS ONE 2016, 11, e0151820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schillaci, D.; Cusimano, M.G.; Spinello, A.; Barone, G.; Russo, D.; Vitale, M.; Parrinello, D.; Arizza, V. Paracentrin 1, a Synthetic Antimicrobial Peptide from the Sea-Urchin Paracentrotus lividus, Interferes with Staphylococcal and Pseudomonas aeruginosa Biofilm Formation. AMB Express 2014, 4, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinello, A.; Cusimano, M.G.; Schillaci, D.; Inguglia, L.; Barone, G.; Arizza, V. Antimicrobial and Antibiofilm Activity of a Recombinant Fragment of β-Thymosin of Sea Urchin Paracentrotus lividus. Mar. Drugs 2018, 16, 366. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone Pigments from Sea Urchins: Chemistry and Pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, C.M.M. Acetylated Triterpene Glycosides and Their Biological Activity from Holothuroidea Reported in the Past Six Decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, A.J.; Afifi, R.; Ahmed, M.; Khalifa, S.; Paget, T. Bioactivity as an Options Value of Sea Cucumbers in the Egyptian Red Sea. Conserv. Biol. 2010, 24, 217–225. [Google Scholar] [CrossRef]
- Yamagishi, M.; Hosoda-Yabe, R.; Tamai, H.; Konishi, M.; Imamura, A.; Ishida, H.; Yabe, T.; Ando, H.; Kiso, M. Structure-Activity Relationship Study of the Neuritogenic Potential of the Glycan of Starfish Ganglioside LLG-3. Mar. Drugs 2015, 13, 7250–7274. [Google Scholar] [CrossRef] [Green Version]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasseur, L.; Hennebert, E.; Fievez, L.; Caulier, G.; Bureau, F.; Tafforeau, L.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. The Roles of Spinochromes in Four Shallow Water Tropical Sea Urchins and Their Potential as Bioactive Pharmacological Agents. Mar. Drugs 2017, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of Spinochrome Pigments in Echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Amarowicz, R.; Synowiecki, J.; Shahidi, F. Sephadex LH-20 Separation of Pigments from Shells of Red Sea Urchin (Strongylocentrotus franciscanus). Food Chem. 1994, 51, 227–229. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, First Aminated Hydroxynaphthazarins from the Sea Urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Shestak, O.P.; Anufriev, V.P.; Novikov, V.L. Preparative Production of Spinochrome E, a Pigment of Different Sea Urchin Species. Nat. Prod. Commun. 2014, 9, 953–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.; Moore, R.E.; Chang, C.W.; Scheuer, P.J. The Synthesis of Spinochromes A, C, D, and E. J. Am. Chem. Soc. 1965, 87, 4023–4024. [Google Scholar] [CrossRef]
- Kuwahara, R.; Hatate, H.; Yuki, T.; Murata, H.; Tanaka, R.; Hama, Y. Antioxidant Property of Polyhydroxylated Naphthoquinone Pigments from Shells of Purple Sea Urchin Anthocidaris crassispina. LWT Food Sci. Technol. 2009, 42, 1296–1300. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a Naturally Occurring Iron Chelator and Free Radical Scavenger in Artificial and Natural Membrane Systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Iron Chelators and Free Radical Scavengers in Naturally Occurring Polyhydroxylated 1,4-Naphthoquinones. Hemoglobin 2008, 32, 165–179. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Mar. Drugs 2018, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ageenko, N.V.; Kiselev, K.V.; Dmitrenok, P.S.; Odintsova, N.A. Pigment Cell Differentiation in Sea Urchin Blastula-Derived Primary Cell Cultures. Mar. Drugs 2014, 12, 3874–3891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishibori, K. Isolation of Echinochrome A from the Spines of the Sea Urchin, Stomopneustes variolaris (Lamarck). Nature 1961, 192, 1293–1294. [Google Scholar] [CrossRef]
- Anufriev, V.P.; Novikov, V.L.; Maximov, O.B.; Elyakov, G.B.; Levitsky, D.O.; Lebedev, A.V.; Sadretdinov, S.M.; Shvilkin, A.V.; Afonskaya, N.I.; Ruda, M.Y.; et al. Synthesis of Some Hydroxynaphthazarins and Their Cardioprotective Effects under Ischemia-Reperfusion in Vivo. Bioorg. Med. Chem. Lett. 1998, 8, 587–592. [Google Scholar] [CrossRef]
- Buĭmov, G.A.; Maksimov, I.V.; Perchatkin, V.A.; Repin, A.N.; Afanas’ev, S.A.; Markov, V.A.; Karpov, R.S. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. Arkhiv 2002, 74, 12–16. [Google Scholar]
- Zakirova, A.N.; Ivanova, M.V.; Golubiatnikov, V.B.; Mishchenko, N.P.; Kol’tsova, E.A.; Fedoreev, S.A.; Krasnovid, N.I.; Lebedev, A.V. Pharmacokinetics and clinical efficacy of histochrome in patients with acute myocardial infarction. Eksp. Klin. Farmacol. 1997, 60, 21–24. [Google Scholar]
- Lebedev, A.V.; Levitskaya, E.L.; Tikhonova, E.V.; Ivanova, M.V. Antioxidant Properties, Autooxidation, and Mutagenic Activity of Echinochrome a Compared with Its Etherified Derivative. Biochemistry 2001, 66, 885–893. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome a Increases Mitochondrial Mass and Function by Modulating Mitochondrial Biogenesis Regulatory Genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef]
- Kim, H.K.; Youm, J.B.; Jeong, S.H.; Lee, S.R.; Song, I.-S.; Ko, T.H.; Pronto, J.R.; Ko, K.S.; Rhee, B.D.; Kim, N.; et al. Echinochrome A Regulates Phosphorylation of Phospholamban Ser16 and Thr17 Suppressing Cardiac SERCA2A Ca2+ Reuptake. Pflug. Arch. 2015, 467, 2151–2163. [Google Scholar] [CrossRef]
- Kim, H.K.; Cho, S.W.; Heo, H.J.; Jeong, S.H.; Kim, M.; Ko, K.S.; Rhee, B.D.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; et al. A Novel Atypical PKC-Iota Inhibitor, Echinochrome A, Enhances Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells. Mar. Drugs 2018, 16, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.-B.; Kim, M.-J.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Lee, H.S.; Kim, D.; Jeong, J.-Y. Echinochrome A Promotes Ex Vivo Expansion of Peripheral Blood-Derived CD34+ Cells, Potentially through Downregulation of ROS Production and Activation of the Src-Lyn-P110δ Pathway. Mar. Drugs 2019, 17, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Lee, N.-K.; Lim, H.J.; Mazumder, S.; Kumar Rethineswaran, V.; Kim, Y.-J.; Jang, W.B.; Ji, S.T.; Kang, S.; Kim, D.Y.; et al. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Mar. Drugs 2019, 17, 368. [Google Scholar] [CrossRef] [Green Version]
- Agafonova, I.G.; Kotel’nikov, V.N.; Mischenko, N.P.; Kolosova, N.G. Evaluation of Effects of Histochrome and Mexidol on Structural and Functional Characteristics of the Brain in Senescence-Accelerated OXYS Rats by Magnetic Resonance Imaging. Bull. Exp. Biol. Med. 2011, 150, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, I.G.; Bogdanovich, R.N.; Kolosova, N.G. Assessment of Nephroprotective Potential of Histochrome during Induced Arterial Hypertension. Bull. Exp. Biol. Med. 2015, 160, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kareva, E.N.; Tikhonov, D.A.; Mishchenko, N.P.; Fedoreev, S.A.; Shimanovskii, N.L. Effects of Histochrome on P53 Expression in Mouse Red Bone Marrow Cells in a Model of Chronic Stress. Pharm. Chem. J. 2014, 48, 149–152. [Google Scholar] [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome A Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar. Drugs 2019, 17, 501. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.A.S. Mechanisms of Echinochrome Potency in Modulating Diabetic Complications in Liver. Life Sci. 2016, 151, 41–49. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.-A.S. The Possible Hypoglycemic Mechanisms of Echinochrome. Curr. Diabetes. Rev. 2018, 14, 334–338. [Google Scholar] [CrossRef]
- Oh, S.-J.; Seo, Y.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.M.; Krivoshapko, O.N. Protective Effects of Polar Lipids and Redox-Active Compounds from Marine Organisms at Modeling of Hyperlipidemia and Diabetes. J. Biomed. Sci. Eng. 2013, 6, 543–550. [Google Scholar] [CrossRef]
- Sayed, D.A.; Soliman, A.M.; Fahmy, S.R. Echinochrome Pigment as Novel Therapeutic Agent against Experimentally - Induced Gastric Ulcer in Rats. Biomed. Pharmacother. 2018, 107, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, I.G.; Anufriev, V.P. The Effect of Hydroxynaphtazarin Derivatives on Decrease of Ischemic Area After Damage Focal Cerebral Blood Circulation. Appl. Magn. Reason. 2017, 48, 579–587. [Google Scholar] [CrossRef]
- Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.A.; Mros, S.; Amagase, K.; Bekhit, A.E.-D.A. In Vitro Antioxidant and Antimicrobial Activities, and in Vivo Anti-Inflammatory Activity of Crude and Fractionated PHNQs from Sea Urchin (Evechinus chloroticus). Food Chem. 2020, 316, 126339. [Google Scholar] [CrossRef]
- Service, M.; Wardlaw, A.C. Echinochrome-A as a Bactericidal Substance in the Coelomic Fluid of Echinus Esculentus (L.). Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 79, 161–165. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [Green Version]
- Mishchenko, N.P.; Krylova, N.V.; Iunikhina, O.V.; Vasileva, E.A.; Likhatskaya, G.N.; Pislyagin, E.A.; Tarbeeva, D.V.; Dmitrenok, P.S.; Fedoreyev, S.A. Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes simplex Virus Type 1. Mar Drugs 2020, 18, 550. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Lin, Q.; Gao, Y.; Ye, L.; Xing, Y.; Xi, T. Characterization and Antitumor Activity of a Polysaccharide from Strongylocentrotus nudus Eggs. Carbohydr. Polym. 2007, 67, 313–318. [Google Scholar] [CrossRef]
- Ke, M.; Wang, H.; Zhang, M.; Tian, Y.; Wang, Y.; Li, B.; Yu, J.; Dou, J.; Xi, T.; Zhou, C. The Anti-Lung Cancer Activity of SEP Is Mediated by the Activation and Cytotoxicity of NK Cells via TLR2/4 in Vivo. Biochem. Pharmacol. 2014, 89, 119–130. [Google Scholar] [CrossRef]
- Ke, M.; Wang, H.; Zhou, Y.; Li, J.; Liu, Y.; Zhang, M.; Dou, J.; Xi, T.; Shen, B.; Zhou, C. SEP Enhanced the Antitumor Activity of 5-Fluorouracil by up-Regulating NKG2D/MICA and Reversed Immune Suppression via Inhibiting ROS and Caspase-3 in Mice. Oncotarget 2016, 7, 49509–49526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Zhou, Y.; Wang, X.; Guo, J.; Li, J.; Fan, H.; Dou, J.; Shen, B.; Zhou, C. Enhanced Antitumor Activity of Gemcitabine by Polysaccharide-Induced NK Cell Activation and Immune Cytotoxicity Reduction in Vitro/Vivo. Carbohydr. Polym. 2017, 173, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ye, L.; Xing, Y.; Hu, J.; Xi, T. Combined SEP and Anti-PD-L1 Antibody Produces a Synergistic Antitumor Effect in B16-F10 Melanoma-Bearing Mice. Sci. Rep. 2018, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariya, Y.; Watabe, S.; Hashimoto, K.; Yoshida, K. Occurrence of Chondroitin Sulfate E in Glycosaminoglycan Isolated from the Body Wall of Sea Cucumber Stichopus japonicus. J. Biol. Chem. 1990, 265, 5081–5085. [Google Scholar] [CrossRef]
- Kariya, Y.; Watabe, S.; Kyogashima, M.; Ishihara, M.; Ishii, T. Structure of Fucose Branches in the Glycosaminoglycan from the Body Wall of the Sea Cucumber Stichopus japonicus. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A. Structure of a Fucose-Branched Chondroitin Sulfate from Sea Cucumber. Evidence for the Presence of 3-O-Sulfo-Beta-D-Glucuronosyl Residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef]
- Kylin, H. Zur Biochemie der Meeresalgen. H.-S. Z. Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H.; Mourão, P.A.S. Structure, Biology, Evolution, and Medical Importance of Sulfated Fucans and Galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H. Fucanomics and Galactanomics: Marine Distribution, Medicinal Impact, Conceptions, and Challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H. Review: An Overview about the Structure-Function Relationship of Marine Sulfated Homopolysaccharides with Regular Chemical Structures. Biopolymers 2009, 91, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.C.O.B.; Kozlowski, E.O.; Micheli, K.V.D.A.; Vilela-Silva, A.C.E.S.; Borsig, L.; Pavão, M.S.G. Sulfated Fucans and a Sulfated Galactan from Sea Urchins as Potent Inhibitors of Selectin-Dependent Hematogenous Metastasis. Glycobiology 2018, 28, 427–434. [Google Scholar] [CrossRef]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, H.; Tanaka, Y.; Yamada, S.; Seno, N.; Haslam, S.M.; Morris, H.R.; Dell, A.; Sugahara, K. A Novel Pentasaccharide Sequence GlcA(3-Sulfate)(Beta1-3)GalNAc(4-Sulfate)(Beta1-4)(Fuc Alpha1-3)GlcA(Beta1-3)GalNAc(4-Sulfate) in the Oligosaccharides Isolated from King Crab Cartilage Chondroitin Sulfate K and Its Differential Susceptibility to Chondroitinases and Hyaluronidase. Biochemistry 1997, 36, 3998–4008. [Google Scholar] [CrossRef]
- Higashi, K.; Okamoto, Y.; Mukuno, A.; Wakai, J.; Hosoyama, S.; Linhardt, R.J.; Toida, T. Functional Chondroitin Sulfate from Enteroctopus Dofleini Containing a 3-O-Sulfo Glucuronic Acid Residue. Carbohydr. Polym. 2015, 134, 557–565. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Pomin, V.H. The Sea as a Rich Source of Structurally Unique Glycosaminoglycans and Mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H.; Vignovich, W.P.; Gonzales, A.V.; Vasconcelos, A.A.; Mulloy, B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules 2019, 24, 2803. [Google Scholar] [CrossRef] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Nifantiev, N.E.; Usov, A.I. Structural Analysis of Holothurian Fucosylated Chondroitin Sulfates: Degradation versus Non-Destructive Approach. Carbohydr. Res. 2019, 476, 8–11. [Google Scholar] [CrossRef]
- Dwivedi, R.; Pomin, V.H. Marine Antithrombotics. Mar. Drugs 2020, 18, 514. [Google Scholar] [CrossRef] [PubMed]
- Vessella, G.; Traboni, S.; Laezza, A.; Iadonisi, A.; Bedini, E. (Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides. Mar. Drugs 2020, 18, 293. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Q.; Lv, K.; Ma, H.; Gao, C.; Liu, Y.; Zhang, S.; Zhao, L. Low-Molecular-Weight Fucosylated Glycosaminoglycan and Its Oligosaccharides from Sea Cucumber as Novel Anticoagulants: A Review. Carbohydr. Polym. 2021, 251, 117034. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Tartte, V. Triterpenoid Saponins: A Review On Biosynthesis, Applications And Mechanism Of Their Action. Int. J. Pharm. Pharm. Sci. 2015, 7, 24–28. [Google Scholar]
- Xiao, G.; Shao, X.; Zhu, D.; Yu, B. Chemical Synthesis of Marine Saponins. Nat. Prod. Rep. 2019, 36, 769–787. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, Taxonomic Distribution, Biogenesis and Biological Activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- Komori, T. Toxins from the Starfish Acanthaster planci and Asterina pectinifera. Toxicon 1997, 35, 1537–1548. [Google Scholar] [CrossRef]
- Demeyer, M.; Wisztorski, M.; Decroo, C.; De Winter, J.; Caulier, G.; Hennebert, E.; Eeckhaut, I.; Fournier, I.; Flammang, P.; Gerbaux, P. Inter- and Intra-Organ Spatial Distributions of Sea Star Saponins by MALDI Imaging. Anal. Bioanal. Chem. 2015, 407, 8813–8824. [Google Scholar] [CrossRef]
- Naruse, M.; Suetomo, H.; Matsubara, T.; Sato, T.; Yanagawa, H.; Hoshi, M.; Matsumoto, M. Acrosome Reaction-Related Steroidal Saponin, Co-ARIS, from the Starfish Induces Structural Changes in Microdomains. Dev. Biol. 2010, 347, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Silchenko, A.S.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Fucoidan from Brown Algae Fucus evanescens Potentiates the Anti-Proliferative Efficacy of Asterosaponins from Starfish Asteropsis carinifera in 2D and 3D Models of Melanoma Cells. Int. J. Biol. Macromol. 2021, 185, 31–39. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Effects of Polar Steroids from the Starfish Patiria (=Asterina) pectinifera in Combination with X-Ray Radiation on Colony Formation and Apoptosis Induction of Human Colorectal Carcinoma Cells. Molecules 2019, 24, 3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, J. Excellent Chemical and Material Cellulose from Tunicates: Diversity in Cellulose Production Yield and Chemical and Morphological Structures from Different Tunicate Species. Cellulose 2014, 21, 3427–3441. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, M.J.; Clemons, C.; Reiner, R.; Sabo, R.; Agarwal, U.P.; Bissessur, R.; Sojoudiasli, H.; Carreau, P.J.; Acharya, B. Towards the Scalable Isolation of Cellulose Nanocrystals from Tunicates. Sci. Rep. 2020, 10, 19090. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, W.; Lee, M.; Choi, J.; Song, B.; Son, H.; Lim, Y.; Kang, H.; An, B.; et al. Therapeutic Effects of a Liquid Bandage Prepared with Cellulose Powders from Styela Clava Tunics and Broussonetia kazinoki Bark: Healing of Surgical Wounds on the Skin of Sprague Dawley Rats. Mol. Med. Rep. 2018, 19, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Song, S.H.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Sung, G.Y.; Kwon, S.H.; Son, H.J.; Lee, H.S.; Jung, Y.J.; Hwang, D.Y. Cellulose Film Regenerated from Styela clava Tunics Have Biodegradability, Toxicity and Biocompatibility in the Skin of SD Rats. J. Mater. Sci. Mater. Med. 2014, 25, 1519–1530. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, J.M.; Kang, T.Y.; Kim, Y.S.; Lee, S.K. Purification of Squirt Cellulose Membrane from the Cystic Tunic of Styela Clava and Identification of Its Osteoconductive Effect. Cellulose 2013, 20, 655–673. [Google Scholar] [CrossRef]
- Kim, D.S.; Jung, S.-M.; Yoon, G.H.; Lee, H.C.; Shin, H.S. Development of a Complex Bone Tissue Culture System Based on Cellulose Nanowhisker Mechanical Strain. Colloids. Surf. B Biointerfaces 2014, 123, 838–844. [Google Scholar] [CrossRef]
- Dugan, J.M.; Collins, R.F.; Gough, J.E.; Eichhorn, S.J. Oriented Surfaces of Adsorbed Cellulose Nanowhiskers Promote Skeletal Muscle Myogenesis. Acta Biomater. 2013, 9, 4707–4715. [Google Scholar] [CrossRef]
- Hu, D.; Cui, Y.; Mo, K.; Wang, J.; Huang, Y.; Miao, X.; Lin, J.; Chang, C. Ultrahigh Strength Nanocomposite Hydrogels Designed by Locking Oriented Tunicate Cellulose Nanocrystals in Polymeric Networks. Compos. Part B Eng. 2020, 197, 108118. [Google Scholar] [CrossRef]
- Pavão, M.S.; Aiello, K.R.; Werneck, C.C.; Silva, L.C.; Valente, A.P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A. Highly Sulfated Dermatan Sulfates from Ascidians. Structure versus Anticoagulant Activity of These Glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavão, M.S.G. Ascidian (Chordata-Tunicata) Glycosaminoglycans: Extraction, Purification, Biochemical, and Spectroscopic Analysis. Methods Mol. Biol. 2015, 1229, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.W.; Kammerer, B.; Bayer, E. New Perspectives in the Chemistry and Biochemistry of the Tunichromes and Related Compounds. Chem. Rev. 1997, 97, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wunderer, J.; Salvenmoser, W.; Ederth, T.; Rothbächer, U. Identifying Adhesive Components in a Model Tunicate. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190197. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, X.; Chen, Y.; Li, X.; Zhan, A. Identification and Characterization of Proteins Involved in Stolon Adhesion in the Highly Invasive Fouling Ascidian Ciona Robusta. Biochem. Biophys. Res. Commun. 2019, 510, 91–96. [Google Scholar] [CrossRef]
- Zhan, K.; Kim, C.; Sung, K.; Ejima, H.; Yoshie, N. Tunicate-Inspired Gallol Polymers for Underwater Adhesive: A Comparative Study of Catechol and Gallol. Biomacromolecules 2017, 18, 2959–2966. [Google Scholar] [CrossRef]
- Oh, D.X.; Kim, S.; Lee, D.; Hwang, D.S. Tunicate-Mimetic Nanofibrous Hydrogel Adhesive with Improved Wet Adhesion. Acta Biomater. 2015, 20, 104–112. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Lee, S.; Rho, S.; Lee, H.; Kim, I.S.; Hwang, D.S. Tunichrome-Inspired Pyrogallol Functionalized Chitosan for Tissue Adhesion and Hemostasis. Carbohydr. Polym. 2019, 208, 77–85. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.S.; Shin, J.; Jeon, E.J.; An, S.; Choi, Y.S.; Cho, S.-W. Ascidian-Inspired Fast-Forming Hydrogel System for Versatile Biomedical Applications: Pyrogallol Chemistry for Dual Modes of Crosslinking Mechanism. Adv. Funct. Mater. 2018, 28, 1705244. [Google Scholar] [CrossRef]
- Prajatelistia, E.; Ju, S.W.; Sanandiya, N.D.; Jun, S.H.; Ahn, J.S.; Hwang, D.S. Tunicate-Inspired Gallic Acid/Metal Ion Complex for Instant and Efficient Treatment of Dentin Hypersensitivity. Adv. Healthc. Mater. 2016, 5, 919–927. [Google Scholar] [CrossRef]
- Wang, L.; Gong, T.; Brown, Z.; Randle, C.; Guan, Y.; Ye, W.; Ming, W. Ascidian-Inspired Heparin-Mimetic Magnetic Nanoparticles with Potential for Application in Hemodialysis as Recycling Anticoagulants. ACS Biomater. Sci. Eng. 2020, 6, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E. Novel Marine-Derived Anti-Cancer Agents. Curr. Pharm. Des. 2007, 13, 3417–3426. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Yao, D. Diving for Drugs: Tunicate Anticancer Compounds. Drug Discov. Today 2012, 17, 636–648. [Google Scholar] [CrossRef]
- Dou, X.; Dong, B. Origins and Bioactivities of Natural Compounds Derived from Marine Ascidians and Their Symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, C.; Tulasi, B.R.; Raju, M.; Thakur, N.; Dufossé, L. Marine Natural Products from Tunicates and Their Associated Microbes. Mar. Drugs 2021, 19, 308. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—Ribosomal Cyclic Peptides Produced by Cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Wunderer, J.; Salvenmoser, W.; Hess, M.W.; Ladurner, P.; Rothbächer, U. Papillae Revisited and the Nature of the Adhesive Secreting Collocytes. Dev. Biol. 2019, 448, 183–198. [Google Scholar] [CrossRef]
- Casertano, M.; Menna, M.; Imperatore, C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics 2020, 9, 510. [Google Scholar] [CrossRef]
- Ayuningrum, D.; Liu, Y.; Riyanti; Sibero, M.T.; Kristiana, R.; Asagabaldan, M.A.; Wuisan, Z.G.; Trianto, A.; Radjasa, O.K.; Sabdono, A.; et al. Tunicate-Associated Bacteria Show a Great Potential for the Discovery of Antimicrobial Compounds. PLoS ONE 2019, 14, e0213797. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.-S.; Xu, J.-L.; Shao, C.-L.; Wang, G.-Y. Biological and Chemical Diversity of Ascidian-Associated Microorganisms. Mar. Drugs 2018, 16, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinehart, K.L.; Gloer, J.B.; Hughes, R.G.; Renis, H.E.; McGovren, J.P.; Swynenberg, E.B.; Stringfellow, D.A.; Kuentzel, S.L.; Li, L.H. Didemnins: Antiviral and Antitumor Depsipeptides from a Caribbean Tunicate. Science 1981, 212, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial Production of the Tunicate-Derived Antitumor Cyclic Depsipeptide Didemnin B. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar] [CrossRef]
- Sakai, R.; Rinehart, K.L.; Kishore, V.; Kundu, B.; Faircloth, G.; Gloer, J.B.; Carney, J.R.; Namikoshi, M.; Sun, F.; Hughes, R.G.; et al. Structure−Activity Relationships of the Didemnins. J. Med. Chem. 1996, 39, 2819–2834. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Raymond, E.; Faivre, S. Aplidine: A Paradigm of How to Handle the Activity and Toxicity of a Novel Marine Anticancer Poison. Curr. Pharm. Des. 2007, 13, 3427–3439. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, Development, and Potential Place in Therapy. Drug Des. Dev. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Taddei, M.L.; Chiarugi, P.; Cuevas, C.; Ramponi, G.; Raugei, G. Oxidation and Inactivation of Low Molecular Weight Protein Tyrosine Phosphatase by the Anticancer Drug Aplidin. Int. J. Cancer 2006, 118, 2082–2088. [Google Scholar] [CrossRef]
- Xu, Y.; Kersten, R.D.; Nam, S.-J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.-Y. Bacterial Biosynthesis and Maturation of the Didemnin Anti-Cancer Agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef] [Green Version]
- Le, V.H.; Inai, M.; Williams, R.M.; Kan, T. Ecteinascidins. A Review of the Chemistry, Biology and Clinical Utility of Potent Tetrahydroisoquinoline Antitumor Antibiotics. Nat. Prod. Rep. 2015, 32, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Moss, C.; Green, D.; MacKenzie, J.D.; Perez, B.; Velasco, A.; Henriques, R. Intracellular Bacteria Associated with the Ascidian Ecteinascidia turbinata: Phylogenetic and in Situ Hybridisation Analysis. Mar. Biol. 2003, 99–110. [Google Scholar] [CrossRef]
- Pérez-Matos, A.E.; Rosado, W.; Govind, N.S. Bacterial Diversity Associated with the Caribbean Tunicate Ecteinascidia turbinata. Antonie Van Leeuwenhoek 2007, 92, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Rath, C.M.; Janto, B.; Earl, J.; Ahmed, A.; Hu, F.Z.; Hiller, L.; Dahlgren, M.; Kreft, R.; Yu, F.; Wolff, J.J.; et al. Meta-Omic Characterization of the Marine Invertebrate Microbial Consortium That Produces the Chemotherapeutic Natural Product ET-743. ACS Chem. Biol. 2011, 6, 1244–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azumi, K.; Yokosawa, H.; Ishii, S. Halocyamines: Novel Antimicrobial Tetrapeptide-like Substances Isolated from the Hemocytes of the Solitary Ascidian Halocynthia roretzi. Biochemistry 1990, 29, 159–165. [Google Scholar] [CrossRef]
- Fedders, H.; Michalek, M.; Grötzinger, J.; Leippe, M. An Exceptional Salt-Tolerant Antimicrobial Peptide Derived from a Novel Gene Family of Haemocytes of the Marine Invertebrate Ciona intestinalis. Biochem. J. 2008, 416, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedders, H.; Leippe, M. A Reverse Search for Antimicrobial Peptides in Ciona intestinalis: Identification of a Gene Family Expressed in Hemocytes and Evaluation of Activity. Dev. Comp. Immunol. 2008, 32, 286–298. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: A New Antimicrobial Peptide from Hemocytes of the Solitary Tunicate, Halocynthia aurantium. FEBS Lett. 2002, 521, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.H.; Zhao, C.; Nguyen, T.; Menzel, L.; Waring, A.J.; Sherman, M.A.; Lehrer, R.I. Clavaspirin, an Antibacterial and Haemolytic Peptide from Styela clava. J. Pept. Res. 2001, 58, 445–456. [Google Scholar]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Styelins, Broad-Spectrum Antimicrobial Peptides from the Solitary Tunicate, Styela clava. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 515–521. [Google Scholar] [CrossRef]
- Taylor, S.W.; Craig, A.G.; Fischer, W.H.; Park, M.; Lehrer, R.I.; Styelin, D. An Extensively Modified Antimicrobial Peptide from Ascidian Hemocytes. J. Biol. Chem. 2000, 275, 38417–38426. [Google Scholar] [CrossRef] [Green Version]
- Tincu, J.A.; Menzel, L.P.; Azimov, R.; Sands, J.; Hong, T.; Waring, A.J.; Taylor, S.W.; Lehrer, R.I. Plicatamide, an Antimicrobial Octapeptide from Styela plicata Hemocytes. J. Biol. Chem. 2003, 278, 13546–13553. [Google Scholar] [CrossRef] [Green Version]
- Ballarin, L.; Cima, F.; Floreani, M.; Sabbadin, A. Oxidative Stress Induces Cytotoxicity during Rejection Reaction in the Compound Ascidian Botryllus schlosseri. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 411–418. [Google Scholar] [CrossRef]
- Menzel, L.P.; Lee, I.H.; Sjostrand, B.; Lehrer, R.I. Immunolocalization of Clavanins in Styela clava Hemocytes. Dev. Comp. Immunol. 2002, 26, 505–515. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Andrew Tincu, J.; Taylor, S.W.; Menzel, L.P.; Waring, A.J. Natural Peptide Antibiotics from Tunicates: Structures, Functions and Potential Uses1. Integr. Comp. Biol. 2003, 43, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liaw, L.; Lee, I.H.; Lehrer, R.I. CDNA Cloning of Three Cecropin-like Antimicrobial Peptides (Styelins) from the Tunicate, Styela clava. FEBS Lett. 1997, 412, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, M.D.; Fedders, H.; Leippe, M.; De Leo, G. A preliminary study on antimicrobial peptides in the naturally damaged tunic of Ciona intestinalis (Tunicata). In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1003–1007. [Google Scholar]
- Fedders, H.; Podschun, R.; Leippe, M. The Antimicrobial Peptide Ci-MAM-A24 Is Highly Active against Multidrug-Resistant and Anaerobic Bacteria Pathogenic for Humans. Int. J. Antimicrob. Agents 2010, 36, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Bauman, K.D.; Butler, K.S.; Moore, B.S.; Chekan, J.R. Genome Mining Methods to Discover Bioactive Natural Products. Nat. Prod. Rep. 2021, 38, 2100–2129. [Google Scholar] [CrossRef]
- Machado, H.; Tuttle, R.N.; Jensen, P.R. Omics-Based Natural Product Discovery and the Lexicon of Genome Mining. Curr. Opin. Microbiol. 2017, 39, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Scherlach, K.; Hertweck, C. Mining and Unearthing Hidden Biosynthetic Potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef]
- Williams, A.N.; Sorout, N.; Cameron, A.J.; Stavrinides, J. The Integration of Genome Mining, Comparative Genomics, and Functional Genetics for Biosynthetic Gene Cluster Identification. Front. Genet. 2020, 11, 600116. [Google Scholar] [CrossRef]
- Leão, T.; Wang, M.; Moss, N.; da Silva, R.; Sanders, J.; Nurk, S.; Gurevich, A.; Humphrey, G.; Reher, R.; Zhu, Q.; et al. A Multi-Omics Characterization of the Natural Product Potential of Tropical Filamentous Marine Cyanobacteria. Mar. Drugs 2021, 19, 20. [Google Scholar] [CrossRef]
- Bumpus, S.B.; Evans, B.S.; Thomas, P.M.; Ntai, I.; Kelleher, N.L. A Proteomics Approach to Discovery of Natural Products and Their Biosynthetic Pathways. Nat. Biotechnol. 2009, 27, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverter, M.; Rohde, S.; Parchemin, C.; Tapissier-Bontemps, N.; Schupp, P.J. Metabolomics and Marine Biotechnology: Coupling Metabolite Profiling and Organism Biology for the Discovery of New Compounds. Front. Mar. Sci. 2020, 7, 613471. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grkovic, T.; Akee, R.K.; Thornburg, C.C.; Trinh, S.K.; Britt, J.R.; Harris, M.J.; Evans, J.R.; Kang, U.; Ensel, S.; Henrich, C.J.; et al. National Cancer Institute (NCI) Program for Natural Products Discovery: Rapid Isolation and Identification of Biologically Active Natural Products from the NCI Prefractionated Library. ACS Chem. Biol. 2020, 15, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.M.; Soule, M.C.K.; Kujawinski, E.B. Extraction Efficiency and Quantification of Dissolved Metabolites in Targeted Marine Metabolomics. Limnol. Oceanogr. Methods 2017, 15, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Snyder, N.W.; Khezam, M.; Mesaros, C.A.; Worth, A.; Blair, I.A. Untargeted Metabolomics from Biological Sources Using Ultraperformance Liquid Chromatography-High Resolution Mass Spectrometry (UPLC-HRMS). J. Vis. Exp. 2013, e50433. [Google Scholar] [CrossRef] [Green Version]
- Viant, M.R.; Rosenblum, E.S.; Tjeerdema, R.S. NMR-Based Metabolomics: A Powerful Approach for Characterizing the Effects of Environmental Stressors on Organism Health. Environ. Sci. Technol. 2003, 37, 4982–4989. [Google Scholar] [CrossRef]
- Amberg, A.; Riefke, B.; Schlotterbeck, G.; Ross, A.; Senn, H.; Dieterle, F.; Keck, M. NMR and MS Methods for Metabolomics. Methods Mol. Biol. 2017, 1641, 229–258. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Nuzillard, J.-M.; van der Hooft, J.J.J.; Renault, J.-H.; Bertrand, S. Accelerating Metabolite Identification in Natural Product Research: Toward an Ideal Combination of Liquid Chromatography–High-Resolution Tandem Mass Spectrometry and NMR Profiling, in Silico Databases, and Chemometrics. Anal. Chem. 2019, 91, 704–742. [Google Scholar] [CrossRef]
- Blunt, J.W.; Munro, M.H.G. (Eds.) Dictionary of Marine Natural Products with CD-ROM; Chapman and Hall/CRC: New York, NY, USA, 2007; ISBN 978-0-429-18643-1. [Google Scholar]
- Johnson, S.R.; Lange, B.M. Open-Access Metabolomics Databases for Natural Product Research: Present Capabilities and Future Potential. Front Bioeng Biotechnol 2015, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The Marine Biodiscovery Pipeline and Ocean Medicines of Tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Betancur, L.A.; Naranjo-Gaybor, S.J.; Vinchira-Villarraga, D.M.; Moreno-Sarmiento, N.C.; Maldonado, L.A.; Suarez-Moreno, Z.R.; Acosta-González, A.; Padilla-Gonzalez, G.F.; Puyana, M.; Castellanos, L.; et al. Marine Actinobacteria as a Source of Compounds for Phytopathogen Control: An Integrative Metabolic-Profiling/Bioactivity and Taxonomical Approach. PLoS ONE 2017, 12, e0170148. [Google Scholar] [CrossRef]
- Bose, U.; Ortori, C.A.; Sarmad, S.; Barrett, D.A.; Hewavitharana, A.K.; Hodson, M.P.; Fuerst, J.A.; Shaw, P.N.; Boden, R. Production of N-Acyl Homoserine Lactones by the Sponge-Associated Marine Actinobacteria Salinispora arenicola and Salinispora Pacifica. FEMS Microbiol. Lett. 2017, 364, fnx002. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Abdelmohsen, U.R. Isolation of Petrocidin A, a New Cytotoxic Cyclic Dipeptide from the Marine Sponge-Derived Bacterium Streptomyces sp. SBT348. Mar. Drugs 2017, 15, 383. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Othman, E.M.; Stopper, H.; Fouad, M.A. Metabolomic Profiling and Biological Investigation of the Marine Sponge-Derived Bacterium Rhodococcus Sp. UA13. Phytochem. Anal. 2018, 29, 543–548. [Google Scholar] [CrossRef]
- Paul, V.J.; Freeman, C.J.; Agarwal, V. Chemical Ecology of Marine Sponges: New Opportunities through “-Omics”. Integr. Comp. Biol. 2019, 59, 765–776. [Google Scholar] [CrossRef]
- Villegas-Plazas, M.; Wos-Oxley, M.L.; Sanchez, J.A.; Pieper, D.H.; Thomas, O.P.; Junca, H. Variations in Microbial Diversity and Metabolite Profiles of the Tropical Marine Sponge Xestospongia muta with Season and Depth. Microb. Ecol. 2019, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.W.A.; Gutierrez, A.P.; Cortés-Araya, Y.; Houston, R.D.; Bean, T.P. Developments in Marine Invertebrate Primary Culture Reveal Novel Cell Morphologies in the Model Bivalve Crassostrea gigas. PeerJ 2020, 8, e9180. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Toullec, G.; Fricano, C.; Chapron, L.; Meunier, V.; Röttinger, E.; Furla, P.; Barnay-Verdier, S. Cnidarian Primary Cell Culture as a Tool to Investigate the Effect of Thermal Stress at Cellular Level. Mar. Biotechnol. 2018, 20, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Conkling, M.; Hesp, K.; Munroe, S.; Sandoval, K.; Martens, D.E.; Sipkema, D.; Wijffels, R.H.; Pomponi, S.A. Breakthrough in Marine Invertebrate Cell Culture: Sponge Cells Divide Rapidly in Improved Nutrient Medium. Sci. Rep. 2019, 9, 17321. [Google Scholar] [CrossRef] [Green Version]
- Urban-Gedamke, E.; Conkling, M.; McCarthy, P.J.; Wills, P.S.; Pomponi, S.A. 3-D Culture of Marine Sponge Cells for Production of Bioactive Compounds. Mar. Drugs 2021, 19, 569. [Google Scholar] [CrossRef]
- De Caralt, S.; Uriz, M.J.; Wijffels, R.H. Cell Culture from Sponges: Pluripotency and Immortality. Trends Biotechnol. 2007, 25, 467–471. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Böhm, M.; Batel, R.; De Rosa, S.; Tommonaro, G.; Müller, I.M.; Schröder, H.C. Application of Cell Culture for the Production of Bioactive Compounds from Sponges: Synthesis of Avarol by Primmorphs from Dysidea avara. J. Nat. Prod. 2000, 63, 1077–1081. [Google Scholar] [CrossRef]
- Glaser, K.B.; Mayer, A.M.S. A Renaissance in Marine Pharmacology: From Preclinical Curiosity to Clinical Reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef]

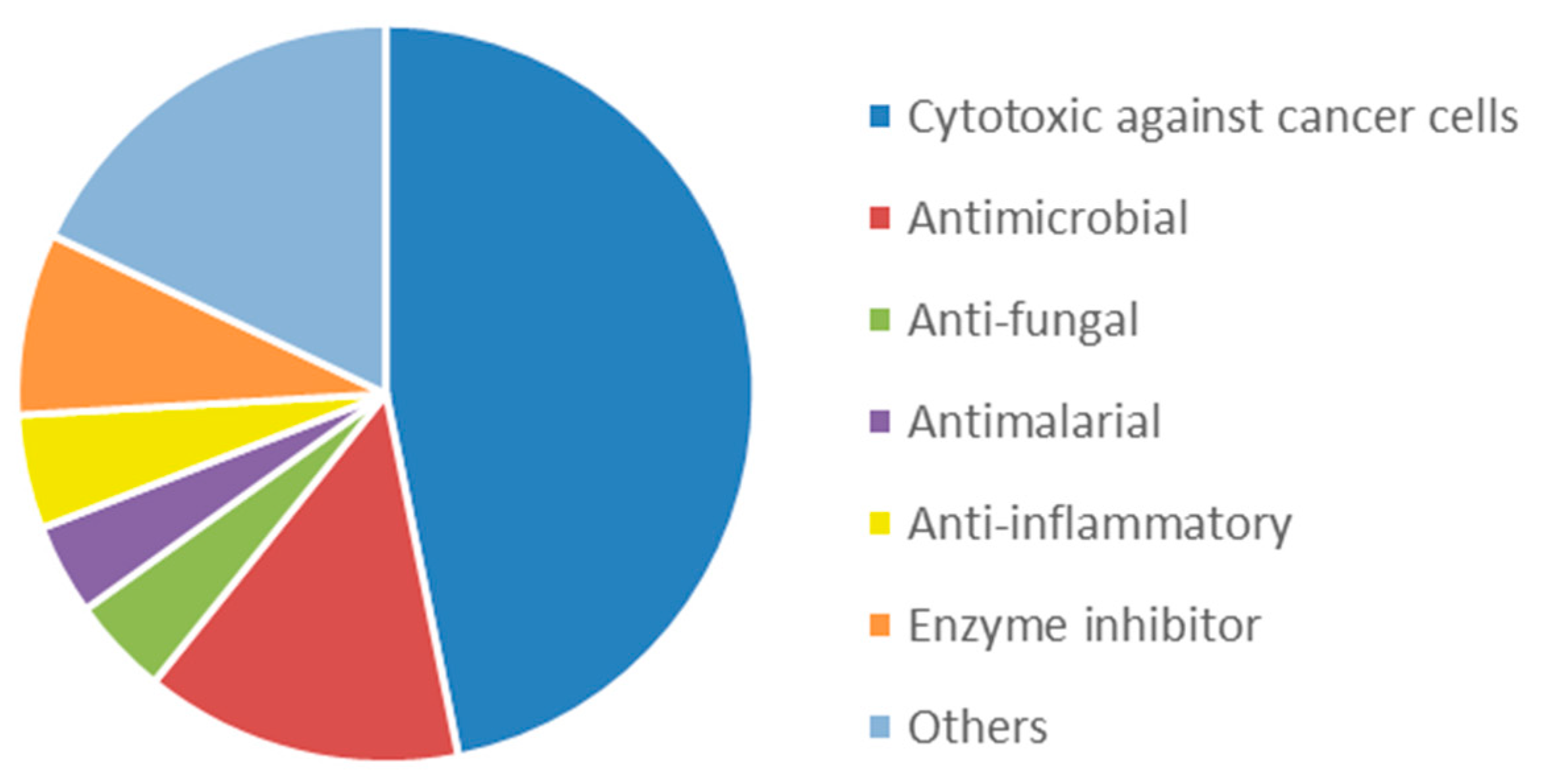
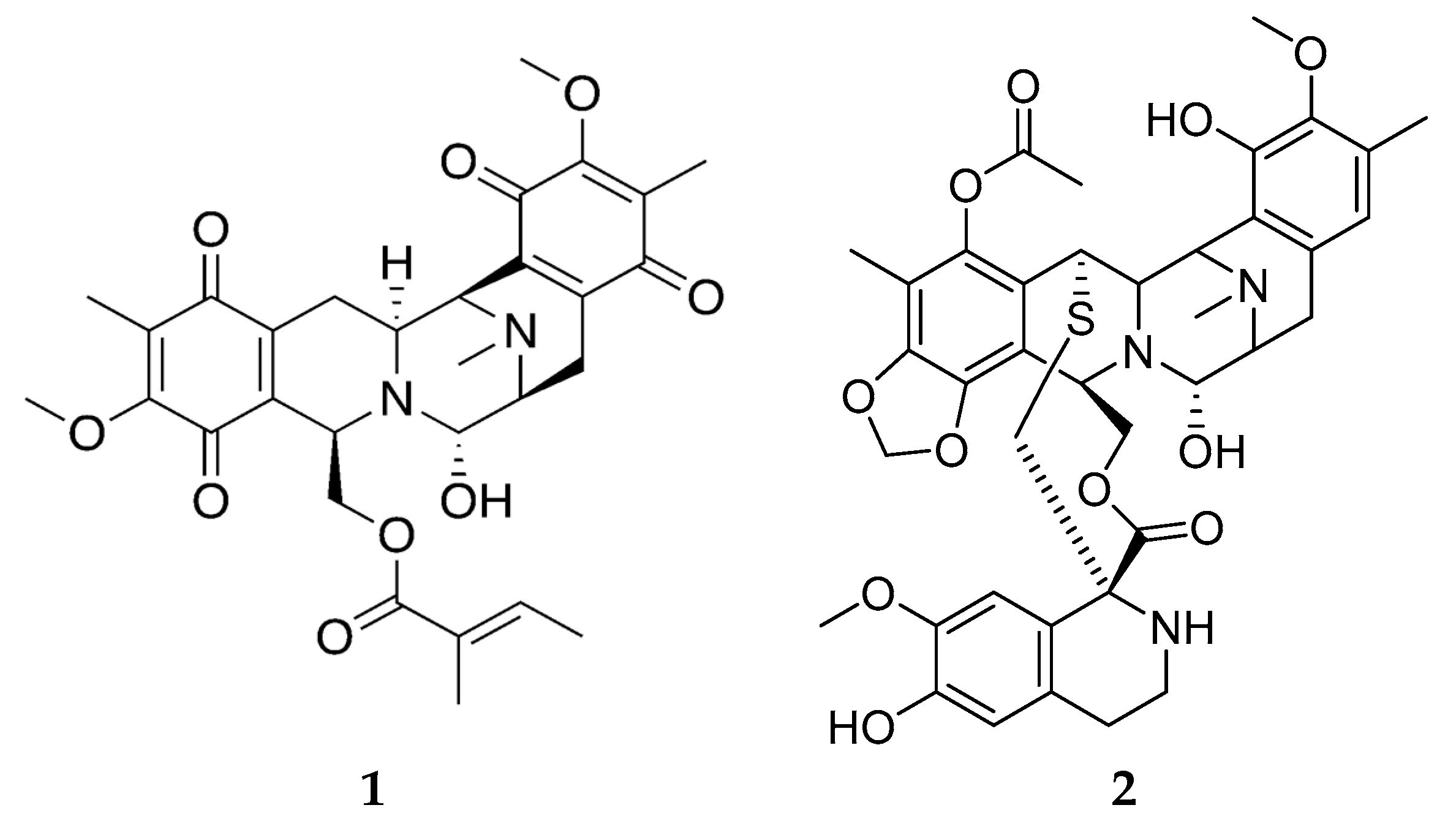
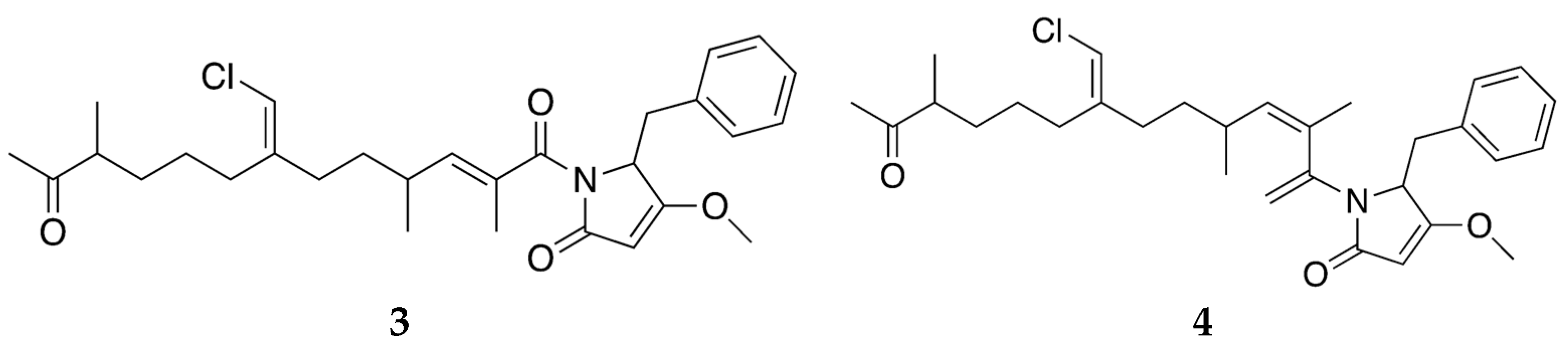

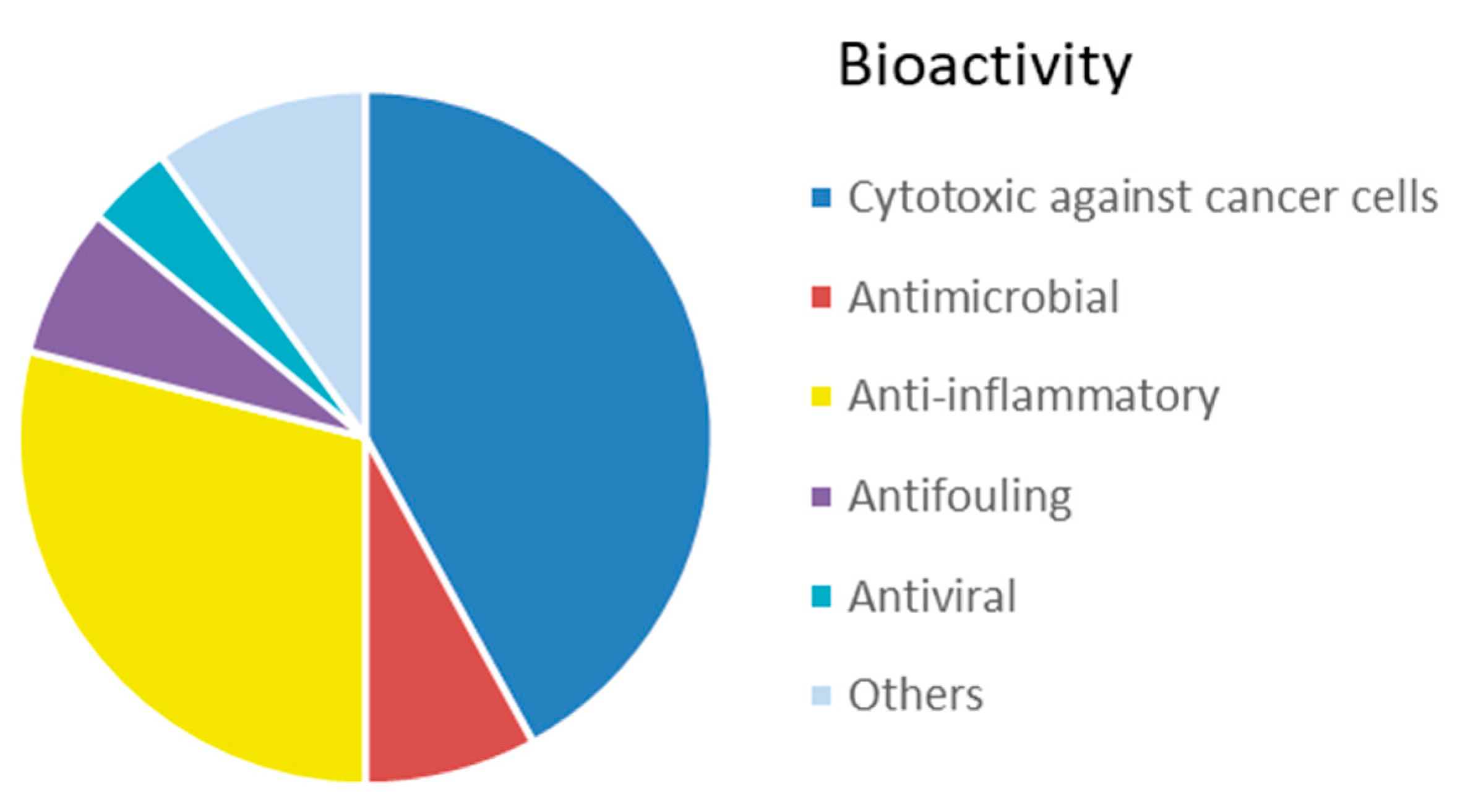
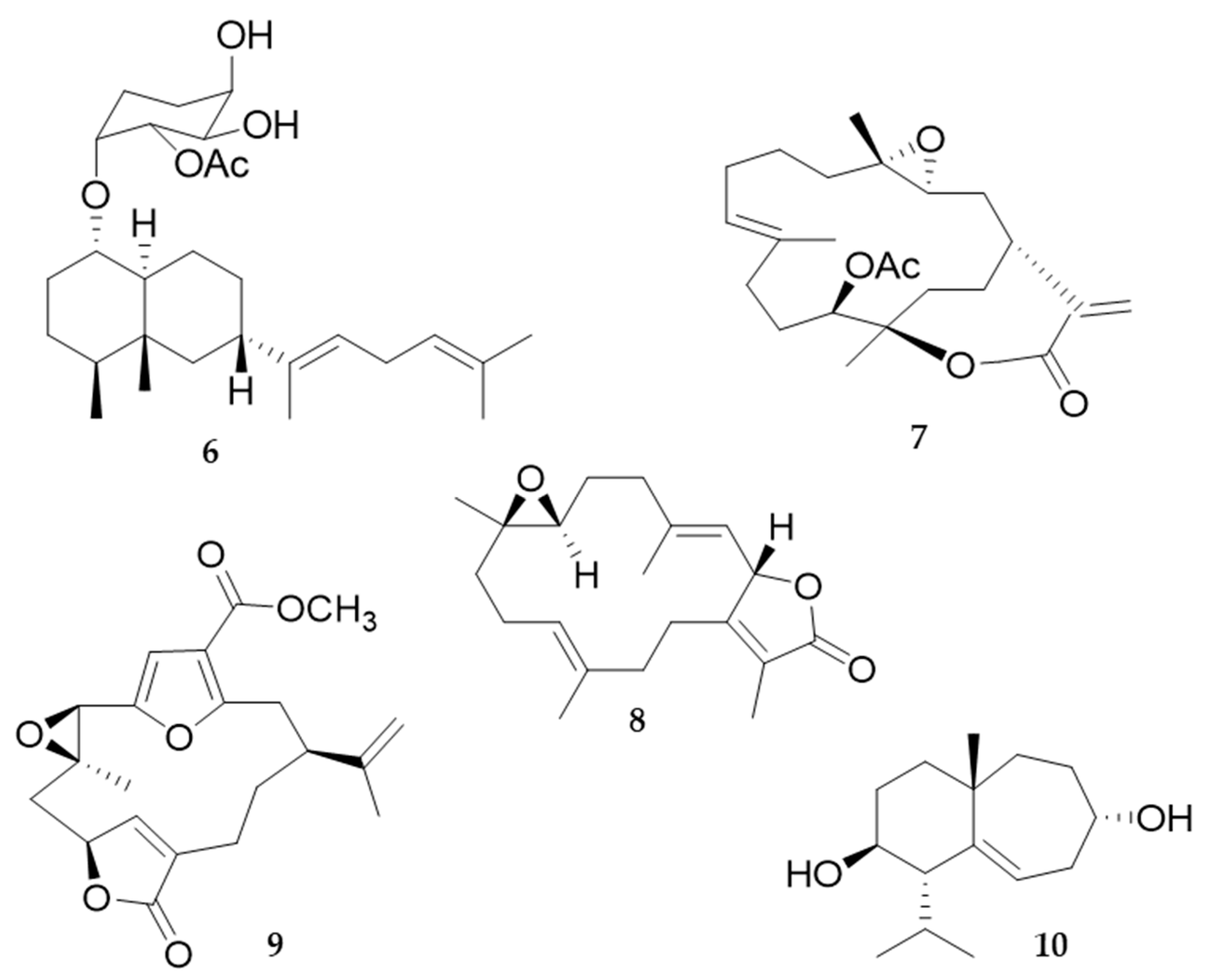
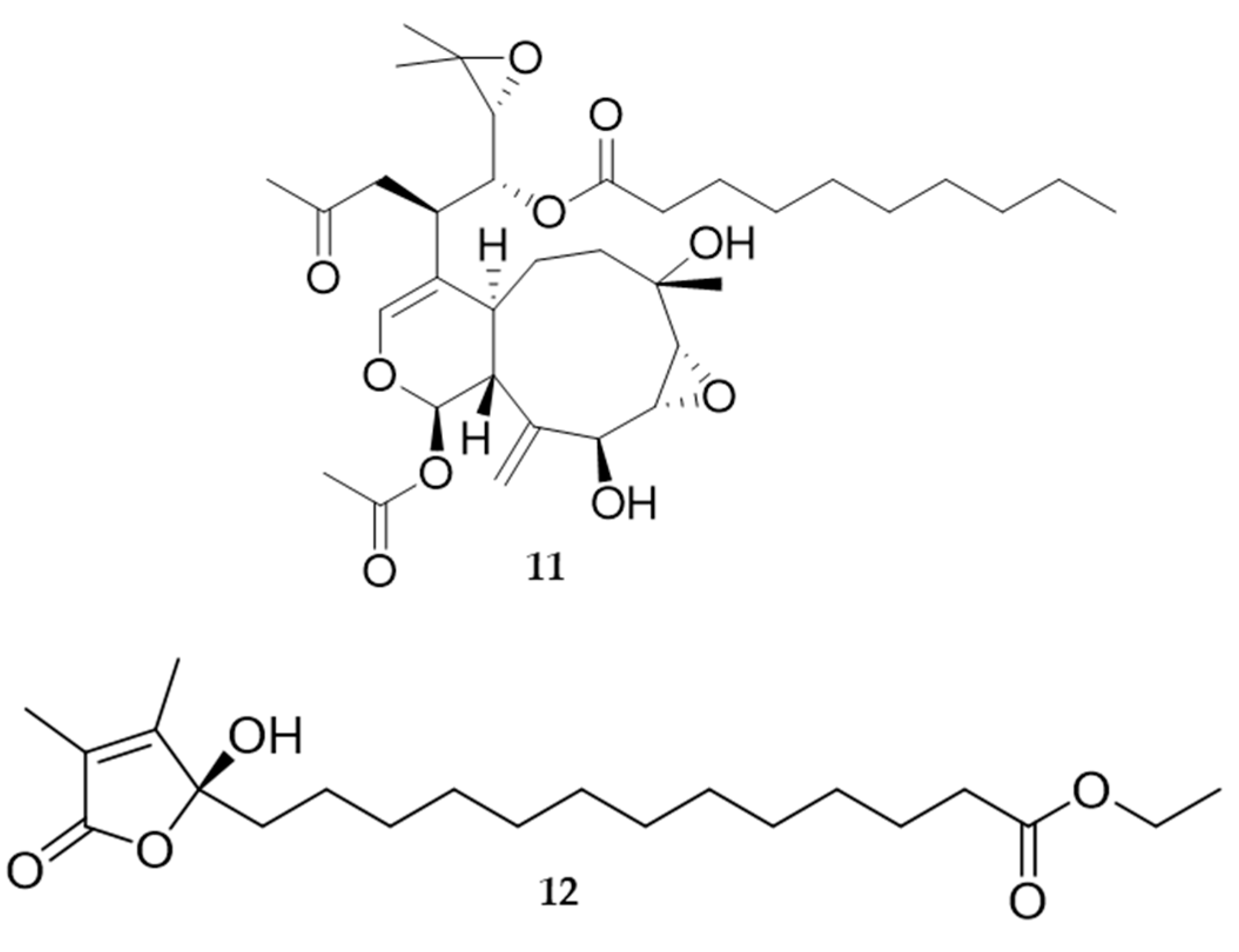

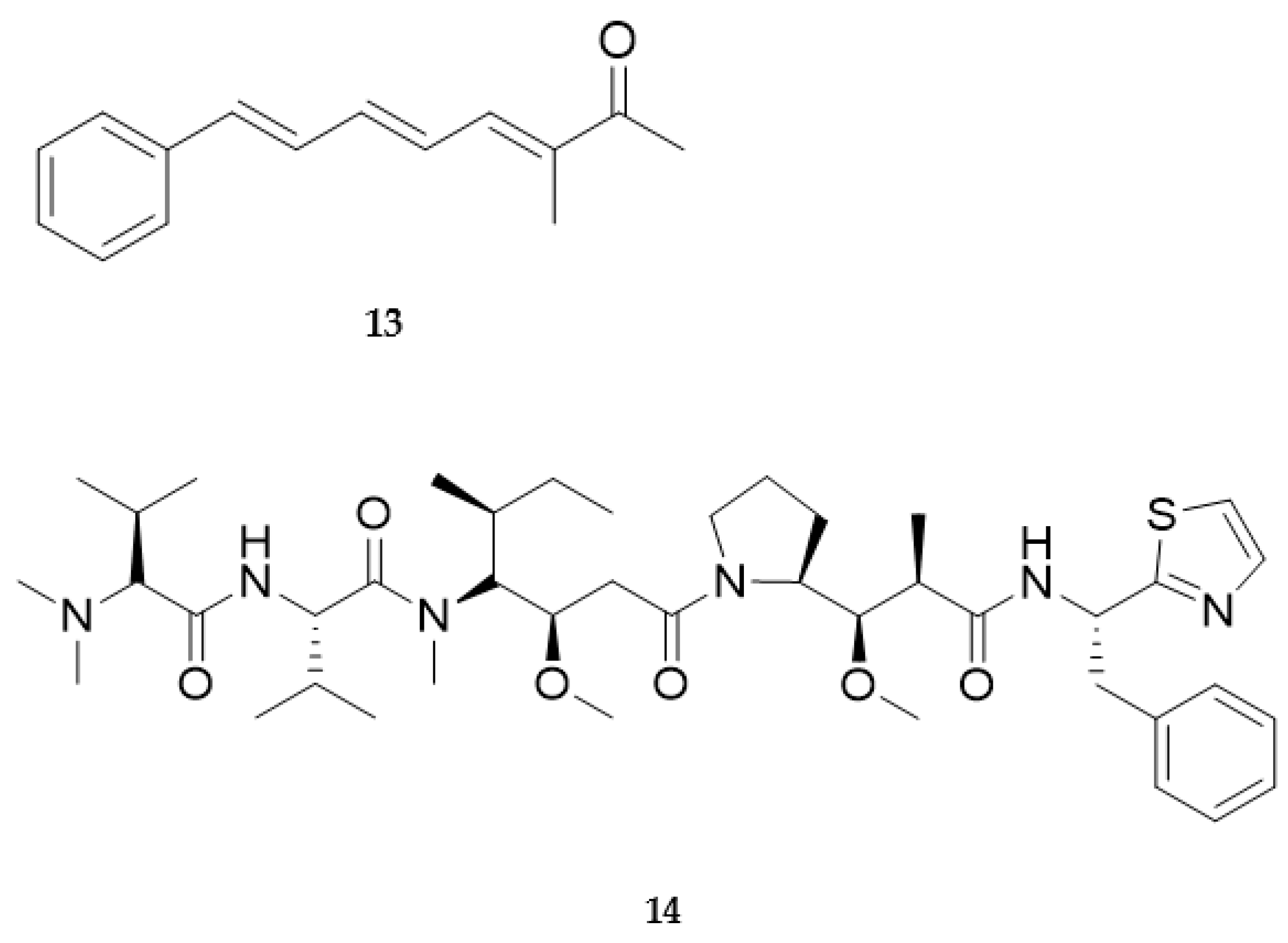



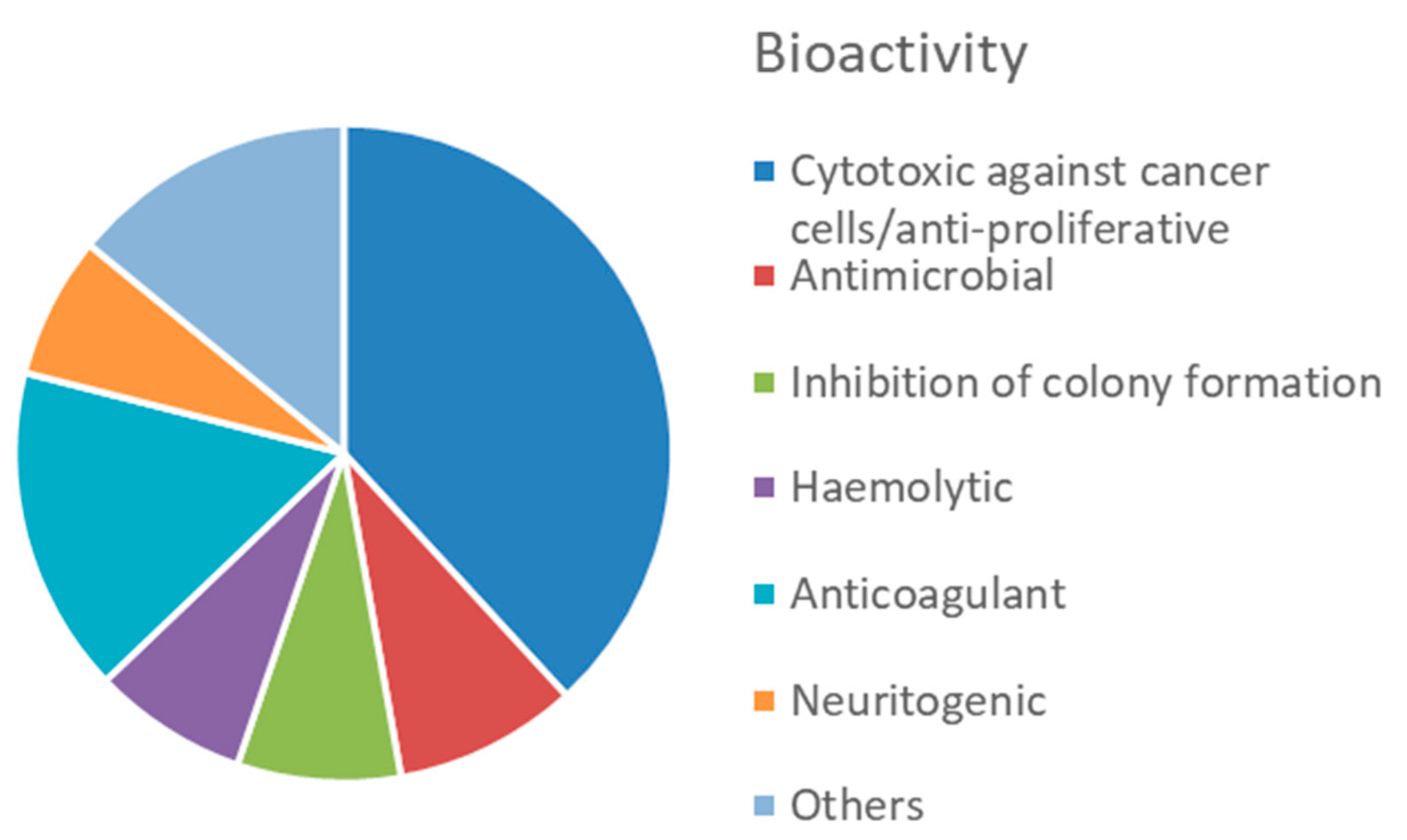
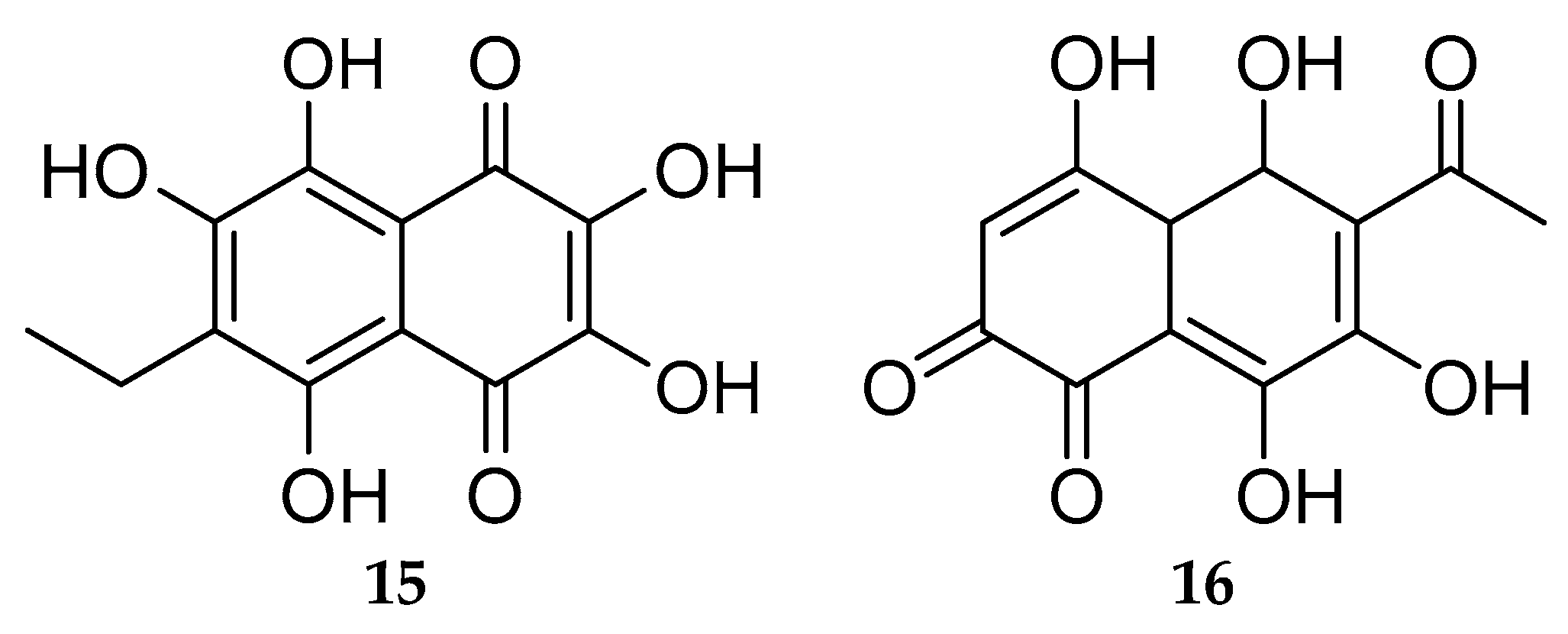


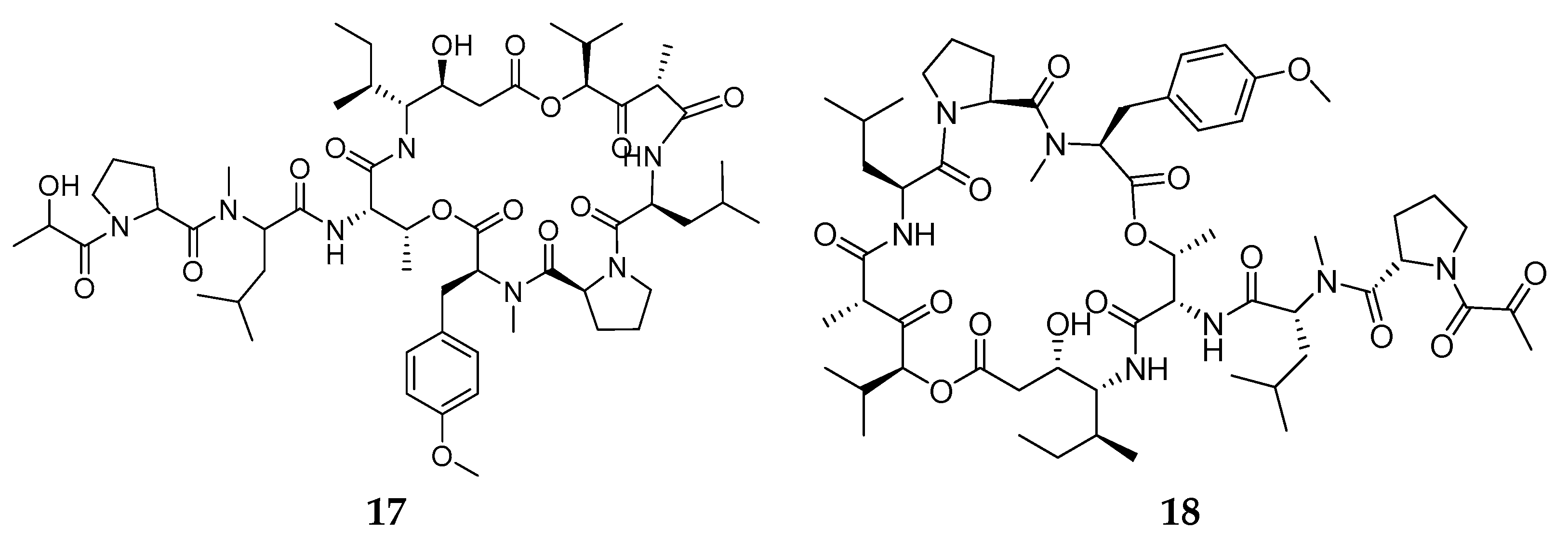
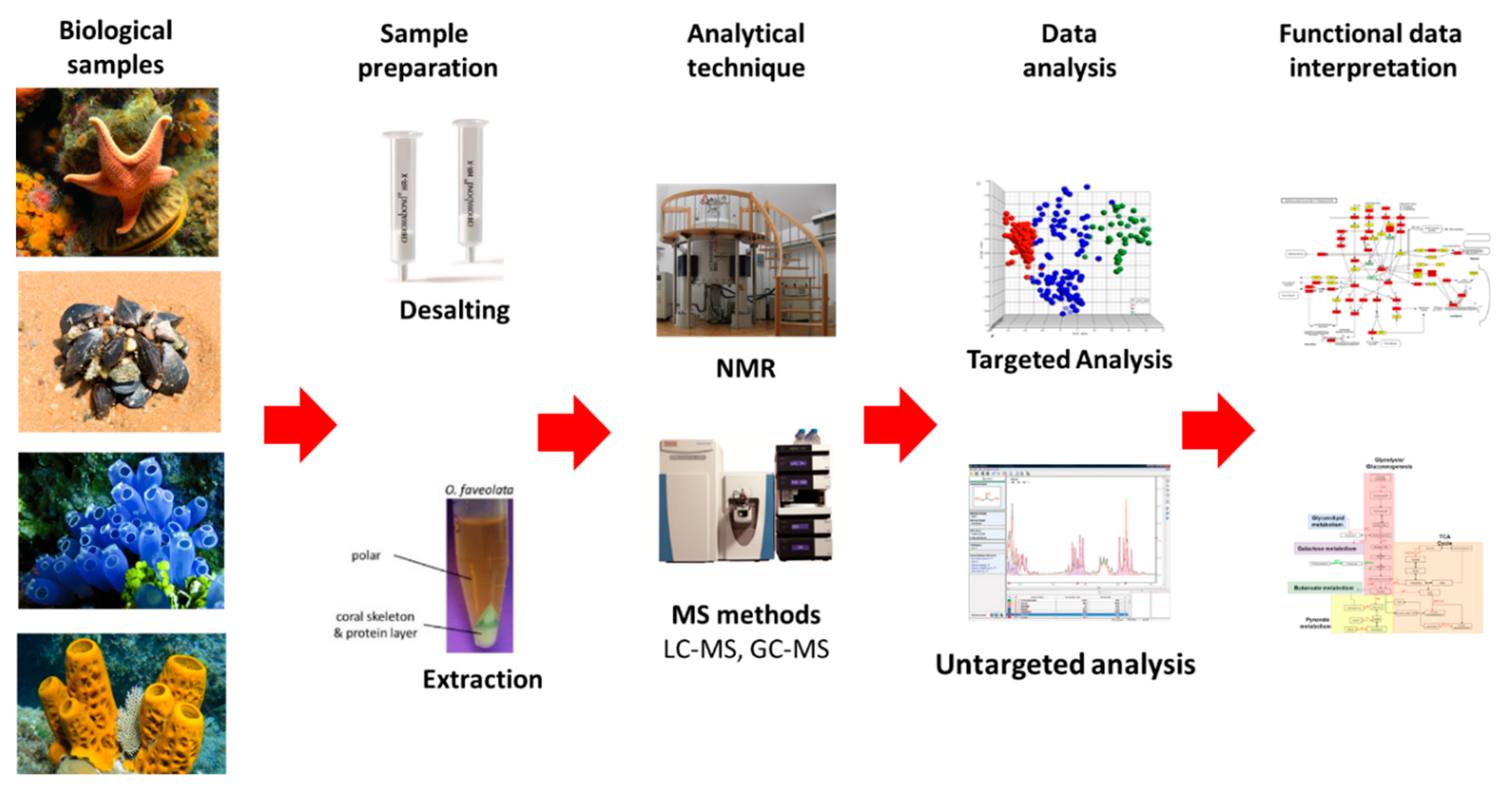

| Class | Producer Species | Class of Biomaterial | Biomaterial | Origin/Structural Component | Possible Applications | References |
|---|---|---|---|---|---|---|
| Demospongiae | Petrosia ficidormis, | Bioceramics | Silicate, Calcium carbonates Silicate/calcium salts | whole body | Support for tissue regeneration | [19] |
| Demospongiae | Petrosia ficidormis, Chondrosia reniformis and Agelas oroides | Bioceramics | Calcium phosphate (hydroxyapatite) | skeleton | Substitutes for synthetic Bioglass® | [20] |
| Demospongiae | Petrosia ficidormis | Inorganic polymer | Biosilica | whole body | 3D support for osteoblast adhesion and growth | [21] |
| Demospongiae | Spongia agaricina, Spongia officinalis, Spongia zimocca | Inorganic polymer | Hydroxyapatite | whole body | Bone substitute material | [22] |
| Demospongiae | Suberites domuncula | Inorganic polymer | Biosilica | skeleton | Stimulates mineralizing activity | [23] |
| n.a. | n.a. | Inorganic polymer | Silicate | skeleton | Stimulates osteogenesis in vivo | [24] |
| n.a. | n.a. | Inorganic polymer | Silica/silicatein | skeleton | Regeneration of bone tissue | [25] |
| n.a. | n.a. | Inorganic polymer | Biosilica/polyphosphate | skeleton | Promotes growth and differentiation of hMSCs *; 3D tissue printing of hMSCs *; Delivery of hMSCs * in fractures | [25] |
| Demospongiae | Spongia agaricina | Inorganic polymer | Hydroxyapatite | whole body | Bone tissue engineering | [26] |
| Demospongiae | Spongia agaricina | Inorganic polymer | Hydroxyapatite | whole body | Tissue engineering (bone scaffolds) | [22] |
| Demospongiae | Ianthella labyrinthus | Polysaccharides | Chitin | skeleton | Scaffolds to culture cardiomyocytes differentiated from human-induced pluripotent stem cells (ipsc-cms); 3D tissue engineering | [27] |
| Demospongiae | Ianthella flabelliformis | Polysaccharides | Chitin | skeleton | Drug delivery biomaterial | [28] |
| Demospongiae | Ianthella basta | Polysaccharides | Chitin | skeleton | Tissue engineering and regenerative medicine | [29] |
| Demospongiae | Pseudoceratina purpurea | Polysaccharides | Chitin | skeleton | Biomedicine | [30] |
| Demospongiae | Mycale euplectellioides | Polysaccharides | Chitin | skeleton | Biomedicine | [31] |
| Demospongiae | Acarnus wolffgangi and Echinoclathria gibbosa | Polysaccharides | Chitin | skeleton | Biomedicine | [32] |
| Demospongiae | Pseudoceratina arabica | Polysaccharides | Chitin | skeleton | Biomedicine | [33] |
| Demospongiae | Aplysina aerophoba | Polysaccharides | Chitin | whole body | 3D microporous chitinous scaffolds for hMSCs * in vitro | [34] |
| Demospongiae | Ianthella basta | Polysaccharides | Chitin | whole body | Scaffolds for human mesenchymal stromal cells | [35] |
| Demospongiae | Aplysina cavernicola, Aplysina cauliformis, Aplysina fulva, Aiolochroia crassa, Plysina aerophoba | Polysaccharides | Chitin | whole body | Chitin scaffolds for chondrocytes attachment | [34] |
| Demospongiae | Aplysina aerophoba | Polysaccharides | Chitin | whole body | Ready-to-use scaffolds for cultivation of cardiomyocytes | [36] |
| Demospongiae | Spongia lamella, Spongia officinalis Hippospongia communis Sarcotragus spinosulus | Polysaccharides/Proteins | Collagen/ proteoglycan | skeletons | Bio-based dressing for topical drug delivery | [16] |
| Demospongiae | Aplysina archeri | Polysaccharides | Chitin | skeletal fibres | Ready-to-use 3D chitin scaffolds | [37] |
| Demospongiae | Aplysina fulva Aplysina aerophoba Ianthella basta | Polysaccharides | Chitin | skeletons | Directed differentiation of human adipose tissue-derived hMSCs * within chitin-based skeletons | [38] |
| Demospongiae | Chondrosia reniformis | Proteins | Collagen | whole body | Support and promote the migration, adhesion, and growth of epithelial cells | [39] |
| Demospongiae | Biemna fortis | Proteins | Collagen | whole body | Bone repair and bone augmentation | [40] |
| Demospongiae | Chondrosia reniformis | Proteins | Collagen | whole body | Sponge collagenous membranes | [41] |
| Demospongiae | Callyspongiidae | Proteins | Collagen | skeletons | Scaffold for use in bone tissue engineering | [42] |
| Demospongiae | Ircinia fusca | Proteins | Collagen | whole body | Composite scaffolds (marine collagen + chitosan + hydroxyapatite) for matrix-based bone repair and bone augmentation | [43] |
| Demospongiae | Aplysina fulva | Proteins | Spongin | whole body | Spongin-enriched biosilicate scaffolds to support bone formation | [44] |
| Class | Producer Species | Family/Class of Biomaterial | Biomaterial | Origin/Structural Component | Possible Applications | References |
|---|---|---|---|---|---|---|
| not identified | Polysaccharides | Cellulose | tunic | Scaffold for bone tissue engineering | [271] | |
| Ascidiacea | Styela clava | Polysaccharides | Cellulose | tunic | Biomaterial for treatment of bone defect | [270] |
| Ascidiella aspersa | Polysaccharides | Cellulose | tunic | Biomaterial for skeletal muscle tissue engineering | [272] | |
| Styela clava | Polysaccharides | Cellulose | tunic | Membrane for wound healing | [124] | |
| Styela clava | Polysaccharides | Cellulose | tunic | Film for wound healing | [269] | |
| Halocynthia roretzi | Polysaccharides | Cellulose | tunic | Hydrogel for biomedical applications | [273] | |
| Styela clava Broussonetia kazinoki | Polysaccharides | Cellulose | tunic | Liquid bandage for wound healing | [268] | |
| not identified | Proteins | TOPA 1 proteins | tunic | Adhesive hydrogel for biomedical applications | [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. https://doi.org/10.3390/md20040219
Romano G, Almeida M, Varela Coelho A, Cutignano A, Gonçalves LG, Hansen E, Khnykin D, Mass T, Ramšak A, Rocha MS, et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Marine Drugs. 2022; 20(4):219. https://doi.org/10.3390/md20040219
Chicago/Turabian StyleRomano, Giovanna, Mariana Almeida, Ana Varela Coelho, Adele Cutignano, Luis G Gonçalves, Espen Hansen, Denis Khnykin, Tali Mass, Andreja Ramšak, Miguel S. Rocha, and et al. 2022. "Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications" Marine Drugs 20, no. 4: 219. https://doi.org/10.3390/md20040219
APA StyleRomano, G., Almeida, M., Varela Coelho, A., Cutignano, A., Gonçalves, L. G., Hansen, E., Khnykin, D., Mass, T., Ramšak, A., Rocha, M. S., Silva, T. H., Sugni, M., Ballarin, L., & Genevière, A.-M. (2022). Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Marine Drugs, 20(4), 219. https://doi.org/10.3390/md20040219











