Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula
Abstract
:1. Introduction
2. Results and Discussion
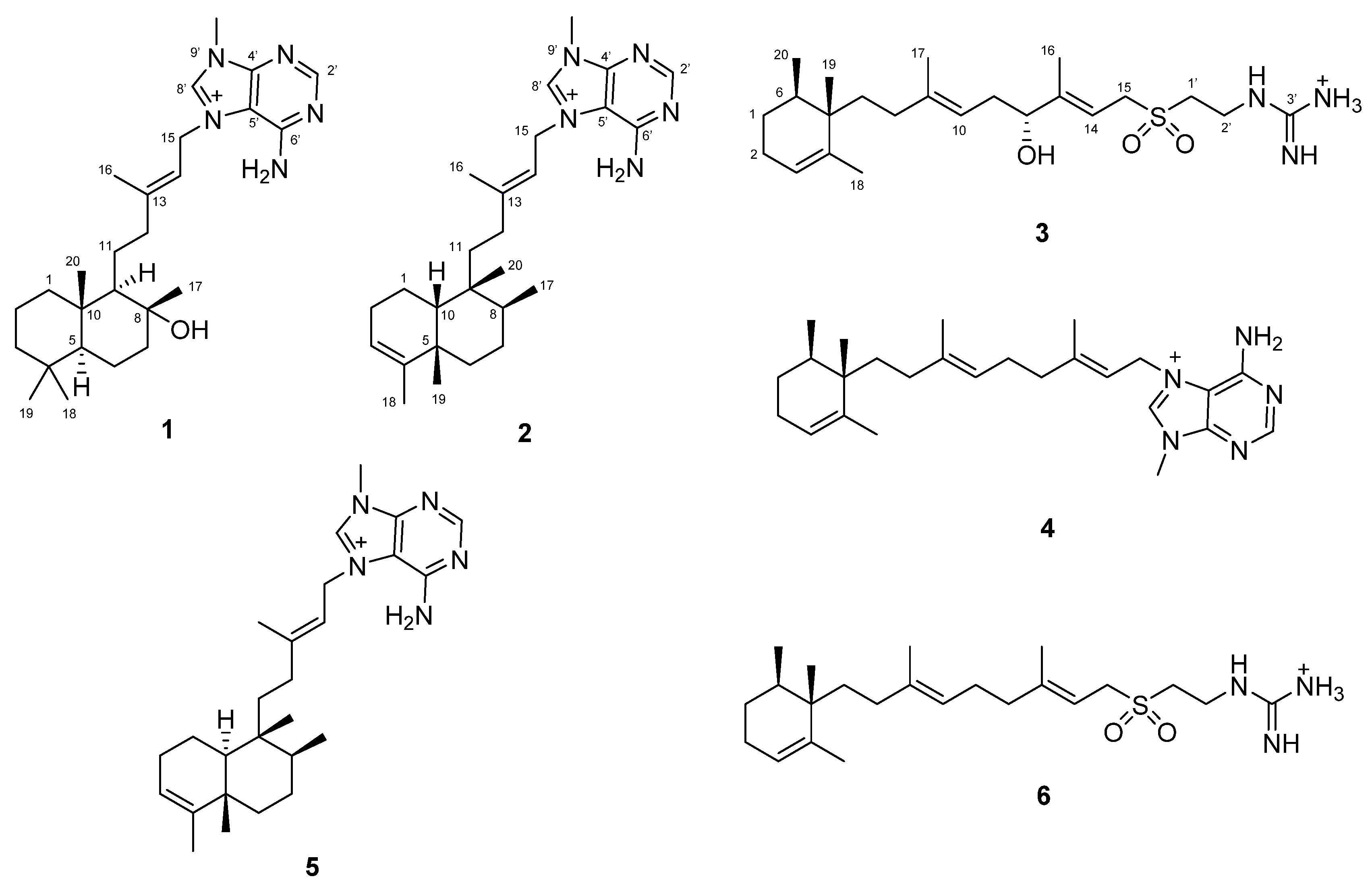
2.1. Isolation and Identification of Agelasines
2.2. Antibacterial Activity of Agelasines
3. Materials and Methods
3.1. General Experimental Chemical Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Structural Characterization
3.5. Preparation of the MTPA Esters
3.6. Antibacterial Activity Assays
3.6.1. Bacterial Strains and Culture Preparation
3.6.2. Microdilution Method: Minimum Inhibitory Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manconi, R.; Pronzato, R.; Perino, E. A New Species of Agelas from the Zanzibar Archipelago, Western Indian Ocean (Porifera, Demospongiae). ZooKeys 2016, 553, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.-J.; Li, M.; Ma, H.; Li, P.-L.; Li, G.-Q. Secondary Metabolites from Marine Sponges of the Genus Agelas: A Comprehensive Update Insight on Structural Diversity and Bioactivity. RSC Adv. 2022, 12, 7789–7820. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D. Two New Naturally Occurring Pyrrole Derivatives from the Tropical Marine Sponge Agelas oroides. Nat. Prod. Lett. 1994, 5, 141–146. [Google Scholar] [CrossRef]
- Sauleau, P.; Moriou, C.; al Mourabit, A. Metabolomics Approach to Chemical Diversity of the Mediterranean Marine Sponge Agelas oroides. Nat. Prod. Res. 2017, 31, 1625–1632. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Chen, J.; Wang, H.; Tenney, K.; Crews, P. Bioactive Secondary Metabolites from the Marine Sponge Genus Agelas. Mar. Drugs 2017, 15, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pech-Puch, D.; Pérez-Povedano, M.; Martinez-Guitian, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Bou, G.; Rodríguez, J.; Beceiro, A.; Jimenez, C. In Vitro and In Vivo Assessment of the Efficacy of Bromoageliferin, an Alkaloid Isolated from the Sponge Agelas dilatata, against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 326. [Google Scholar] [CrossRef]
- Jiménez, C.; Crews, P. Mauritamide A and Accompanying Oroidin Alkaloids from the Sponge Agelas mauritiana. Tetrahedron Lett. 1994, 35, 1375–1378. [Google Scholar] [CrossRef]
- Hong, L.-L.; Sun, J.-B.; Yang, F.; Liu, M.; Tang, J.; Sun, F.; Jiao, W.-H.; Wang, S.-P.; Zhang, W.; Lin, H.-W. New Diterpene Alkaloids from the Marine Sponge Agelas mauritiana. RSC Adv. 2017, 7, 23970–23976. [Google Scholar] [CrossRef] [Green Version]
- Pech-Puch, D.; Joseph-Nathan, P.; Burgueño-Tapia, E.; González-Salas, C.; Martínez-Matamoros, D.; Pereira, D.M.; Pereira, R.B.; Jiménez, C.; Rodríguez, J. Absolute Configuration by Vibrational Circular Dichroism of Anti-Inflammatory Macrolide Briarane Diterpenoids from the Gorgonian Briareum asbestinum. Sci. Rep. 2021, 11, 496. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Pérez-Povedano, M.; Gómez, P.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Novoa-Olmedo, M.L.; Guillén-Hernández, S.; Villegas-Hernández, H.; Bou, G.; et al. Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds. Mar. Drugs 2020, 18, 369. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Berastegui-Cabrera, J.; Pérez-Povedano, M.; Villegas-Hernández, H.; Guillén-Hernández, S.; Cautain, B.; Reyes, F.; Pachón, J.; Gómez, P.; Rodríguez, J.; et al. Antiviral and Antiproliferative Potential of Marine Organisms from the Yucatan Peninsula, Mexico. Front. Mar. Sci. 2020, 7, 607. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Rodríguez, J.; Cautain, B.; Sandoval-Castro, C.A.; Jiménez, C. Cytotoxic Furanoditerpenes from the Sponge Spongia tubulifera Collected in the Mexican Caribbean. Mar. Drugs 2019, 17, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal Diterpene Alkaloids from the Caribbean Sponge Agelas citrina: Unified Configurational Assignments of Agelasidines and Agelasines. Eur. J. Org. Chem. 2012, 2012, 5131–5135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cychon, C.; Lichte, E.; Köck, M. The Marine Sponge Agelas citrina as a Source of the New Pyrrole–Imidazole Alkaloids Citrinamines A–D and N -Methylagelongine. Beilstein J. Org. Chem. 2015, 11, 2029–2037. [Google Scholar] [CrossRef] [Green Version]
- Anta, C.; González, N.; Rodríguez, J.; Jiménez, C. A New Secosterol from the Indonesian Octocoral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1357–1359. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Agelasines O–U, New Diterpene Alkaloids with a 9-N-Methyladenine Unit from a Marine Sponge Agelas sp. Tetrahedron 2012, 68, 9738–9744. [Google Scholar] [CrossRef]
- Bendall, J.; Cambie, R.; Moratti, S.; Rutledge, P.; Woodgate, P. Synthesis of a Novel Diterpenoid Rearrangement Product. Aust. J. Chem. 1995, 48, 1747. [Google Scholar] [CrossRef]
- Millán, R.E.; Rodríguez, J.; Sarandeses, L.A.; Gómez-Bengoa, E.; Sestelo, J.P. Indium(III)-Catalyzed Stereoselective Synthesis of Tricyclic Frameworks by Cascade Cycloisomerization Reactions of Aryl 1,5-Enynes. J. Org. Chem. 2021, 86, 9515–9529. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Physiologically Active Marine Natural Products from Porifera. VIII. Agelasidines. Novel Hypotaurocyamine Derivatives from the Okinawan Sea Sponge Agelas nakamurai Hoshino. J. Org. Chem. 1985, 50, 2494–2497. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Structures and Biological Evaluations of Agelasines Isolated from the Okinawan Marine Sponge Agelas nakamurai. J. Nat. Prod. 2015, 78, 1428–1433. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–118. [Google Scholar] [CrossRef]
- Proszenyák, Á.; Brændvang, M.; Charnock, C.; Gundersen, L.-L. The First Synthesis of Ent-Agelasine F. Tetrahedron 2009, 65, 194–199. [Google Scholar] [CrossRef]




| Post. | 1 a | 2 b | 5 b | |||
|---|---|---|---|---|---|---|
| δH, Mult, (J in Hz) | δC, Type | δH, Mult, (J in Hz) | δC, Type | δH, Mult, (J in Hz) | δC, Type | |
| 1 | 1.58, m 0.89, m | 39.2, CH2 | 1.64 m 1.33, m | 19.7, CH2 | 1.38, m 1.15, m | 18.2, CH2 |
| 2 | 1.53, m | 19.8, CH2 | 1.98, m | 22.9, CH2 | 1.49, m | 26.8, CH2 |
| 1.19, m | 1.91, m | 1.29, m | ||||
| 3 | 1.33, m | 41.3, CH2 | 5.30, brs | 120.7, CH | 5.16, brs | 120.5, CH |
| 1.10, m | ||||||
| 4 | 32.9, C | 136.2, C | 144.3, C | |||
| 5 | 0.81, m | 55.3, CH | 40.5, C | 38.3, C | ||
| 6 | 1.34, m | 17.7, CH2 | 1.80, m 1.71, m | 33.7, CH2 | 1.70, d, (12.6) 1.14 m | 36.7, CH2 |
| 7 | 1.70, dt, (11.8, 3.0) 1.30, m | 43.7, CH2 | 1.64, m 1.42, m | 29.2, CH2 | 1.43 m | 27.4, CH2 |
| 8 | 72.1, C | 1.31, m | 33.5, CH | 1.41, m | 36.3, CH | |
| 9 | 0.98, m | 59.9, CH | 39.1, C | 38.9, C | ||
| 10 | 38.5, C | 2.28, brd, (12.9) | 42.0, CH | 1.29 m | 46.4, CH | |
| 11 | 1.55, m | 22.7, CH2 | 1.35, m | 30.0, CH2 | 1.60 m | 32.5, CH2 |
| 1.27, m | 1.25, m | 1.45 m | ||||
| 12 | 2.22, td, (12.2, 6.1) | 42.2, CH2 | 2.03, dt, (11.8, 5.6) | 35.1, CH2 | 2.00 m | 33.0, CH2 |
| 2.09, td, (12.2, 4.9) | 1.97, m | 1.92 m | ||||
| 13 | 147.0, C | 149.2, C | 148.8, C | |||
| 14 | 5.44, t, (7.0) | 114.6, CH | 5.45, t, (6.4) | 115.0, CH | 5.43, t, (5.6) | 115.0, CH |
| 15 | 5.13, d, (7.3) | 46.6, CH2 | 5.25, d, (6.5) | 48.6, CH2 | 5.34, d, (6.0) | 48.9, CH2 |
| 16 | 1.79, s | 16.5, CH3 | 1.86, s | 17.2, CH3 | 1.85, s | 17.2, CH3 |
| 17 | 1.00, s | 23.6, CH3 | 1.07, d, (7.4) | 18.7, CH3 | 0.78, d, (5.9) | 15.9, CH3 |
| 18 | 0.84, s | 33.0, CH3 | 1.62, s | 21.7, CH3 | 1.56, s | 18.0, CH3 |
| 19 | 0.75, s | 21.1, CH3 | 0.86, s | 17.2, CH3 | 0.98, s | 20.0, CH3 |
| 20 | 0.74, s | 15.0, CH3 | 0.94, s | 15.6, CH3 | 0.70, s | 18.3, CH3 |
| 2′ | 8.46, s | 155.4, CH | 8.54, s | 155.8, CH | 8.52, s | 155.2, CH |
| 4′ | 149.0, C | 149.7, C | 149.6, C | |||
| 5′ | 109.2, C | 110.0, C | 110.5, C | |||
| 6′ | 152.4, C | 151.8, C | 151.7, C | |||
| 8′ | 9.51, s | 140.8, CH | 9.87, s | 142.2, C | 10.06, s | 142.3, CH |
| 9′-N-Me | 3.88, s | 31.1, CH3 | 4.06, s | 31.9, CH3 | 4.07, s | 32.2, CH3 |
| NH2 | 7.92, s | |||||
| Position | 3 | |
|---|---|---|
| δH, Mult (J in Hz) | δC, Type | |
| 1 | 1.44, m | 27.2, CH2 |
| 2 | 1.95, m | 25.7, CH2 |
| 3 | 5.40, brs | 124.4, CH |
| 4 | 139.7, C | |
| 5 | 40.5, C | |
| 6 | 1.71, m | 33.3, CH |
| 7 | 1.43, m | 35.3, CH2 |
| 8 | 1.95, m; 1.48, m | 34.6, CH2 |
| 9 | 140.1, C | |
| 10 | 5.06, t, (6.5) | 118.6, CH |
| 11 | 2.27, t, (6.5) | 34.0, CH2 |
| 12 | 4.09, t, (6.5) | 76.3, CH |
| 13 | 149.2, C | |
| 14 | 5.52, t, (8.0) | 110.0, CH |
| 15 | 3.84, dd, (14.5, 8.0); 3.80, dd, (14.5, 8.0) | 53.8, CH2 |
| 16 | 1.71, brs | 13.1, CH3 |
| 17 | 1.61, brs | 16.6, CH3 |
| 18 | 1.60, brs | 19.3, CH3 |
| 19 | 0.84, s | 16.0, CH3 |
| 20 | 0.85, d, (6.7) | 21.2, CH3 |
| 1′ | 3.28, brs | 50.3, CH2 |
| 2′ | 3.70, brs | 34.5, CH2 |
| 3′ | 157.5, C | |
| Compound | S. aureus ATCC 29213 | S. aureus USA300LAC | S. pneumoniae ATCC 49619 | S. pneumoniae 549 CHUAC | E. faecalis ATCC 29212 | E. faecalis 256 CHUAC | E. faecium 214 CHUAC |
|---|---|---|---|---|---|---|---|
| (+)-8-epiagelasine T (1) | 16 | 16 | 16 | 32 | 32 | ≥64 | 32 |
| (+)-10-epiagelasine B (2) | 1 | 2 | 4 | 8 | 4 | 4 | 4 |
| (+)-12-hydroxyagelasidine C (3) | 8 | 8 | 16 | - | 16 | 32 | 8 |
| (+)-ent-agelasine F (4) | 4 | 4 | 4 | - | 8 | 8 | 8 |
| (+)-agelasine B (5) | 2 | 2 | 4 | 16 | 8 | 8 | 4 |
| (+)-agelasidine C (6) | 8 | 8 | 4 | - | 8 | 8 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Puch, D.; Forero, A.M.; Fuentes-Monteverde, J.C.; Lasarte-Monterrubio, C.; Martinez-Guitian, M.; González-Salas, C.; Guillén-Hernández, S.; Villegas-Hernández, H.; Beceiro, A.; Griesinger, C.; et al. Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Mar. Drugs 2022, 20, 298. https://doi.org/10.3390/md20050298
Pech-Puch D, Forero AM, Fuentes-Monteverde JC, Lasarte-Monterrubio C, Martinez-Guitian M, González-Salas C, Guillén-Hernández S, Villegas-Hernández H, Beceiro A, Griesinger C, et al. Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Marine Drugs. 2022; 20(5):298. https://doi.org/10.3390/md20050298
Chicago/Turabian StylePech-Puch, Dawrin, Abel M. Forero, Juan Carlos Fuentes-Monteverde, Cristina Lasarte-Monterrubio, Marta Martinez-Guitian, Carlos González-Salas, Sergio Guillén-Hernández, Harold Villegas-Hernández, Alejandro Beceiro, Christian Griesinger, and et al. 2022. "Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula" Marine Drugs 20, no. 5: 298. https://doi.org/10.3390/md20050298
APA StylePech-Puch, D., Forero, A. M., Fuentes-Monteverde, J. C., Lasarte-Monterrubio, C., Martinez-Guitian, M., González-Salas, C., Guillén-Hernández, S., Villegas-Hernández, H., Beceiro, A., Griesinger, C., Rodríguez, J., & Jiménez, C. (2022). Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Marine Drugs, 20(5), 298. https://doi.org/10.3390/md20050298









