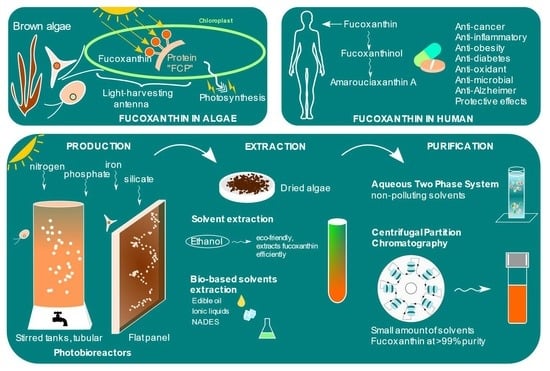
Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes
Abstract
:1. Introduction
2. Isomers of Fucoxanthin
3. Biological Function
3.1. In Algae
3.2. In Human
4. Biosynthesis in Algae
5. Bioavailability
6. Producing Species
6.1. Best Producers
6.2. Measurement of Fucoxanthin
7. Culture
7.1. Reactors, Temperature, pH and Salinity
7.2. Light
7.3. Nutrients
7.3.1. Nitrogen
7.3.2. Phosphorus
7.3.3. Silicate
7.3.4. Carbon
8. Extraction and Purification
8.1. Ultrasound Pretreatment
8.2. Conventional Solvents
8.3. Microwave, US, Pressurized Liquid, Enzyme, Sub and Supercritical Fluid, Electrotechnology
8.4. Bio-Based Solvents
8.4.1. Edible Oil
8.4.2. Ionic Liquids
8.4.3. Natural Deep Eutectic Solvents
8.5. HPLC and Supercritical Anti-Solvent
8.6. Aqueous Two-Phase System
8.7. Centrifugal Partition Chromatography
8.8. Stability of Fucoxanthin
9. Global Market of Fucoxanthin
10. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Liaaen-Jensen, S. Chapter 1—Marine Carotenoids. In Marine Natural Products; Scheuer, P.J., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 1–73. ISBN 978-0-12-624002-3. [Google Scholar]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yu, L.-J.; Xu, C.; Tomizaki, T.; Zhao, S.; Umena, Y.; Chen, X.; Qin, X.; Xin, Y.; Suga, M.; et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science 2019, 363, eaav0365. [Google Scholar] [CrossRef] [PubMed]
- Junghans, A.; Sies, H.; Stahl, W. Macular Pigments Lutein and Zeaxanthin as Blue Light Filters Studied in Liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of Apoptosis Induced by Fucoxanthin in Human Promyelocytic Leukemia Cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef]
- Paiva, S.A.R.; Russell, R.M. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Englert, G.; Bjørnland, T.; Liaaen-Jensen, S. 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Org. Magn. Reson. 1990, 28, 519–528. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Koo, S.Y.; Hwang, J.-H.; Yang, S.-H.; Um, J.-I.; Hong, K.W.; Kang, K.; Pan, C.-H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [Green Version]
- Kawee-Ai, A.; Kim, S.M. Application of Microalgal Fucoxanthin for the Reduction of Colon Cancer Risk: Inhibitory Activity of Fucoxanthin against β-Glucuronidase and DLD-1 Cancer Cells. NPC Nat. Prod. Commun. 2014, 5, 921–924. [Google Scholar]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef] [PubMed]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2018, 31, 281–299. [Google Scholar] [CrossRef]
- Mordi, R.C. Mechanism of Beta-Carotene Degradation. Biochem. J. 1993, 292, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Schieber, A.; Carle, R. Effects of Heating and Illumination on Trans−Cis Isomerization and Degradation of β-Carotene and Lutein in Isolated Spinach Chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Achir, N.; Randrianatoandro, V.A.; Bohuon, P.; Laffargue, A.; Avallone, S. Kinetic study of β-carotene and lutein degradation in oils during heat treatment. Eur. J. Lipid Sci. Technol. 2010, 112, 349–361. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI PRESS: Washington, DC, USA, 2001; 71p. [Google Scholar]
- Böhm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of α-Carotene, β-Carotene, Lycopene, and Zeaxanthin. J. Agric. Food Chem. 2001, 50, 221–226. [Google Scholar] [CrossRef]
- Kawee-Ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and Antioxidant Activities of Fucoxanthin Purified from the Microalga Phaeodactylum Tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative Evaluation of the Radical-Scavenging Activities of Fucoxanthin and Its Stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Premvardhan, L.; Bordes, L.; Beer, A.; Büchel, C.; Robert, B. Carotenoid Structures and Environments in Trimeric and Oligomeric Fucoxanthin Chlorophyll a/c2 Proteins from Resonance Raman Spectroscopy. J. Phys. Chem. B 2009, 113, 12565–12574. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Shannon, E. Seaweed Carotenoid, Fucoxanthin, as Functional Food. In Microbial Functional Foods and Nutraceuticals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 39–64. ISBN 978-1-119-04896-1. [Google Scholar]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Rochaix, J.-D. Regulation and Dynamics of the Light-Harvesting System. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Schmitz, C.; Corrêa, R.; Herrera, C.; Ramlov, F.; Oliveira, E.; Pizzato, A.; Varela, L.; Cabral, D.; Yunes, R.; et al. Chapter 8—In vitro fucoxanthin production by the Phaeodactylum tricornutum diatom. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 211–242. [Google Scholar] [CrossRef]
- Polívka, T.; Sundström, V. Ultrafast Dynamics of Carotenoid Excited States−From Solution to Natural and Artificial Systems. Chem. Rev. 2004, 104, 2021–2072. [Google Scholar] [CrossRef]
- Zigmantas, D.; Hiller, R.G.; Sharples, F.P.; Frank, H.A.; Sundström, V.; Polívka, T. Effect of a conjugated carbonyl group on the photophysical properties of carotenoids. Phys. Chem. Chem. Phys. 2004, 6, 3009–3016. [Google Scholar] [CrossRef]
- Premvardhan, L.; Sandberg, D.J.; Fey, H.; Birge, R.R.; Büchel, C.; van Grondelle, R. The Charge-Transfer Properties of the S2 State of Fucoxanthin in Solution and in Fucoxanthin Chlorophyll-a/c2 Protein (FCP) Based on Stark Spectroscopy and Molecular-Orbital Theory. J. Phys. Chem. B 2008, 112, 11838–11853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelzinis, A.; Butkus, V.; Songaila, E.; Augulis, R.; Gall, A.; Büchel, C.; Robert, B.; Abramavicius, D.; Zigmantas, D.; Valkunas, L. Mapping energy transfer channels in fucoxanthin–chlorophyll protein complex. Biochim. Biophys. Acta 2014, 1847, 241–247. [Google Scholar] [CrossRef]
- Polikva, T.; Zigmantas, D.; Hiller, R.G.; Sundström, V. Excited State Dynamics of the Carotenoid Peridinin. In Femtochemistry and Femtobiology: Ultrafast Events in Molecular Science; Martin, M.M., Hynes, J.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 445–452. ISBN 978-0-08-050626-5. [Google Scholar]
- Di Valentin, M.; Büchel, C.; Giacometti, G.M.; Carbonera, D. Chlorophyll triplet quenching by fucoxanthin in the fucoxanthin–chlorophyll protein from the diatom Cyclotella meneghiniana. Biochem. Biophys. Res. Commun. 2012, 427, 637–641. [Google Scholar] [CrossRef]
- Nagao, R.; Yokono, M.; Akimoto, S.; Tomo, T. High Excitation Energy Quenching in Fucoxanthin Chlorophyll a/c-Binding Protein Complexes from the Diatom Chaetoceros gracilis. J. Phys. Chem. B 2013, 117, 6888–6895. [Google Scholar] [CrossRef]
- Goss, R.; Pinto, E.A.; Wilhelm, C.; Richter, M. The importance of a highly active and ΔpH-regulated diatoxanthin epoxidase for the regulation of the PS II antenna function in diadinoxanthin cycle containing algae. J. Plant Physiol. 2006, 163, 1008–1021. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef]
- Tiwari, A.; Melchor-Martínez, E.M.; Saxena, A.; Kapoor, N.; Singh, K.J.; Saldarriaga-Hernández, S.; Parra-Saldívar, R.; Iqbal, H.M. Therapeutic attributes and applied aspects of biological macromolecules (polypeptides, fucoxanthin, sterols, fatty acids, polysaccharides, and polyphenols) from diatoms—A review. Int. J. Biol. Macromol. 2021, 171, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, M. Functional Foods and Their Role in Cancer Pre-vention and Health Promotion: A Comprehensive Review. Am. J. Cancer Res. 2017, 30, 740–769. [Google Scholar]
- Irvani, N.; Hajiaghaee, R.; Zarekarizi, A.R. A Review on Biosynthesis, Health Benefits and Extraction Methods of Fucoxanthin, Particular Marine Carotenoids in Algae. J. Med. Plants 2018, 17, 25. [Google Scholar]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A.; Shamsollahi, Z. Production of fucoxanthin by the microalga Tisochrysis lutea: A review of recent developments. Aquaculture 2019, 516, 734637. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.; Garcia-Oliveira, P.; Prieto, M.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2014, 184, 355–362. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Mazli, N.A.I.N.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R. The Critical Studies of Fucoxanthin Research Trends from 1928 to June 2021: A Bibliometric Review. Mar. Drugs 2021, 19, 606. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorption by Caco-2 Human Intestinal Cells and Mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Takahashi, S.; Nishimura, R.; Kubota, A.; Kojima, H.; Ohta, T.; Hamada, J.; Kuramitsu, Y.; Maeda, H.; Miyashita, K.; et al. A Marine Carotenoid of Fucoxanthinol Accelerates the Growth of Human Pancreatic Cancer PANC-1 Cells. Nutr. Cancer 2021, 74, 357–371. [Google Scholar] [CrossRef]
- Terasaki, M.; Inoue, T.; Murase, W.; Kubota, A.; Kojima, H.; Kojoma, M.; Ohta, T.; Maeda, H.; Miyashita, K.; Mutoh, M.; et al. Fucoxanthinol Induces Apoptosis in a Pancreatic Intraepithelial Neoplasia Cell Line. Cancer Genom. Proteom. 2021, 18, 133–146. [Google Scholar] [CrossRef]
- Terasaki, M.; Nishizaka, Y.; Murase, W.; Kubota, A.; Kojima, H.; Kojoma, M.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M.; et al. Effect of Fucoxanthinol on Pancreatic Ductal Adenocarcinoma Cells from an N-Nitrosobis(2-oxopropyl)amine-initiated Syrian Golden Hamster Pancreatic Carcinogenesis Model. Cancer Genom. Proteom. 2021, 18 (Suppl. 3), 407–423. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Nascimiento, L.D.S.; Tredici, M.; Luceri, C. A Comparative In Vitro Evaluation of the Anti-Inflammatory Effects of a Tisochrysis lutea Extract and Fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Gomes, A.; Campos, A.M.; Falé, P.; Afonso, C.; Bandarra, N.M. Bioprospection of Isochrysis galbanaand its potential as a nutraceutical. Food Funct. 2019, 10, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, N.; Morandi, A.C.; Bolin, A.; Otton, R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int. Immunopharmacol. 2014, 22, 41–50. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Fucoxanthin Protects Cultured Human Keratinocytes against Oxidative Stress by Blocking Free Radicals and Inhibiting Apoptosis. Biomol. Ther. 2013, 21, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Hamoya, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin Prevents Colorectal Cancer Development in Dextran Sodium Sulfate-treated ApcMin/+ Mice. Anticancer Res. 2021, 41, 1299–1305. [Google Scholar] [CrossRef]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2020, 42, 210–219. [Google Scholar] [CrossRef]
- Sui, Y.; Gu, Y.; Lu, Y.; Yu, C.; Zheng, J.; Qi, H. Fucoxanthin@Polyvinylpyrrolidone Nanoparticles Promoted Oxidative Stress-Induced Cell Death in Caco-2 Human Colon Cancer Cells. Mar. Drugs 2021, 19, 92. [Google Scholar] [CrossRef]
- Yokoyama, R.; Kojima, H.; Takai, R.; Ohta, T.; Maeda, H.; Miyashita, K.; Mutoh, M.; Terasaki, M. Effects of CLIC4 on Fucoxanthinol-Induced Apoptosis in Human Colorectal Cancer Cells. Nutr. Cancer 2020, 73, 889–898. [Google Scholar] [CrossRef]
- Iyappan, P. Fucoxanthin induced apoptotic cell death in oral squamous carcinoma (KB) cells. Bioinformation 2021, 17, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pruteanu, L.-L.; Kopanitsa, L.; Módos, D.; Kletnieks, E.; Samarova, E.; Bender, A.; Gomez, L.D.; Bailey, D.S. Transcriptomics predicts compound synergy in drug and natural product treated glioblastoma cells. PLoS ONE 2020, 15, e0239551. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2020, 265, 113302. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.R.; Kusmita, L.; Wink, M. Bixin and fucoxanthin sensitize human lung cancer and cervical cancer cell to cisplatin in vitro. BMC Res. Notes 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Long, Y.; Cao, X.; Zhao, R.; Gong, S.; Jin, L.; Feng, C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef]
- Wu, S.-J.; Liou, C.-J.; Chen, Y.-L.; Cheng, S.-C.; Huang, W.-C. Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Jiang, Y.-F.; Lu, Y.-A.; Yu, J.-B.; Kang, M.-C.; Jeon, Y.-J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Takatani, N.; Taya, D.; Katsuki, A.; Beppu, F.; Yamano, Y.; Wada, A.; Miyashita, K.; Hosokawa, M. Identification of Paracentrone in Fucoxanthin-Fed Mice and Anti-Inflammatory Effect against Lipopolysaccharide-Stimulated Macrophages and Adipocytes. Mol. Nutr. Food Res. 2020, 65, e2000405. [Google Scholar] [CrossRef]
- Jin, W.; Yang, L.; Yi, Z.; Fang, H.; Chen, W.; Hong, Z.; Zhang, Y.; Zhang, G.; Li, L. Anti-Inflammatory Effects of Fucoxanthinol in LPS-Induced RAW264.7 Cells through the NAAA-PEA Pathway. Mar. Drugs 2020, 18, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Q.; Sun, G.; Xin, T.; Zhang, R.; Liu, C. Fucoxanthin attenuates behavior deficits and neuroinflammatory response in 1-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine-induced parkinson’s disease in mice. Pharmacogn. Mag. 2020, 16, 51. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Liu, K.; Li, M.; Luo, H.; Cui, L.; Huang, L.; Luo, L. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MyD88 signaling axis. Aging 2020, 13, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.; Rachkovskaya, L.; Surovtseva, M.; Kim, I.; Gevorgiz, R.; Zheleznova, S.; Korolev, M.; Letyagin, A.; Poveshchenko, O. In Vitro and In Vivo Effect of the Composition of Fucoxanthin with Porous Aluminum-Silicon Career on Cells. Biointerface Res. Appl. Chem. 2021, 11, 9467–9476. [Google Scholar]
- Lee, A.-H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Lin, C.-H.; Lee, A.-S.; Liu, H.-J.; Li, C.-C.; Tseng, K.-W. Protective Effects of Fucoxanthin Dampen Pathogen-Associated Molecular Pattern (PAMP) Lipopolysaccharide-Induced Inflammatory Action and Elevated Intraocular Pressure by Activating Nrf2 Signaling and Generating Reactive Oxygen Species. Antioxidants 2021, 10, 1092. [Google Scholar] [CrossRef]
- Huang, L.L.; Huang, Z.Q.; Zhang, X.Q.; Liu, J.; Zhang, Y.P.; Zhao, H.Y.; Huang, M.Q. Effect of Fucoxanthin on Insulin Re-sistance in Obese Mice Induced by High Fat Diet. Zhongguo Zhong Yao Za Zhi 2021, 46, 171–176. [Google Scholar] [CrossRef]
- Du Preez, R.; Magnusson, M.; Majzoub, M.; Thomas, T.; Praeger, C.; Glasson, C.; Panchal, S.; Brown, L. Brown Seaweed Sargassum siliquosum as an Intervention for Diet-Induced Obesity in Male Wistar Rats. Nutrients 2021, 13, 1754. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin During Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef]
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.-M.; Kim, H.-S.; Kim, T.H.; Kim, H.-M.; Lee, J.J.; Kang, M.-C.; Jeon, Y.-J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. Food Funct. 2020, 11, 2299–2308. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2021, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Han, H.; Liu, J.; Tang, M.; Wu, X.; Cao, X.; Zhao, T.; Lu, Y.; Niu, T.; Chen, J.; et al. Fucoxanthin Prevents 6-OHDA-Induced Neurotoxicity by Targeting Keap1. Oxidative Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Mou, C.; Bao, Y.; Xie, Y.; Jin, H.; Shen, H.; Zhou, W.; Zhang, J.; He, S.; Chen, B.; et al. Fucoxanthin alleviates methamphetamine-induced neurotoxicity possibly via the inhibition of interaction between Keap1 and Nrf2. J. Funct. Foods 2021, 86, 104713. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Li, W.; Wu, R.; Zheng, M.; Kong, A.-N. Epigenomic, Transcriptomic, and Protective Effect of Carotenoid Fucoxanthin in High Glucose-Induced Oxidative Stress in Mes13 Kidney Mesangial Cells. Chem. Res. Toxicol. 2021, 34, 713–722. [Google Scholar] [CrossRef] [PubMed]
- El Bakary, N.M.; Thabet, N.M.; El Fatih, N.M.; Abdel-Rafei, M.K.; El Tawill, G.; Azab, K.S. Fucoxanthin alters the apelin-13/APJ pathway in certain organs of γ-irradiated mice. J. Radiat. Res. 2021, 62, 600–617. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Tsai, C.-H.; Chen, H.-Y.; Wang, K.-L.; Chang, H.-Y.; Huang, Y.-J.; Hong, Y.-H.; Ali, M.; Shieh, T.-M.; Huang, T.-C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Lee, A.-S.; Lin, C.-H.; Tseng, K.-W. Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Mar. Drugs 2021, 19, 114. [Google Scholar] [CrossRef]
- Natsume, C.; Aoki, N.; Aoyama, T.; Senda, K.; Matsui, M.; Ikegami, A.; Tanaka, K.; Azuma, Y.-T.; Fujita, T. Fucoxanthin Ameliorates Atopic Dermatitis Symptoms by Regulating Keratinocytes and Regulatory Innate Lymphoid Cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef] [Green Version]
- Guvatova, Z.; Dalina, A.; Marusich, E.; Pudova, E.; Snezhkina, A.; Krasnov, G.; Kudryavtseva, A.; Leonov, S.; Moskalev, A. Protective effects of carotenoid fucoxanthin in fibroblasts cellular senescence. Mech. Ageing Dev. 2020, 189, 111260. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, L.; Xiong, Y.; Jiang, G.; Liu, X. Fucoxanthin Attenuates Oxidative Damage by Activating the Sirt1/Nrf2/HO-1 Signaling Pathway to Protect the Kidney from Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2022, 2022, 1–28. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, J.; Zhao, B.; Zhu, T.; Qi, H. Photoprotective Mechanism of Fucoxanthin in Ultraviolet B Irradiation-Induced Retinal Müller Cells Based on Lipidomics Analysis. J. Agric. Food Chem. 2022, 70, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Shen, Y.; Wu, Z.; Zhang, X.; Ge, S. Effects of algae subtype and extraction condition on extracted fucoxanthin antioxidant property: A 20-year meta-analysis. Algal Res. 2020, 53, 102161. [Google Scholar] [CrossRef]
- Mishra, B.; Tiwari, A. Cultivation of Anabena variabilis, Synechococcus elongatus, Spirulina platensis for the production of C-Phycocyanin, C-Phycoerythrin and Thalassiosira, Skeletonema, Chaetoceros for fucoxanthin. Syst. Microbiol. Biomanuf. 2021, 1, 356–361. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What Do We Know about Antimicrobial Activity of Astaxanthin and Fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate In Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef]
- Ha, Y.-J.; Choi, Y.; Oh, Y.; Kang, E.; Khang, G.; Park, Y.-B.; Lee, Y. Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling. Mar. Drugs 2021, 19, 132. [Google Scholar] [CrossRef]
- Wang, R.; Younis, E.M.; Veeraraghavan, V.P.; Tian, C. Antiurolithiatic effect of Fucoxanthin on ethylene glycol-induced renal calculus in experimental rats. J. King Saud Univ.-Sci. 2020, 32, 1896–1901. [Google Scholar] [CrossRef]
- Li, Y.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Fucoxanthin metabolites exert anti-fibrogenic and antioxidant effects in hepatic stellate cells. J. Agric. Food Res. 2021, 6, 100245. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Vuong, T.T.; Choi, J.; Lee, T.S.; Um, J.-I.; Koo, S.Y.; Hwang, K.T.; Kim, S.M. Fucoxanthin biosynthesis has a positive correlation with the specific growth rate in the culture of microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2021, 33, 1473–1485. [Google Scholar] [CrossRef]
- Gaidarenko, O.; Mills, D.W.; Vernet, M.; Hildebrand, M. Overexpression of Thalassiosira Pseudonana Violaxanthin de-Epoxidase-like 2 (VDL2) Increases Fucoxanthin While Stoichiometrically Reducing Diadinoxanthin Cycle Pigment Abundance. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef]
- Dautermann, O.; Lyska, D.; Andersen-Ranberg, J.; Becker, M.; Fröhlich-Nowoisky, J.; Gartmann, H.; Krämer, L.C.; Mayr, K.; Pieper, D.; Rij, L.M.; et al. An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Sci. Adv. 2020, 6, eaaw9183. [Google Scholar] [CrossRef] [Green Version]
- Beuzenberg, V.; Goodwin, E.O.; Puddick, J.; Romanazzi, D.; Adams, S.; Packer, M.A. Optimising conditions for growth and xanthophyll production in continuous culture of Tisochrysis lutea using photobioreactor arrays and central composite design experiments. N. Z. J. Bot. 2016, 55, 64–78. [Google Scholar] [CrossRef]
- De Oliveira-Júnior, R.G.; Grougnet, R.; Bodet, P.-E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated pigment composition of Tisochrysis lutea and purification of fucoxanthin using centrifugal partition chromatography coupled to flash chromatography for the chemosensitization of melanoma cells. Algal Res. 2020, 51, 102035. [Google Scholar] [CrossRef]
- Hao, T.-B.; Lu, Y.; Zhang, Z.-H.; Liu, S.-F.; Wang, X.; Yang, W.-D.; Balamurugan, S.; Li, H.-Y. Hyperaccumulation of fucoxanthin by enhancing methylerythritol phosphate pathway in Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2021, 105, 8783–8793. [Google Scholar] [CrossRef]
- Handelman, G.J. The evolving role of carotenoids in human biochemistry. Nutrition 2001, 17, 818–822. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Available online: https://www.hindawi.com/journals/ecam/2015/723515/ (accessed on 2 April 2020).
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of Fucoxanthinol into Amarouciaxanthin a in Mice and hepg2 Cells: Formation and Cytotoxicity of Fucoxanthin Metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2020, 533, 736074. [Google Scholar] [CrossRef]
- Gao, F.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Fucoxanthin and docosahexaenoic acid production by cold-adapted Tisochrysis lutea. New Biotechnol. 2021, 66, 16–24. [Google Scholar] [CrossRef]
- Gao, F.; Sá, M.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Improved fucoxanthin and docosahexaenoic acid productivities of a sorted self-settling Tisochrysis lutea phenotype at pilot scale. Bioresour. Technol. 2021, 325, 124725. [Google Scholar] [CrossRef]
- Gao, F.; Cabanelas, I.I.T.; Wijffels, R.H.; Barbosa, M.J. Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour. Technol. 2020, 315, 123894. [Google Scholar] [CrossRef]
- Gao, F.; Cabanelas, I.I.T.; Ferrer-Ledo, N.; Wijffels, R.H.; Barbosa, M.J. Production and high throughput quantification of fucoxanthin and lipids in Tisochrysis lutea using single-cell fluorescence. Bioresour. Technol. 2020, 318, 124104. [Google Scholar] [CrossRef]
- Gao, F.; Woolschot, S.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Light spectra as triggers for sorting improved strains of Tisochrysis lutea. Bioresour. Technol. 2020, 321, 124434. [Google Scholar] [CrossRef]
- Gao, F.; Sá, M.; Cabanelas, I.I.T.; Wijffels, R.H.; Barbosa, M.J. Production and monitoring of biomass and fucoxanthin with brown microalgae under outdoor conditions. Biotechnol. Bioeng. 2020, 118, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Sá, M.; Maia, I.; Rodrigues, A.; Teles, I.; Wijffels, R.H.; Navalho, J.; Barbosa, M. Fucoxanthin production from Tisochrysis lutea and Phaeodactylum tricornutum at industrial scale. Algal Res. 2021, 56, 102322. [Google Scholar] [CrossRef]
- Kanamoto, A.; Kato, Y.; Yoshida, E.; Hasunuma, T.; Kondo, A. Development of a Method for Fucoxanthin Production Using the Haptophyte Marine Microalga Pavlova sp. OPMS 30543. Mar. Biotechnol. 2021, 23, 331–341. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2021, 347, 126401. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, D. Improving Fucoxanthin Production in Mixotrophic Culture of Marine Diatom Phaeodactylum tricornutum by LED Light Shift and Nitrogen Supplementation. Front. Bioeng. Biotechnol. 2020, 8, 820. [Google Scholar] [CrossRef] [PubMed]
- Derwenskus, F.; Weickert, S.; Lewandowski, I.; Schmid-Staiger, U.; Hirth, T. Economic evaluation of up- and downstream scenarios for the co-production of fucoxanthin and eicosapentaenoic acid with P. tricornutum using flat-panel airlift photobioreactors with artificial light. Algal Res. 2020, 51, 102078. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Wang, P.-K.; Ma, Y.; Lin, J.-X.; Wang, C.-L.; Zhao, Y.-X.; Zhang, X.-Y.; Huang, B.-C.; Zhao, S.-G.; Gao, L.; et al. Laminaria japonica hydrolysate promotes fucoxanthin accumulation in Phaeodactylum tricornutum. Bioresour. Technol. 2021, 344, 126117. [Google Scholar] [CrossRef]
- Bárcenas-Pérez, D.; Střížek, A.; Hrouzek, P.; Kopecký, J.; Barradas, M.; Sierra-Ramirez, A.; Fernandez-Marcos, P.J.; Cheel, J. Production of Fucoxanthin from Phaeodactylum tricornutum Using High Performance Countercurrent Chromatography Retaining Its FOXO3 Nuclear Translocation-Inducing Effect. Mar. Drugs 2021, 19, 517. [Google Scholar] [CrossRef]
- Rehmanji, M.; Nesamma, A.; Khan, N.; Fatma, T.; Jutur, P. Media Engineering in Marine Diatom Phaeodactylum Tricornutum Employing Cost-Effective Substrates for Sustainable Production of High Value Renewables. Authorea 2022. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, J.; Zhang, H.; Zhang, Z.; Du, Y.; Cheng, Z.; Feng, B.; Yao, T.; Zhang, A.; Zhao, Z. Potential integration of wastewater treatment and natural pigment production by Phaeodactylum tricornutum: Microalgal growth, nutrient removal, and fucoxanthin accumulation. J. Appl. Phycol. 2022, 1–12. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ooi, C.W.; Chew, K.W.; Foo, S.C.; Show, P.L. Bioprocessing of Chaetoceros calcitrans for the recovery of fucoxanthin using CO2-based alkyl carbamate ionic liquids. Bioresour. Technol. 2020, 322, 124520. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, D.; Archer, L.; Fleming, G.T.; Gillespie, E.; Touzet, N. The effect of nutrient and phytohormone supplementation on the growth, pigment yields and biochemical composition of newly isolated microalgae. Process Biochem. 2020, 92, 61–68. [Google Scholar] [CrossRef]
- Parkes, R.; Archer, L.; Mc Gee, D.; Smyth, T.J.; Gillespie, E.; Touzet, N. Differential responses in EPA and fucoxanthin production by the marine diatom Stauroneis sp. under varying cultivation conditions. Biotechnol. Prog. 2021, 37, e3197. [Google Scholar] [CrossRef] [PubMed]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, P.; Cai, Q.; Zhang, C.; Gao, B. Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor. Bioresour. Technol. 2021, 344, 126260. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, A.; Karataş, A.B.; Demirel, Z.; Dalay, M.C. Purification of fucoxanthin from the diatom Amphora capitellata by preparative chromatography after its enhanced productivity via oxidative stress. J. Appl. Phycol. 2021, 34, 301–309. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Couto, C.; Hernández, C.P.; Sobrinho, R.C.M.A.; Mendes, C.R.B.; Roselet, F.; Abreu, P.C. Optimization of a low-cost fertilizer-based medium for large-scale cultivation of the coastal diatom Conticribra weissflogii using response surface methodology and its effects on biomass composition. J. Appl. Phycol. 2021, 33, 2767–2781. [Google Scholar] [CrossRef]
- Gérin, S.; Delhez, T.; Corato, A.; Remacle, C.; Franck, F. A novel culture medium for freshwater diatoms promotes efficient photoautotrophic batch production of biomass, fucoxanthin, and eicosapentaenoic acid. J. Appl. Phycol. 2020, 32, 1581–1596. [Google Scholar] [CrossRef]
- Tachihana, S.; Nagao, N.; Katayama, T.; Hirahara, M.; Yusoff, F.M.; Banerjee, S.; Shariff, M.; Kurosawa, N.; Toda, T.; Furuya, K. High Productivity of Eicosapentaenoic Acid and Fucoxanthin by a Marine Diatom Chaetoceros gracilis in a Semi-Continuous Culture. Front. Bioeng. Biotechnol. 2020, 8, 602721. [Google Scholar] [CrossRef]
- Boeing, P. Larval Feed Alternatives. Glob. Aquac. Advocate 1999, 3, 48–50. [Google Scholar]
- Aghzar, A.; Miñambres, M.; Alvarez, P.; Presa, P. A Cost-Benefit Assessment of Two Multi-Species Algae Diets for Juveniles of Mytilus Galloprovincialis. Thalassas 2013, 29, 9–16. [Google Scholar]
- Fernandez, F.G.A.; Hall, D.O.; Guerrero, E.C.; Rao, K.K.; Grima, E.M. Outdoor production of Phaeodactylum tricornutum biomass in a helical reactor. J. Biotechnol. 2003, 103, 137–152. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Leal, E.; de Beyer, L.; O’Connor, W.; Dove, M.; Ralph, P.J.; Pernice, M. Production optimisation of Tisochrysis lutea as a live feed for juvenile Sydney rock oysters, Saccostrea glomerata, using large-scale photobioreactors. Aquaculture 2020, 533, 736077. [Google Scholar] [CrossRef]
- Rico-Villa, B.; Le Coz, J.; Mingant, C.; Robert, R. Influence of phytoplankton diet mixtures on microalgae consumption, larval development and settlement of the Pacific oyster Crassostrea gigas (Thunberg). Aquaculture 2006, 256, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2013, 26, 151–161. [Google Scholar] [CrossRef]
- Delbrut, A.; Albina, P.; Lapierre, T.; Pradelles, R.; Dubreucq, E. Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process. Molecules 2018, 23, 874. [Google Scholar] [CrossRef] [Green Version]
- Alkhamis, Y.; Qin, J.G. Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. J. Appl. Phycol. 2015, 28, 35–42. [Google Scholar] [CrossRef]
- Chen, G.; Chen, F. Growing Phototrophic Cells without Light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Saoudi-Helis, L.; Dubacq, J.P.; Marty, Y.; Samain, J.F.; Gudin, C. Influence of growth rate on pigment and lipid composition of the microalga Isochrysis aff.galbana clone T.iso. J. Appl. Phycol. 1994, 6, 315–322. [Google Scholar] [CrossRef]
- Fret, J.; Roef, L.; Diels, L.; Tavernier, S.; Vyverman, W.; Michiels, M. Combining medium recirculation with alternating the microalga production strain: A laboratory and pilot scale cultivation test. Algal Res. 2020, 46, 101763. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as Sustainable Renewable Energy Feedstock for Biofuel Production. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Torzillo, G.; Faraloni, C.; Silva, A.M.; Kopecký, J.; Pilný, J.; Masojidek, J. Photoacclimation of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in photobioreactors and open ponds. Eur. J. Phycol. 2012, 47, 169–181. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.-Y.; Varjani, S.; Chang, J.-S. Producing fucoxanthin from algae—Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2021, 344, 126170. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Kavadikeri, S.; Manokaran, S.; Reddy, A.H.M. Extraction and Characterization of Microalgal Oil and Fucoxanthin from Diatom. J. Pharm. Sci. 2020, 12, 5. [Google Scholar]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Transition from start-up to scale-up for fucoxanthin production in flat plate photobioreactor. J. Appl. Phycol. 2019, 31, 1525–1533. [Google Scholar] [CrossRef]
- Gao, B.; Chen, A.; Zhang, W.; Li, A.; Zhang, C. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean Univ. China 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Comparison of different photobioreactor configurations and empirical computational fluid dynamics simulation for fucoxanthin production. Algal Res. 2018, 37, 195–204. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Xie, X.; Huan, L.; Zheng, Z.; Zhao, P.; Kuang, J.; Liu, X.; Wang, G. Selection of optimal flocculant for effective harvesting of the fucoxanthin-rich marine microalga Isochrysis galbana. J. Appl. Phycol. 2015, 28, 1579–1588. [Google Scholar] [CrossRef]
- Fitt, W.K.; Dunne, R.P.; Gibb, S.; Cummings, D.G.; Ambarsari, I.; Brown, B.E.; Warner, M.E. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: Evidence for photoinhibition and photoprotection. Coral Reefs 1999, 18, 99–105. [Google Scholar] [CrossRef]
- Anning, T.; MacIntyre, H.L.; Pratt, S.M.; Sammes, P.J.; Gibb, S.; Geider, R.J. Photoacclimation in the marine diatom Skeletonema costatum. Limnol. Oceanogr. 2000, 45, 1807–1817. [Google Scholar] [CrossRef]
- Lavaud, J.; Rousseau, B.; van Gorkom, H.J.; Etienne, A.-L. Influence of the Diadinoxanthin Pool Size on Photoprotection in the Marine Planktonic Diatom Phaeodactylum tricornutum. Plant Physiol. 2002, 129, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Garab, G.; Adams, W.W. Govindjee Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2014; Volume 40, ISBN 978-94-017-9031-4. [Google Scholar]
- Zigman, M.; Dubinsky, Z.; Iluz, D. The Xanthophyll Cycle in Aquatic Phototrophs and Its Role in the Mitigation of Photoin-hibition and Photodynamic Damage. In Applied Photosynthesis; Najafpour, M., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0061-4. [Google Scholar]
- Zhao, D.; Kim, S.-M.; Pan, C.-H.; Chung, D. Effects of heating, aerial exposure and illumination on stability of fucoxanthin in canola oil. Food Chem. 2014, 145, 505–513. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, R.; Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.N.; Scanlan, D.J.; Geider, R.J. Responses of Emiliania huxleyi (Prymnesiophyceae) to step changes in photon flux density. Eur. J. Phycol. 2009, 44, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Muüller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Verma, S.K.; Said, I.H.; Thomsen, L.; Ullrich, M.S.; Kuhnert, N. Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb. Cell Factories 2018, 17, 110. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S. Fucoxanthin pigment markers of marine phytoplankton analysed by HPLC and HPTLC. Mar. Ecol. Prog. Ser. 1987, 38, 259–266. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Gómez, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2015, 28, 849–860. [Google Scholar] [CrossRef]
- Gupta, P.; Lee, S.-M.; Choi, H.-J. Integration of microalgal cultivation system for wastewater remediation and sustainable biomass production. World J. Microbiol. Biotechnol. 2016, 32, 139. [Google Scholar] [CrossRef]
- Flynn, K.; Fasham, M.J.R.; Hipkin, C.R. Modelling the interactions between ammonium and nitrate uptake in marine phytoplankton. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 1625–1645. [Google Scholar] [CrossRef]
- Bhaya, D.; Schwarz, R.; Grossman, A.R. Molecular Responses to Environmental Stress. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 397–442. [Google Scholar] [CrossRef]
- Fuggi, A.; Rigano, V.D.M.; Vona, V.; Rigano, C. Nitrate and ammonium assimilation in algal cell-suspensions and related pH variations in the external medium, monitored by electrodes. Plant Sci. Lett. 1981, 23, 129–138. [Google Scholar] [CrossRef]
- Mairet, F.; Bernard, O.; Lacour, T.; Sciandra, A. Modelling microalgae growth in nitrogen limited photobiorector for estimating biomass, carbohydrate and neutral lipid productivities. IFAC Proc. Vol. 2011, 44, 10591–10596. [Google Scholar] [CrossRef] [Green Version]
- Bougaran, G.; Bernard, O.; Sciandra, A. Modeling continuous cultures of microalgae colimited by nitrogen and phosphorus. J. Theor. Biol. 2010, 265, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; Assis, D.; de Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2019, 46, 153–158. [Google Scholar] [CrossRef]
- Moussa, I.D.-B.; Chtourou, H.; Karray, F.; Sayadi, S.; Dhouib, A. Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour. Technol. 2017, 238, 325–332. [Google Scholar] [CrossRef]
- Geider, R.; Intyre, M.; Graziano, L.; McKay, R. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol. 1998, 33, 315–332. [Google Scholar] [CrossRef]
- De Wever, A.; Muylaert, K.; Langlet, D.; Alleman, L.; Descy, J.-P.; André, L.; Cocquyt, C.; Vyverman, W. Differential response of phytoplankton to additions of nitrogen, phosphorus and iron in Lake Tanganyika. Freshw. Biol. 2007, 53, 264–277. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosci. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef]
- Sahin, M.S.; Khazi, M.I.; Demirel, Z.; Dalay, M.C. Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal. Agric. Biotechnol. 2019, 17, 390–398. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Z.; Su, Y.; Cherek, P.; Nelson, D.R.; Lin, J.; Rolfsson, O.; Wu, H.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Combined artificial high-silicate medium and LED illumination promote carotenoid accumulation in the marine diatom Phaeodactylum tricornutum. Microb. Cell Factories 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Chew, K.W.; Yen, H.-W.; Lim, J.W.; Lam, M.K.; Show, P.L. Cultivation of Oily Microalgae for the Production of Third-Generation Biofuels. Sustainability 2019, 11, 5424. [Google Scholar] [CrossRef] [Green Version]
- Moreno-García, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; He, Y.; Guo, B.; Sun, H.; Chen, F. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour. Technol. 2019, 294, 122145. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.C.; Mirón, A.S.; Sevilla, J.M.F.; Grima, E.M.; Garcia-Camacho, F. Mixotrophic growth of the microalga Phaeodactylum tricornutum: Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- Cerón-García, M.; Fernández-Sevilla, J.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Mixotrophic growth of Phaeodactylum tricornutum on fructose and glycerol in fed-batch and semi-continuous modes. Bioresour. Technol. 2013, 147, 569–576. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Bioactive significance of fucoxanthin and its effective extraction. Biocatal. Agric. Biotechnol. 2020, 26, 101639. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Gao, B.; Huang, L.; Zhang, C. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Siahaan, E.A.; Chun, B.S. Innovative Alternative Technology for Fucoxanthin Recovery. In Encyclopedia of Marine Biotechnology; John Wiley & Sons, Inc.: New York, NY, USA, 2020; pp. 3213–3227. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem. Eng. J. 2019, 153, 107403. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Cheng, P.; Zhu, J.; Hou, Y.; Li, Y.; Zhang, J.; Yan, X. A simple and efficient strategy for fucoxanthin extraction from the microalga Phaeodactylum tricornutum. Algal Res. 2021, 61, 102610. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.-B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef]
- Taucher, J.; Baer, S.; Schwerna, P.; Hofmann, D.; Hümmer, M.; Becker, R.B.A.A. Cell Disruption and Pressurized Liquid Extraction of Carotenoids from Microalgae. J. Thermodyn. Catal. 2016, 7, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Billakanti, J.; Catchpole, O.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Salinas, F.; Medina, E.; Rincón, B.; Martín, M.; Gutiérrez, M.C.; Cerezal-Mezquita, P. Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion. Mar. Drugs 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.T.A.D. Pulsed electric fields as a pretreatment technique in drying processes. Stewart Postharvest Rev. 2006, 2, 1–6. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Menegol, T.; Rech, R.; Mercali, G.D.; Marczak, L.D.F. Carotenoid and lipid extraction from Heterochlorella luteoviridis using moderate electric field and ethanol. Process Biochem. 2016, 51, 1636–1643. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ooi, C.W.; Chew, K.W.; Chia, S.R.; Foo, S.C.; Ng, H.S.; Show, P.L. Extraction of fucoxanthin from Chaetoceros calcitrans by electropermeabilization-assisted liquid biphasic flotation system. J. Chromatogr. A 2022, 1668, 462915. [Google Scholar] [CrossRef]
- Pierobon, S.C.; Cheng, X.; Graham, P.J.; Nguyen, B.; Karakolis, E.G.; Sinton, D. Emerging microalgae technology: A review. Sustain. Energy Fuels 2017, 2, 13–38. [Google Scholar] [CrossRef]
- Teramukai, K.; Kakui, S.; Beppu, F.; Hosokawa, M.; Miyashita, K. Effective extraction of carotenoids from brown seaweeds and vegetable leaves with edible oils. Innov. Food Sci. Emerg. Technol. 2020, 60, 102302. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decreases Blood Glucose in Obese/Diabetic KK-Ay Mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef]
- Choi, S.-A.; Oh, Y.-K.; Lee, J.; Sim, S.J.; Hong, M.E.; Park, J.-Y.; Kim, M.-S.; Kim, S.W.; Lee, J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2018, 274, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tian, M.; Zhou, J.; Row, K.H. Task-specific ionic liquid-assisted extraction and separation of astaxanthin from shrimp waste. J. Chromatogr. B 2010, 878, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Costa Gomes, M. Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Springer International Publishing: New York, NY, USA, 2021. [Google Scholar]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents to Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Pozharitskaya, O.; Zakharova, L.; Daurtseva, A.; Flisyuk, E.; Shikov, A. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Gallego, R.; Tardif, C.; Parreira, C.; Guerra, T.; Alves, M.J.; Ibáñez, E.; Herrero, M. Simultaneous extraction and purification of fucoxanthin from Tisochrysis lutea microalgae using compressed fluids. J. Sep. Sci. 2020, 43, 1967–1977. [Google Scholar] [CrossRef]
- Chen, C.-R.; Lin, D.-M.; Chang, C.-M.J.; Chou, H.-N.; Wu, J.-J. Supercritical carbon dioxide anti-solvent crystallization of fucoxanthin chromatographically purified from Hincksia mitchellae P.C. Silva. J. Supercrit. Fluids 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Gonzalez-Valdez, J.; Rito-Palomares, M. Insights on the downstream purification of fucoxanthin, a microalgal carotenoid, from an aqueous two-phase system stream exploiting ultrafiltration. J. Appl. Phycol. 2014, 27, 1517–1523. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Espitia-Saloma, E.; Villegas, P.V.; Aguilar, O.; Rito-Palomares, M. Continuous aqueous two-phase systems devices for the recovery of biological products. Food Bioprod. Process. 2014, 92, 101–112. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous Two-Phase Systems: Methods and Protocols; Springer Science & Business Media: New York, NY, USA, 2000; ISBN 978-1-59259-028-5. [Google Scholar]
- Albertsson, P.A. Partition of cell particles and macromolecules in polymer two-phase systems. Adv. Protein Chem. 1970, 24, 309–341. [Google Scholar] [PubMed]
- Chen, J.-P.; Lee, M.-S. Enhanced production of Serratia marcescens chitinase in PEG/dextran aqueous two-phase systems. Enzym. Microb. Technol. 1995, 17, 1021–1027. [Google Scholar] [CrossRef]
- Helfrich, M.R.; El-Kouedi, M.; Etherton, M.R.; Keating, C.D. Partitioning and Assembly of Metal Particles and Their Bioconjugates in Aqueous Two-Phase Systems. Langmuir 2005, 21, 8478–8486. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; Rito-Palomares, M. Simplified two-stage method to B-phycoerythrin recovery from Porphyridium cruentum. J. Chromatogr. B 2006, 844, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Ventom, A.; Asenjo, J. Partitioning and purification of α-amylase in aqueous two-phase systems. Enzym. Microb. Technol. 1994, 16, 131–142. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; González-Valdez, J.; González-González, M.; Benavides, J.; Rito-Palomares, M. Practical experiences from the bench-scale implementation of a bioprocess for fucoxanthin production. J. Chem. Technol. Biotechnol. 2017, 93, 2033–2039. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Partition behavior of fucoxanthin in ethanol-potassium phosphate two-phase systems. J. Chem. Technol. Biotechnol. 2014, 89, 1637–1645. [Google Scholar] [CrossRef]
- Kim, S.M.; Shang, Y.F.; Um, B.-H. A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem. Anal. 2011, 22, 322–329. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-Week Oral Toxicity Study of Fucoxanthin Oil from Microalgae in Rats. Food Hyg. Saf. Sci. 2011, 52, 183–189. [Google Scholar] [CrossRef]
- Yusof, Z.; Khong, N.M.H.; Choo, W.S.; Foo, S.C. Opportunities for the marine carotenoid value chain from the perspective of fucoxanthin degradation. Food Chem. 2022, 383, 132394. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Nakamura, Y.; Yu, C.; Qi, H. Fucoxanthin activities motivate its nano/micro-encapsulation for food or nutraceutical application: A review. Food Funct. 2020, 11, 9338–9358. [Google Scholar] [CrossRef] [PubMed]
- Cordenonsi, L.M.; Santer, A.; Sponchiado, R.M.; Wingert, N.R.; Raffin, R.P.; Schapoval, E.E.S. Amazonia Products in Novel Lipid Nanoparticles for Fucoxanthin Encapsulation. AAPS PharmSciTech 2019, 21, 32. [Google Scholar] [CrossRef]
- Foo, S.C.; Khong, N.M.; Yusoff, F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.-K.; Pan, C.-H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Y.; Ma, Y.; Liu, Y.; Wang, S.; Guo, Z.; Li, J.; Wang, Y.; Tan, B.; Wei, Y. Fabricating hydrophilic fatty acid-protein particles to encapsulate fucoxanthin: Fatty acid screening, structural characterization, and thermal stability analysis. Food Chem. 2022, 382, 132311. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Comparative study of oil-in-water emulsions encapsulating fucoxanthin formulated by microchannel emulsification and high-pressure homogenization. Food Hydrocoll. 2020, 108, 105977. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Double encapsulation of fucoxanthin using porous starch through sequential coating modification with maltodextrin and gum Arabic. Food Sci. Nutr. 2020, 8, 1226–1236. [Google Scholar] [CrossRef]
- Rehman, A.Q.T.; Jafari, S.M.; Assadpour, E.Q.S.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Sajid, B.; Mushtaq, W.A. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Dąbek, P.; Gloc, M.; Golubeva, A.; Dobrucka, R.; Kurzydłowski, K.; Witkowski, A. Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles. Materials 2021, 14, 4094. [Google Scholar] [CrossRef]
- Wang, C.; Ren, J.; Song, H.; Chen, X.; Qi, H. Characterization of whey protein-based nanocomplex to load fucoxanthin and the mechanism of action on glial cells PC12. LWT 2021, 151, 112208. [Google Scholar] [CrossRef]
- Bayu, A.; Rachman, A.; Noerdjito, D.R.; Putra, M.Y.; Widayatno, W.B. High-value chemicals from marine diatoms: A biorefinery approach. IOP Conf. Series Earth Environ. Sci. 2020, 460, 012012. [Google Scholar] [CrossRef]
- Centre d’Etude et de Valorisation des Algues. Macroalgues et Microalgues Alimentaires—Statut Règlementaire En France et En Europe: Synthèse CEVA; CEVA: Pleubian, France, 2019. [Google Scholar]
- Faisal Hossain, M.; Mamoon, R.; Thomas, B.; Mohammed, A.; Winnie, W.; Adeshina, K.K.; Rajjit, S.; Michael, J.; Shamly, A. Evaluation of Fucoxanthin Content in Popular Weight Loss Supplements: The Case for Stricter Regulation of Dietary Supplements. J. Obes. Weight. Medicat. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Nam, G.-W. Sunscreen Boosting Effect by Solid Lipid Nanoparticles-Loaded Fucoxanthin Formulation. Cosmetics 2020, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-T.; Lee, Y.; Jeong, J.-H.; Jo, B.-K. New Anti-Wrinkle Cosmetics. J. Soc. Cosmet. Sci. Korea 2002, 28, 71–79. [Google Scholar]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17, 196–199. [Google Scholar]
- Himms-Hagen, J. Brown adipose tissue thermogenesis: Interdisciplinary studies. FASEB J. 1990, 4, 2890–2898. [Google Scholar] [CrossRef]
- Martinez-Teipel, B.; Bouhrir, S. Reshaping Body Care with Biotech Microalgae. Personal Care 2021, 22, 59–63. [Google Scholar]
- Market Reports World Global Fucoxanthin Market. 2017. Available online: https://www.marketreportsworld.com/global-fucoxanthin-market-10565168 (accessed on 18 September 2019).






| Property | Source of Fucoxanthin | Target | Reference |
|---|---|---|---|
| Anti-cancer | p | Mice pancreatic cancer cells | [45] |
| p | Hamster pancreatic cancer cells | [46] | |
| p | Mice colorectal cancer cells | [52,53] | |
| p | Human colon cancer cells | [54,55] | |
| p | Oral squamous cancer cells (KB) | [56] | |
| p | Human glioblastoma cells (U87MG) | [57] | |
| Laminaria japonica | Human lung cancer cells | [58] | |
| p | Human lung and cervical cancer cells | [59] | |
| p | Nasopharyngeal carcinoma cells | [60] | |
| Anti-inflammatory | p | Inflammation of mice tracheal epithelial cells | [61] |
| p | Inflammation in non-alcoholic fatty liver disease | [62,63] | |
| Sargassum fusiformis | Particulate matter-induced inflammation | [64] | |
| Tisochrysis lutea, brown seaweeds | Lipopolysaccharide (LPS)- stimulated RAW264.7 macrophages | [47,65,66] | |
| p | Neuroinflammatory response in induced-Parkinson’s disease | [67] | |
| p | Acute lung injury inflammation | [68] | |
| Cylindrotheca closterium | Immunocytes, enterocytes, mesenchymal stem cells | [69] | |
| Phaeodactylum tricornutum | Pro-inflammatory cytokines | [70] | |
| p | PAMP lipopolysaccharide-induced uveitis inflammation | [71] | |
| Anti-obesity | p | Insulin resistance of obese mice | [72] |
| Sargassum siliquosum | Diet-induced obesity in rats | [73] | |
| p | Gut microbiota in high-fat diet-fed mice | [74] | |
| Plocamium telfairiae | High-fat diet-fed mice | [75] | |
| Anti-diabetes | Sargassum angustifolium | Streptozotocin-nicotinamide-induced type 2 diabetic mice | [76] |
| Protective effects | p | dexamethasone-induced myotubes atrophy | [77] |
| p | Neurodegenerative disorders | [78] | |
| p | 6-OHDA-Induced Neurotoxicity | [79] | |
| Sargassum honeri | Methamphetamine-induced neurotoxicity | [80] | |
| p | High glucose-induced oxidative stress | [81] | |
| p | Irradiated mice | [82] | |
| p | Calcification of heart valve interstitial cells | [83] | |
| p | Macular degeneration and retinal pigment epithelial cell senescence | [84] | |
| p | Atopic dermatitis symptoms | [85] | |
| p | Fibroblasts cellular senescence | [86] | |
| p | Ischemia-reperfusion injury in kidney | [87] | |
| p | UV-B irradiation induced retinal Müller cells | [88] | |
| Anti-oxidant | 20-year meta-analysis review | [89] | |
| Anti-microbial | Thalassiosira sp., Chaetoceroes sp. | Pathogenic bacteria (Staphylococcus aureus, Escherichia coli) | [90] |
| p | 20 bacterial species (Streptococcus agalactiae, Staphylococcus epidermidis…) | [91] | |
| p | review | [92] | |
| Anti-Alzheimer | Sargassum horneri | Aβ oligomers-induced neurotoxicity | [93] |
| Anti-osteoclastogenesis | p | MAP kinase, Nrf2 signaling | [94] |
| Anti-urolithiatic | p | Ethylene glycol-induced renal calculus in rats | [95] |
| Anti-fibrogenic | p | Hepatic stellate cells | [96] |
| Species | Fx Content (mg·g−1 Dry Weight) | Fx Productivity (mg·L−1·Day−1) | Condition | Reference | |
|---|---|---|---|---|---|
| Tisochrysis lutea | haptophyte | 16.05 | 13.75 | dried | [112] |
| Tisochrysis lutea | haptophyte | 6.66 | 1.82 | dried | [113] |
| Tisochrysis lutea | haptophyte | 10.01 | 9.81 | dried | [114] |
| Tisochrysis lutea | haptophyte | 13.09 | - | dried | [115] |
| Tisochrysis lutea | haptophyte | 16.30 | 2.77 | dried | [116] |
| Tisochrysis lutea | haptophyte | 17.80 | 1.14 | dried | [117] |
| Tisochrysis lutea | haptophyte | 79.40 | - | dried | [111] |
| Tisochrysis lutea | haptophyte | 5.40 | - | dried | [118] |
| Pavlova lutheri | haptophyte | 20.86 | 4.88 | dried | [119] |
| Isochrysis zhangjiangensis | haptophyte | 22.6 | 3.06 | dried | [120] |
| Phaeodactylum tricornutum | diatom | 13.30 | 1.41 | dried | [117] |
| Phaeodactylum tricornutum | diatom | 7.00 | - | dried | [118] |
| Phaeodactylum tricornutum | diatom | 13.00 | 8.22 | dried | [121] |
| Phaeodactylum tricornutum | diatom | 16.30 | - | dried | [122] |
| Phaeodactylum tricornutum | diatom | 17.55 | - | dried | [123] |
| Phaeodactylum tricornutum | diatom | 16.13 | - | dried | [124] |
| Phaeodactylum tricornutum | diatom | 21.90 | - | fresh | [125] |
| Phaeodactylum tricornutum | diatom | 21.20 | - | dried | [126] |
| Chaetoceros calcitrans | diatom | 17.51 | - | dried | [127] |
| Stauroneis sp. | diatom | 11.80 | - | dried | [128] |
| Stauroneis sp. | diatom | 5.90 | - | dried | [129] |
| Thalassiosira weissflogii | diatom | 9.00 | 5.10 | dried | [130] |
| Odontella aurita | diatom | 16.20 | 9.41 | dried | [131] |
| Amphora capitellata | diatom | 41.83 | - | dried | [132] |
| Nitzschia laevis | diatom | 12.20 | - | dried | [133] |
| Conticribra weissflogii | diatom | 10.00 | - | fresh | [134] |
| Sellaphora minima | diatom | 7.60 | 1.2 | fresh | [135] |
| Nitzschia paela | diatom | 5.70 | 0.60 | fresh | [135] |
| Chaetoceros gracilis | diatom | 15.4 | 3.82 | fresh | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. https://doi.org/10.3390/md20040222
Pajot A, Hao Huynh G, Picot L, Marchal L, Nicolau E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Marine Drugs. 2022; 20(4):222. https://doi.org/10.3390/md20040222
Chicago/Turabian StylePajot, Anne, Gia Hao Huynh, Laurent Picot, Luc Marchal, and Elodie Nicolau. 2022. "Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes" Marine Drugs 20, no. 4: 222. https://doi.org/10.3390/md20040222
APA StylePajot, A., Hao Huynh, G., Picot, L., Marchal, L., & Nicolau, E. (2022). Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Marine Drugs, 20(4), 222. https://doi.org/10.3390/md20040222