Comprehensive Genomic Analysis of Marine Strain Streptomyces sp. 891, an Excellent Producer of Chrysomycin A with Therapeutic Potential
Abstract
1. Introduction
2. Results
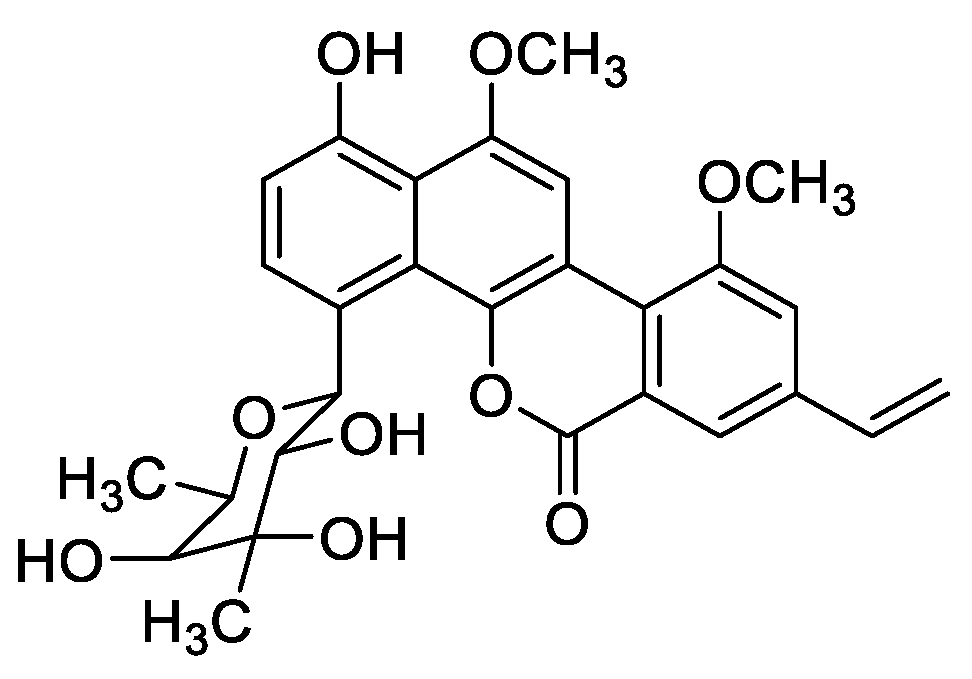
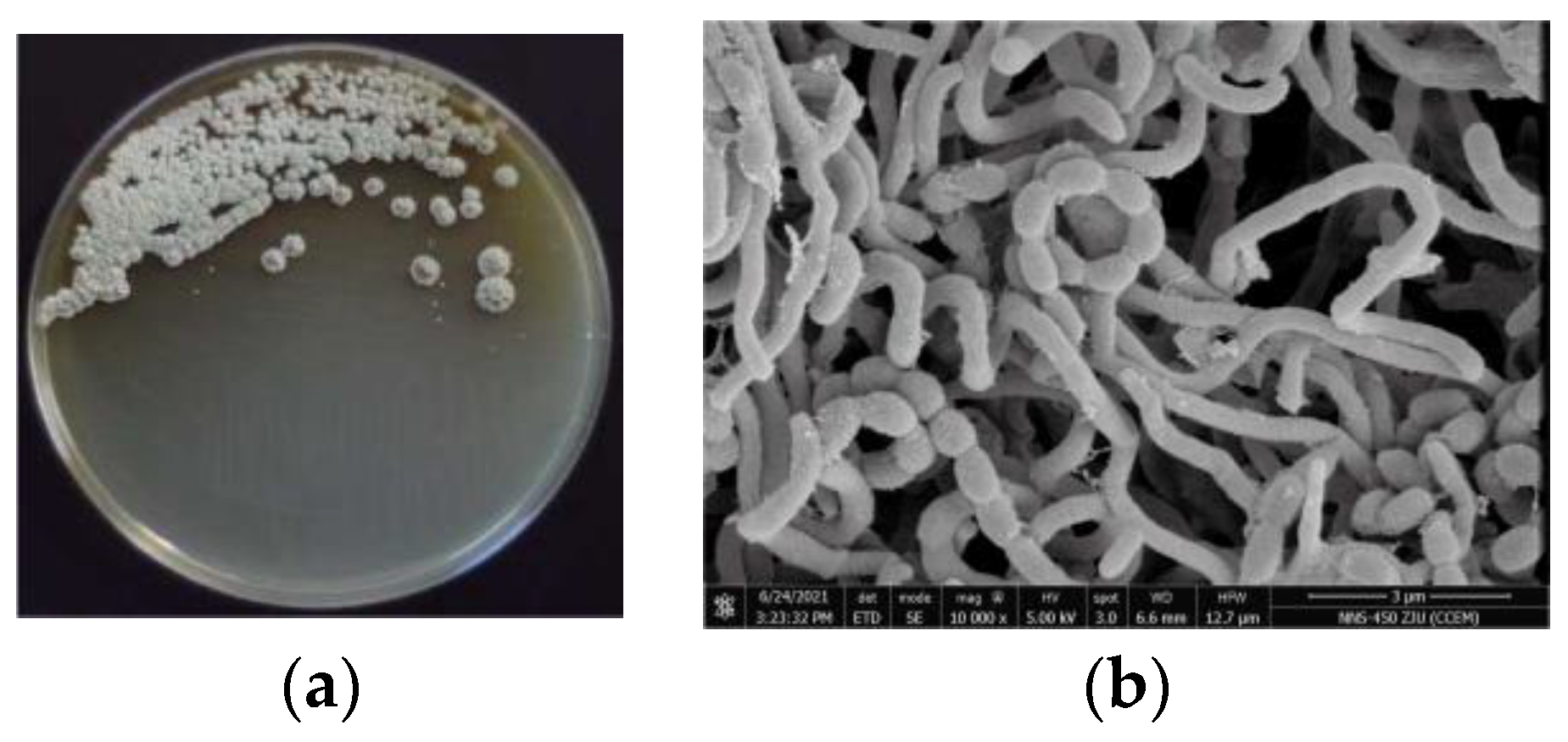
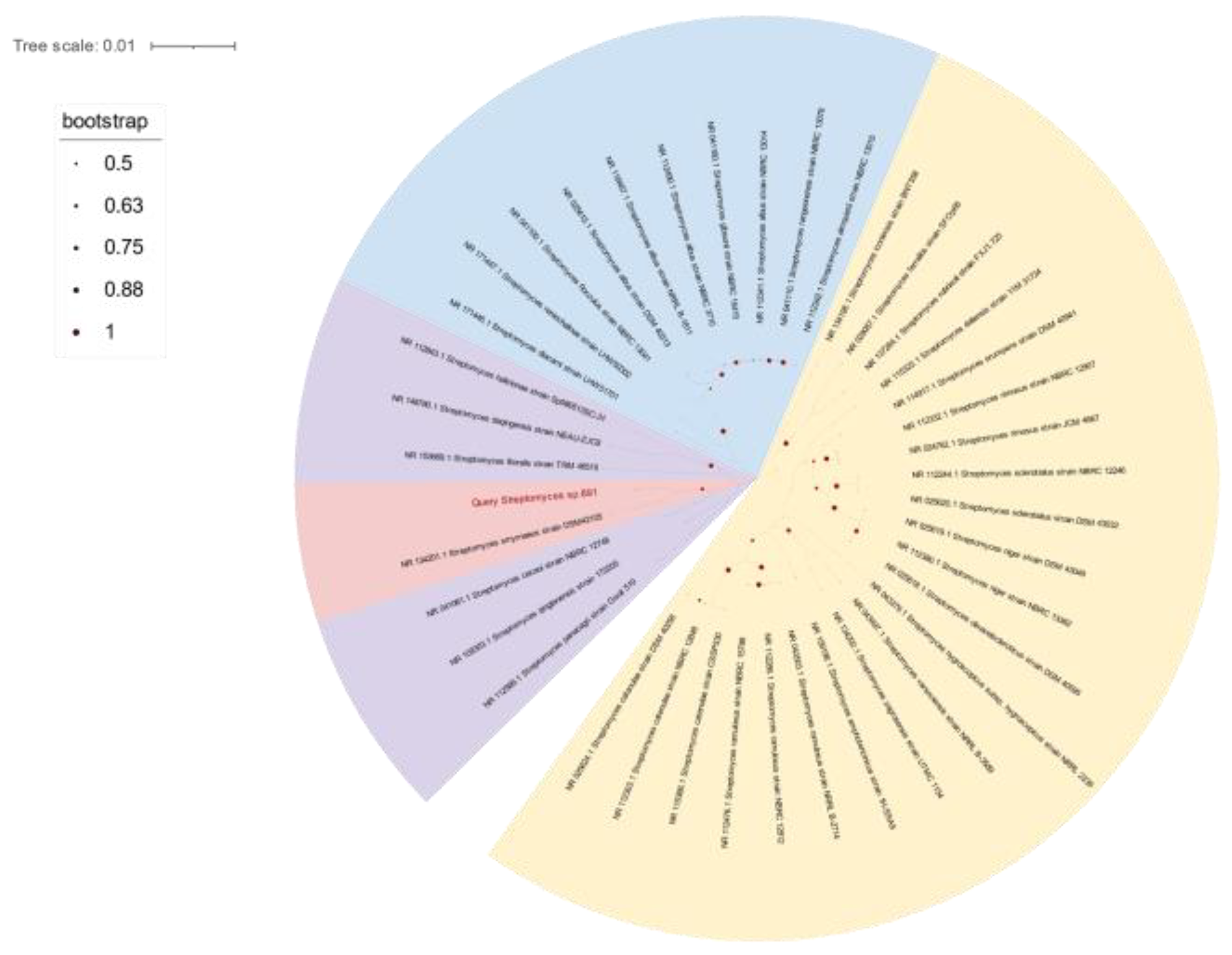
2.1. Morphology, Classification, and Phylogenetic Analysis of Strain 891
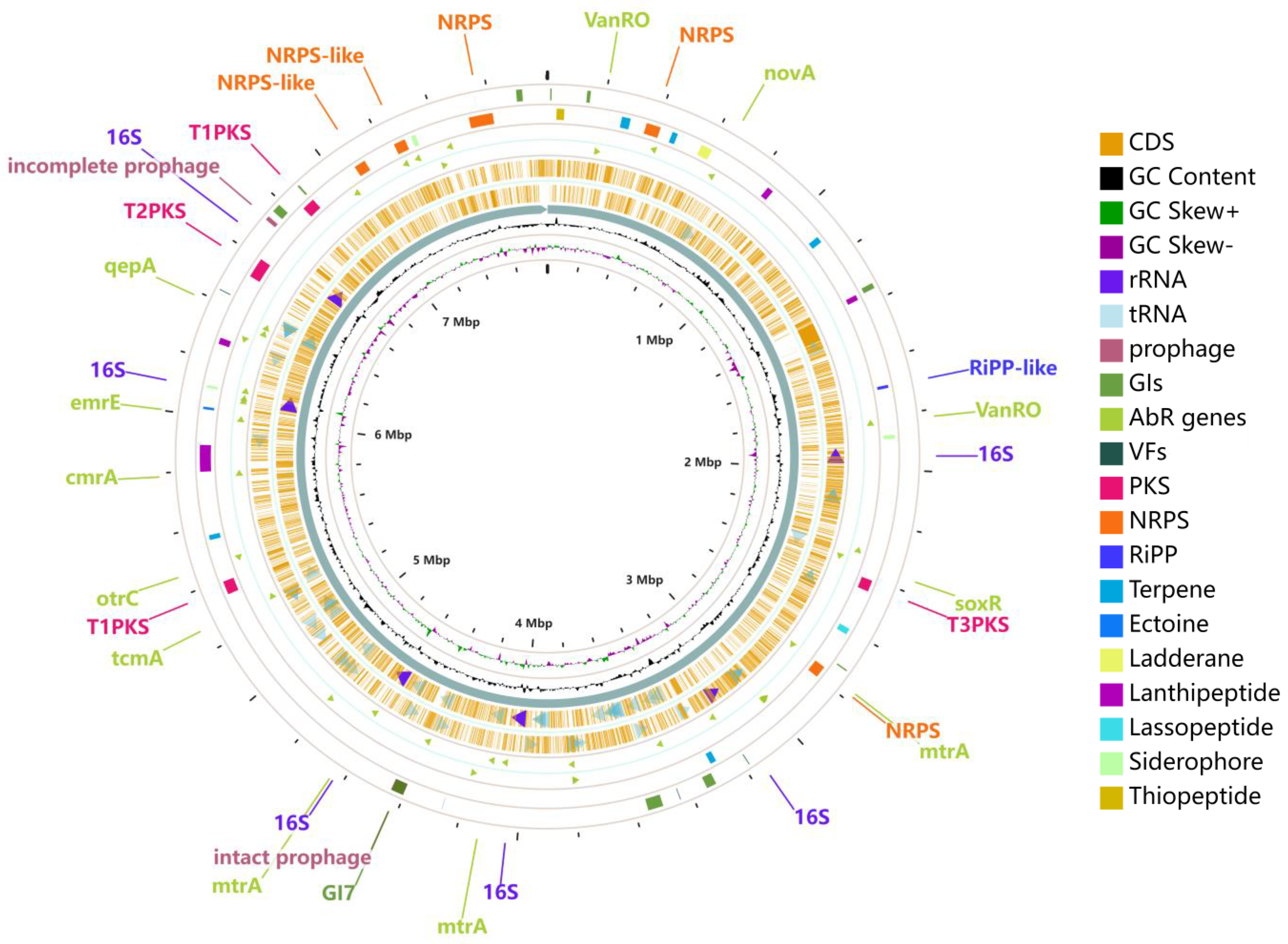
2.2. Genome Features of Strain 891
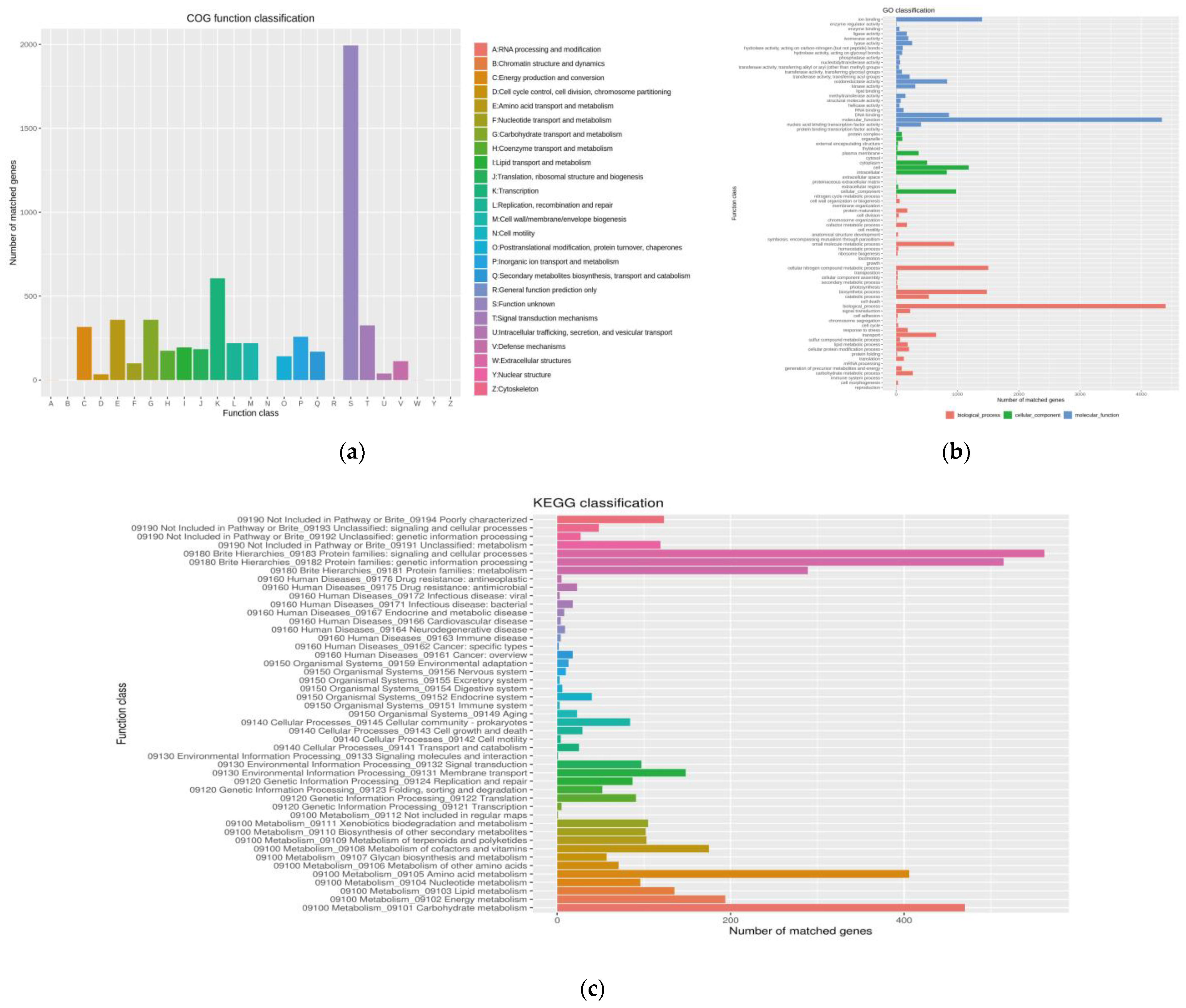
2.3. Genome Sequence Annotation of Strain 891
2.4. Additional Annotation on Prophage, Genomic Islands, Antibiotic Resistance, and Carbohydrate Genes
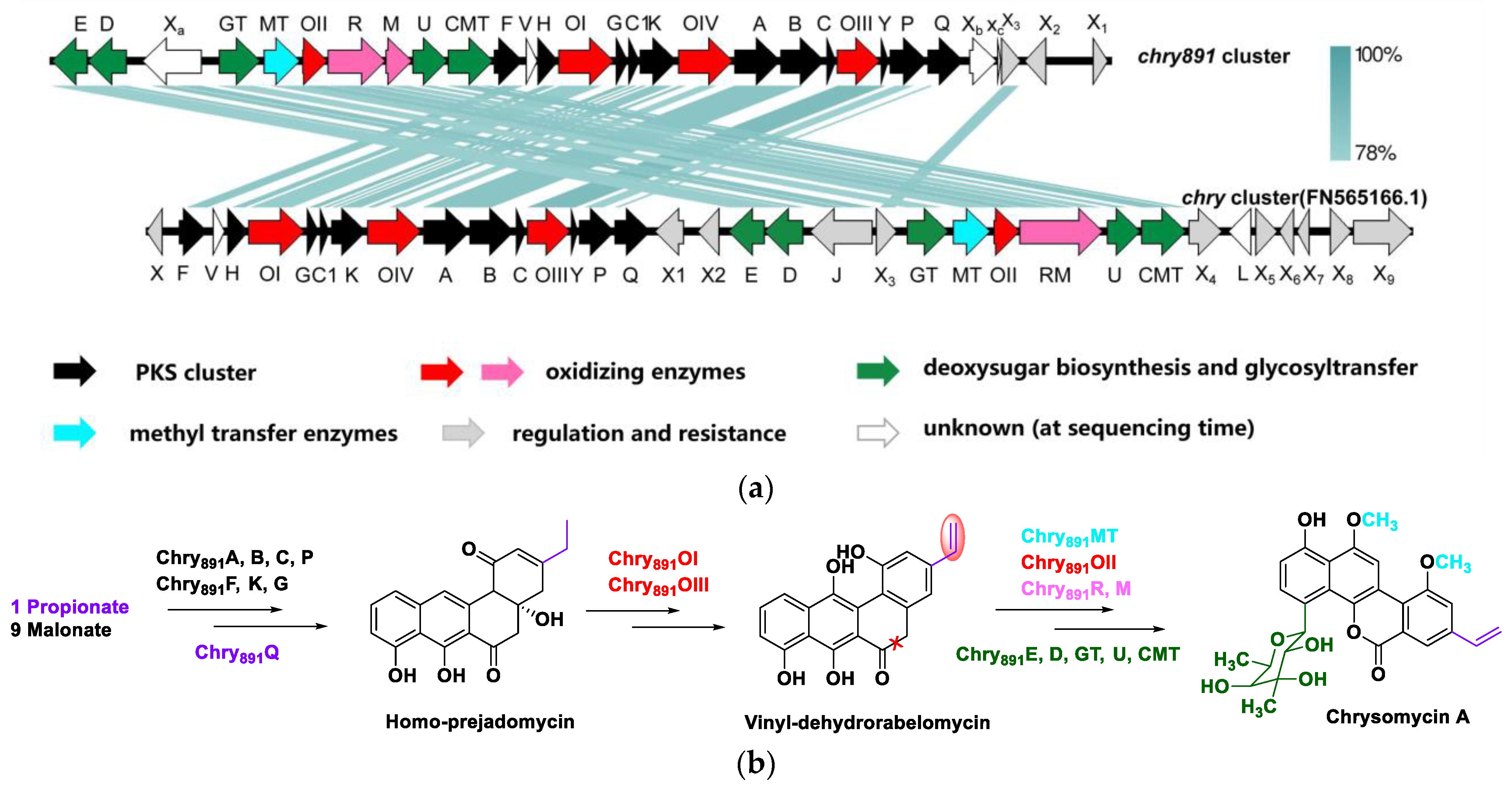
2.5. Analysis of Secondary Metabolite Biosynthetic Gene Clusters
3. Conclusions
4. Materials and Methods
4.1. Microbes and Cultivation
4.2. Phylogenetic Analysis
4.3. Genome Sequencing and Assembly
4.4. Genome Sequence Annotation
4.5. Additional Bioinformatics Analysis
4.6. Analysis of Secondary Metabolite Biosynthetic Gene Clusters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How Different Are Marine Microbial Natural Products Compared to Their Terrestrial Counterparts? Nat. Prod. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Daptomycin: Mechanisms of Action and Resistance, and Biosynthetic Engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Kim, H.; Park, S.R.; Yoon, Y.J. An Overview of Rapamycin: From Discovery to Future Perspectives. J. Ind. Microbiol. Biotechnol. 2017, 44, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, E.-S.; Hwang, Y.-S.; Choi, C.-Y. Avermectin: Biochemical and Molecular Basis of Its Biosynthesis and Regulation. Appl. Microbiol. Biotechnol. 2004, 63, 626–634. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022. [Google Scholar] [CrossRef]
- Medema, M.H.; de Rond, T.; Moore, B.S. Mining Genomes to Illuminate the Specialized Chemistry of Life. Nat. Rev. Genet. 2021, 22, 553–571. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Ishida, K.; Maier, A.; Kelter, G.; Jiang, Y.; Peschel, G.; Menzel, K.-D.; Li, M.; Wen, M.; et al. Plasticity in Gilvocarcin-Type C-Glycoside Pathways: Discovery and Antitumoral Evaluation of Polycarcin V from Streptomyces Polyformus. Org. Biomol. Chem. 2008, 6, 3601. [Google Scholar] [CrossRef] [PubMed]
- Gober, R.; Wheeler, R.; Rohr, J. Post-PKS Enzyme Complexes. Med. Chem. Commun. 2019, 10, 1855–1866. [Google Scholar] [CrossRef]
- Strelitz, F.; Flon, H.; Asheshov, I.N. Chrysomycin: A New Antibiotic Substance for Bacterial Viruses. J. Bacteriol. 1955, 69, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Yoshihira, K.; Highet, R.J.; White, R.J.; Wei, T.T. The Chemistry of the Antibiotics Chrysomycin A and B Antitumor Activity of Chrysomycin A. J. Antibiot. 1982, 35, 1194–1201. [Google Scholar] [CrossRef]
- Matson, J.A.; Rose, W.C.; Bush, J.A.; Myllymaki, R.; Bradner, W.T.; Doyle, T.W. Antitumor Activity of Chrysomycins M and V. J. Antibiot. 1989, 42, 1446–1448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, F.; Zhang, J.; Song, F.; Wang, S.; Guo, H.; Wei, Q.; Dai, H.; Chen, X.; Xia, X.; Liu, X.; et al. Chrysomycin A Derivatives for the Treatment of Multi-Drug-Resistant Tuberculosis. ACS Cent. Sci. 2020, 6, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Sawa, R.; Iwanami, F.; Nagayoshi, M.; Kubota, Y.; Iijima, K.; Hayashi, C.; Shibuya, Y.; Hatano, M.; Igarashi, M.; et al. Structures and Biological Activities of Novel 4′-Acetylated Analogs of Chrysomycins A and B. J. Antibiot. 2017, 70, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, B.; Dan, V.M.; Vinodh, J.S.; Jamsheena, V.; Ramachandran, R.; Thomas, S.; Dastager, S.G.; Kumar, K.S.; Lankalapalli, R.S.; Kumar, R.A. Anti-Microbial Activity of Chrysomycin A Produced by Streptomyces Sp. against Mycobacterium Tuberculosis. RSC Adv. 2017, 7, 36335–36339. [Google Scholar] [CrossRef]
- Muralikrishnan, B.; Edison, L.K.; Dusthackeer, A.; Jijimole, G.R.; Ramachandran, R.; Madhavan, A.; Kumar, R.A. Chrysomycin A Inhibits the Topoisomerase I of Mycobacterium Tuberculosis. J. Antibiot. 2022, 75, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.-J.; Lv, S.-Y.; Sheng, Y.-T.; Wang, H.; Chu, X.-H.; Zhang, H.-W. Optimization of Fermentation Conditions and Medium Compositions for the Production of Chrysomycin a by a Marine-Derived Strain Streptomyces Sp. 891. Prep. Biochem. Biotechnol. 2021, 1–6. [Google Scholar] [CrossRef]
- Tatar, D.; Guven, K.; Spröer, C.; Klenk, H.-P.; Sahin, N. Streptomyces Iconiensis Sp. Nov. and Streptomyces Smyrnaeus Sp. Nov., Two Halotolerant Actinomycetes Isolated from a Salt Lake and Saltern. Int. J. Syst. Evol. Microbiol. 2014, 64, 3126–3133. [Google Scholar] [CrossRef]
- Figueras, M.J.; Beaz-Hidalgo, R.; Hossain, M.J.; Liles, M.R. Taxonomic Affiliation of New Genomes Should Be Verified Using Average Nucleotide Identity and Multilocus Phylogenetic Analysis. Genome Announc. 2014, 2, e00927-14. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.-K. Mini Review: Genome Mining Approaches for the Identification of Secondary Metabolite Biosynthetic Gene Clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Kim, Y.; Jeong, Y.; Roe, J.H.; Kim, B.G.; Cho, B.K. Comparative Genomics Reveals the Core and Accessory Genomes of Streptomyces Species. J. Microbiol. Biotechnol. 2015, 25, 1599–1605. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR-Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbohydrate-Active Enzymes Database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Nybo, S.E.; Shepherd, M.D.; Rohr, J. Cloning and Characterization of the Ravidomycin and Chrysomycin Biosynthetic Gene Clusters. ChemBioChem 2010, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Grove, A. MarR Family Transcription Factors. Curr. Biol. 2013, 23, R142–R143. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, S.; Perozzo, R.; Reinelt, S.; Meyer, M.; Pfister, K.; Scapozza, L.; Bott, M. The Periplasmic Domain of the Histidine Autokinase CitA Functions as a Highly Specific Citrate Receptor. Mol. Microbiol. 1999, 33, 858–872. [Google Scholar] [CrossRef]
- Pasqua, M.; Bonaccorsi di Patti, M.C.; Fanelli, G.; Utsumi, R.; Eguchi, Y.; Trirocco, R.; Prosseda, G.; Grossi, M.; Colonna, B. Host—Bacterial Pathogen Communication: The Wily Role of the Multidrug Efflux Pumps of the MFS Family. Front. Mol. Biosci. 2021, 8, 3274. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.S.; Leplae, R.; Summers, A.O.; Toussaint, A. Mobile Genetic Elements: The Agents of Open Source Evolution. Nat. Rev. Microbiol. 2005, 3, 722–732. [Google Scholar] [CrossRef]
- Gui, C.; Chen, J.; Xie, Q.; Mo, X.; Zhang, S.; Zhang, H.; Ma, J.; Li, Q.; Gu, Y.-C.; Ju, J. CytA, a Reductase in the Cytorhodin Biosynthesis Pathway, Inactivates Anthracycline Drugs in Streptomyces. Commun. Biol. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Almabruk, K.H.; Dinh, L.K.; Philmus, B. Self-Resistance of Natural Product Producers: Past, Present, and Future Focusing on Self-Resistant Protein Variants. ACS Chem. Biol. 2018, 13, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A Toolkit to Classify Genomes with the Genome Taxonomy Database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-S.; Peluso, P.; Sedlazeck, F.J.; Nattestad, M.; Concepcion, G.T.; Clum, A.; Dunn, C.; O’Malley, R.; Figueroa-Balderas, R.; Morales-Cruz, A.; et al. Phased Diploid Genome Assembly with Single Molecule Real-Time Sequencing. Nat. Methods 2016, 13, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptive k-Mer Weighting and Repeat Separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A Self-Training Method for Prediction of Gene Starts in Microbial Genomes. Implications for Finding Sequence Motifs in Regulatory Regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb Database and Tools to Display CRISPRs and to Generate Dictionaries of Spacers and Repeats. BMC Bioinform. 2007, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An Automatic Genome Annotation and Pathway Reconstruction Server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S. Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- Gauze, G.F.; Sveshnikova, M.A.; Maksimova, T.S.; Ol’khovatova, O.L.; Bazhanov, V.S. Formation of New Antibiotic, Virenomycin, by a Culture of Streptomyces virens sp. nev. Antibiotiki 1977, 22, 963–967. [Google Scholar]
- Carter, G.T.; Fantini, A.; James, J.C.; Borders, D.B.; White, R.J. Biosynthesis of Chrysomycins A and B Origin of the Chromophore. J. Antibiot. 1985, 38, 242–248. [Google Scholar] [CrossRef]
- Jain, S.K.; Pathania, A.S.; Parshad, R.; Raina, C.; Ali, A.; Gupta, A.P.; Kushwaha, M.; Aravinda, S.; Bhushan, S.; Bharate, S.B.; et al. Chrysomycins A–C, Antileukemic Naphthocoumarins from Streptomyces Sporoverrucosus. RSC Adv. 2013, 3, 21046. [Google Scholar] [CrossRef]

| Features | Chromosome | Plasmid |
|---|---|---|
| Genome topology | linear | linear |
| Genome size (bp) | 7,804,062 | 35,476 |
| GC content (%) | 71.11 | 68.31 |
| Open reading frames | 6871 | 37 |
| Gene total length (bp) | 6,656,877 | 30,870 |
| Gene density (genes per kb) | 0.88 | 1.043 |
| Longest gene length (bp) | 78,261 | 4968 |
| Gene average length (bp) | 968.84 | 834.32 |
| GC content in gene region (%) | 71.44 | 68.62 |
| rRNA genes | 18 | 0 |
| tRNA genes | 57 | 0 |
| ncRNA genes | 103 | 0 |
| Secondary metabolite BGCs | 26 | 0 |
| Genes assigned to Swiss-Prot | 4236 | 5 |
| Genes assigned to KEGG | 2232 | 2 |
| Genes assigned to GO | 4675 | 7 |
| Genes assigned to NR | 6581 | 25 |
| Genes assigned to COG | 5808 | 11 |
| Genes assigned to CARD | 55 | 0 |
| Genes assigned to CAZy | 250 | 0 |
| CRISPR repeats | 15 | 0 |
| GenBank accession number | CP050693 | CP050694 |
| Positions | Functions | |
|---|---|---|
| CRISPR repeat No.1 | 7,701,174–7,702,715 | CRISPR repeat sequences |
| cas gene | 7,703,460–7,703,765 | CRISPR-associated endoribonuclease Cas6 |
| 7,705,207–7,706,280 | type I-B CRISPR-associated protein Cas7/Cst2/DevR | |
| 7,706,474–7,707,001 | CRISPR-associated protein Cas5 | |
| 7,707,097–7,709,430 | CRISPR-associated helicase/endonuclease Cas3 | |
| 7,709,427–7,709,942 | CRISPR-associated protein Cas4 | |
| 7,709,942–7,710,922 | CRISPR-associated protein Cas1 | |
| 7,711,064–7,711,192 | CRISPR-associated protein Cas2 | |
| CRISPR repeat No.2 | 7,712,784–7,714,256 | CRISPR repeat sequences |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Tang, Y.; Liu, Y.; Pei, X.; Huang, Z.; Song, F.; Zhang, H. Comprehensive Genomic Analysis of Marine Strain Streptomyces sp. 891, an Excellent Producer of Chrysomycin A with Therapeutic Potential. Mar. Drugs 2022, 20, 287. https://doi.org/10.3390/md20050287
Hu X, Tang Y, Liu Y, Pei X, Huang Z, Song F, Zhang H. Comprehensive Genomic Analysis of Marine Strain Streptomyces sp. 891, an Excellent Producer of Chrysomycin A with Therapeutic Potential. Marine Drugs. 2022; 20(5):287. https://doi.org/10.3390/md20050287
Chicago/Turabian StyleHu, Xu, Yuqi Tang, Yuanyuan Liu, Xinwei Pei, Ziwei Huang, Fuhang Song, and Huawei Zhang. 2022. "Comprehensive Genomic Analysis of Marine Strain Streptomyces sp. 891, an Excellent Producer of Chrysomycin A with Therapeutic Potential" Marine Drugs 20, no. 5: 287. https://doi.org/10.3390/md20050287
APA StyleHu, X., Tang, Y., Liu, Y., Pei, X., Huang, Z., Song, F., & Zhang, H. (2022). Comprehensive Genomic Analysis of Marine Strain Streptomyces sp. 891, an Excellent Producer of Chrysomycin A with Therapeutic Potential. Marine Drugs, 20(5), 287. https://doi.org/10.3390/md20050287







