Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo
Abstract
:1. Introduction
2. In Vitro Study of Natural Marine Products against CRC
2.1. Alkaloid
2.2. Peptides
2.3. Terpenes
2.4. Polysaccharides
2.5. Carotenoids
2.6. Other Compounds
| Compound Name | Marine Organism | Species Name | Cell Lines | IC50 | Mechanism | References |
|---|---|---|---|---|---|---|
| Polyphenolic fraction | Seagrass | Thalassia testudinum | HCT-15 | 36.51 ± 4.68 µg/mL, 24 h | Cytotoxicity; apoptosis | [83] |
| 22.47 ± 1.30 µg/mL, 48 h | ||||||
| Thalassiolin B | Seagrass | Thalassia testudinum | HCT-15 | 51.82 ± 8.72 µg/mL, 24 h | Cytotoxicity; apoptosis | [83] |
| 38.75 ± 3.57 µg/mL, 48 h | ||||||
| TTE | Seagrass | Thalassia testudinum | RKO | 251.9 ± 8.8 µg/mL, 48 h 174.9 ± 8.7 µg/mL, 72 h | Anti-proliferative; block EMT; Anti-angiogenesis; ATF4-P53-NF-κB pathway | [84] |
| SW480 | 60.5 ± 7.6 µg/mL, 48 h 58.9 ± 7.9 µg/mL, 72 h | |||||
| Candidaspongiolide (CAN) | Marine sponge | Candidaspongia sp. | HCT-116 | ≈100 nM, 48 h | Apoptosis; caspase 12 pathway | [87] |
| Mycophenolic acid | Marine fungi | Penicillium brevicompactum | HT-29 | 5.47 μM, \ | Cytotoxicity | [98] |
| Neaumycin B | Marine actinomycetes | Micromonospora | HCT-116 | 3.338 μg/mL, 5 days | Cytotoxicity | [101] |
| PM100117 | Marine actinomycetes | Streptomyces caniferus GUA-06-05-006A | HCT-116 | 3.61 μM, 72 h | Cytotoxicity | [102] |
| PM100118 | Marine actinomycetes | Streptomyces caniferus GUA-06-05-006A | HT-29 | 4.09 μM, 72 h | Cytotoxicity | [102] |
| Compound 5 | Marine sponge | Theonella sp. | DLD-1 | 2.50 µM, 24 h | Anti-proliferative | [103] |
| Compound 7 | Marine sponge | Theonella sp. | HCT-116 | 0.78 µM, 24 h | Anti-proliferative | [103] |
| DLD-1 | 0.55 µM, 24 h | Anti-proliferative | ||||
| Ganodermasides A | Marine fungi | Pseudogymnoascus sp. HSX2#-11 | HCT-116 | 25 ± 1.5 µM, 24 h | Cytotoxicity | [104] |
| Ganodermasides B | Marine fungi | Pseudogymnoascus sp. HSX2#-11 | HCT-116 | 23 ± 0.93 µM, 24 h | Cytotoxicity | [104] |
| Compound 1 1 | Marine fungi | Penicillium oxalicum | Caco-2 | 21.4 µM, 24 h | Cytotoxicity | [105] |
| Compound 9 | Marine fungi | Aspergillus flocculosus 01NT-1.1.5 | HCT-15 | 3.0 µM, 48 h | Cytotoxicity | [106] |
| Compound 10 | Marine fungi | Aspergillus flocculosus 01NT-1.1.5 | HCT-15 | 2.8 µM, 48 h | Cytotoxicity | [106] |
| Trichothecin | Marine fungi | Alternaria sp. TZP-11 | HCT-116 | 0.25 µM, 72 h | Anti-proliferation; apoptosis; G0/G1 cell cycle arrest; block EMT; STAT3 pathway | [107] |
| Shellmycin A | Marine actinomycetes | Streptomyces sp. Shell-016 | HT-29 | 4.69 µM, 24 h 0.85 µM, 72 h | Cytotoxicity | [108] |
| Shellmycin B | Marine actinomycetes | Streptomyces sp. Shell-016 | HT-29 | 6.12 µM, 24 h 1.12 µM, 72 h | Cytotoxicity | [108] |
| Shellmycin C | Marine actinomycetes | Streptomyces sp. Shell-016 | HT-29 | 13.0 µM, 24 h 4.33 µM, 72 h | Cytotoxicity | [108] |
| Shellmycin D | Marine actinomycetes | Streptomyces sp. Shell-016 | HT-29 | 5.37 µM, 24 h 1.02 µM, 72 h | Cytotoxicity | [108] |
| Asperphenin A | Marine fungi | Aspergillus sp. | RKO | 0.84 ± 0.26 µM, 72 h | Apoptosis; G2/M cell cycle arrest | [109] |
| Asperphenin B | Marine fungi | Aspergillus sp. | RKO | 1.26 ± 0.43 µM, 72 h | Cytotoxicity | [109] |
| Cladoloside D1 | Sea cucumber | Cladolabes schmeltzii | HT-29 | 16.0 ± 0.7 μM, 24 h | Cytotoxicity | [110] |
| Cladoloside M | Sea cucumber | Cladolabes schmeltzii | HT-29 | 14.8 ± 1.6 μM, 24 h | Cytotoxicity | [110] |
| Cladoloside M1 | Sea cucumber | Cladolabes schmeltzii | HT-29 | 16.9 ± 0.4 μM, 24 h | Cytotoxicity | [110] |
| Cladoloside M2 | Sea cucumber | Cladolabes schmeltzii | HT-29 | 8.5 ± 0.5 μM, 24 h | Cytotoxicity | [110] |
| Cladoloside N | Sea cucumber | Cladolabes schmeltzii | HT-29 | 8.8 ± 0.3 μM, 24 h | Cytotoxicity | [110] |
| Cladoloside Q | Sea cucumber | Cladolabes schmeltzii | HT-29 | 15.0 ± 1.4 μM, 24 h | Cytotoxicity | [110] |
| Compound 2 | Marine actinomycetes | Streptomyces cacaoi | Caco-2 | 7.4 ± 0.3 μM, 48 h | Inhibit autophagy; induce apoptosis | [111] |
| Compound 1 1 | Marine sponge | Aplysinella | HCT-116 | 8.2 ± 0.72 µM, 72 h | Cytotoxicity | [112] |
| Compound 3 | Marine sponge | Aplysinella | HCT-116 | 5.1 ± 0.41 µM, 72 h | Cytotoxicity | [112] |
| Compound 4 | Marine sponge | Aplysinella | HCT-116 | 3.7 ± 0.31 µM, 72 h | Cytotoxicity | [112] |
| Ethanol | Seaweed | Gracilaria verrucosa | HCT-116 | 43.9 μg/mL, 48 h | Cytotoxicity | [113] |
| anthenosides J and K (ratio of 3:1) | Starfish | Anthenea aspera | HT-29 | 40 μM, 24 h | Apoptosis | [114] |
| Fraction D | Marine dinoflagellate | Alexandrium andersoni | HT-29 | ≈3 μg/mL, 48 h | Cytotoxicity; TNF pathway | [115] |
| Ethyl acetate | Seaweed | Eucheuma spinosum | HCT-116 | 16.82 μg/mL, 48 h | Cytotoxicity | [116] |
| Chloroform | Seaweed | Eucheuma spinosum | HCT-116 | 26.87 μg/mL, 48 h | Cytotoxicity | [116] |
| Hexane | Seaweed | Eucheuma cottonii | HCT-116 | 24.83 μg/mL, 48 h | Cytotoxicity | [116] |
| N-Hexane | Brown algae | Halopteris scoparia L. Sauvageau | Caco-2 | 4.53 ± 0.12 μg/mL, 48 h | Cytotoxicity; apoptosis; AKT pathway | [117] |
| Methanol | Brown algae | Halopteris scoparia L. Sauvageau | Caco-2 | 22 ± 0.11 μg/mL, 48 h | Cytotoxicity; apoptosis; AKT pathway | [117] |
| Crude extract | Marine sponge | Latrunculia biformis | HCT-116 | 4.8 µg/mL, 24 h | Cytotoxicity | [118] |
| HT-29 | 4.0 µg/mL, 24 h | |||||
| Ethanol extract | Sea cucumber | Holothuria atra | WiDr | 11.4 µg/mL, 24 h | Cytotoxicity | [119] |
| AVSC4 extract | Marine bacterium | Bacillus flexus | HT-29 | 93.4 µg/mL, 48 h | Cytotoxicity | [120] |
| NB extract | Nudibranch | Dolabella auricularia | HCT-116 | 1.01 ± 0.19 µg/mL, 24 h | Anti-proliferation; apoptosis; G2/M cell cycle arrest; block EMT | [121] |
| Crude containing liposomes | Marine sponge | Coscinoderma sp. | Caco-2 | 1.7 ± 0.18 µg/mL, 24 h | Anti-proliferative | [122] |
| F5 | Marine plant | Fucus vesiculosus | Caco-2 | 97.4 ± 11.6 µg/mL, 48 h | Apoptosis; necrosis | [123] |
| HT-29 | 118.8 ± 19.7 µg/mL, 48 h | Cytotoxicity | ||||
| EtOAc | Marine plant | Fucus vesiculosus | HT-29 | 170.0 ± 2.8 µg/mL, 48 h | Cytotoxicity | [123] |
| Hexane extract | Marine crab | Portunus segnis | HT-29 | 35.27 ± 0.71 µg/mL, 24 h 25.07 ± 0.68 µg/mL, 48 h 19.25 ± 0.22 µg/mL, 72 h | Anti-proliferative | [124] |
| Butanol extract | Marine crab | Portunus segnis | HT-29 | 26.63 ± 0.20 µg/mL, 24 h 15.13 ± 0.21 µg/mL, 48 h 10.12 ± 0.35 µg/mL, 72 h | Anti-proliferative; apoptosis; Caspases-3/7/9 pathway | [124] |
| Ethyl acetate extract | Marine crab | Portunus segnis | HT-29 | 48.14 ± 0.32 µg/mL, 24 h 34.63 ± 0.38 µg/mL, 48 h 22.86 ± 0.51 µg/mL, 72 h | Anti-proliferative | [124] |
| H2O extract | Marine crab | Portunus segnis | HT-29 | 44.33 ± 0.33 µg/mL, 24 h 31.97 ± 0.62 µg/mL, 48 h 19.38 ± 0.23 µg/mL, 72 h | Anti-proliferative | [124] |
3. In Vivo Study of Natural Marine Products against CRC
| Compound Name | Marine Organism | Species Name | Cell Lines | Mode of Tumor Formation | Delivery Way | Doses | Tumor Suppressor | References |
|---|---|---|---|---|---|---|---|---|
| Ohmyungsamycin A | Marine actinomycetes | Streptomyces sp. | HCT-116 | Injected subcutaneously into the flanks of the mice | Intraperitoneal injection | 10 mg/kg; Three times per week | Tumor inhibition rate: 52.1% | [18] |
| Peptide, (P6) | Bvalve mollusk | Arca inflata | HT-29 | Injected subcutaneously into the left armpit of mice | Intraperitoneal injection | 30 mg/kg; Every day | Tumor inhibition rate: 72.66% | [22] |
| Siphonodictyal B | Marine sponge | Aka coralliphaga | HCT-116 | Implanted subcutaneously into the right flanks of mice | Intraperitoneal injection | 20 mg/kg; Every 3 days | Tumor growth inhibition | [28] |
| Polyphenolic fraction | Seagrass | Thalassia testudinum | HCT-15 | Injected subcutaneously into the lower right flank region of mice | Oral gavage | 25 mg/kg; Three days a week | Tumor growth inhibition | [83] |
| TTE | Seagrass | Thalassia testudinum | CT-26 | Injected subcutaneously into the right dorsal side of mice | Oral gavage | 100 mg/kg; Every day | Tumor inhibition rate: 69.39 ± 6.7% | [84] |
| Caulerpin | Green algae | Caulerpa cylindracea | SW480 | Injected subcutaneously into the right flanks of mice | Oral gavage | 30 mg/kg; Every other day | Tumor growth inhibition | [12] |
| Asperphenin A | Marine fungi | Aspergillus sp. | RKO | Injected subcutaneously into the flanks of the mice | Intraperitoneal injection | 8 mg/kg; Three times per week | Tumor inhibition rate: 68.7 ± 17.1% | [107] |
| SPS-CF | Green alga | Capsosiphon fulvescens | HT-29 | Injected subcutaneously into the back of mice | Intraperitoneal injection | 400 mg/kg/day | Tumor inhibition rate: 20% | [131] |
| Fucoxanthin (Fx) | Brown algae | \ | HT-29 | Injected subcutaneously into the right femoral region of mice | Oral gavage | 2.5 mg/kg; Every 2 or 3 days | Tumor growth inhibition | [132] |
4. Clinical Study of Natural Marine Compounds
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. Ca-A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. Ca-A Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Clinical Pipeline Marine Pharmacology. Available online: https://www.marinepharmacology.org/ (accessed on 11 April 2022).
- Tan, L.T. Filamentous tropical marine cyanobacteria: A rich source of natural products for anticancer drug discovery. J. Appl. Phycol. 2010, 22, 659–676. [Google Scholar] [CrossRef]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, X.Y.; Nong, X.H.; Wang, J.; Qi, S.H. Brevianamides and Mycophenolic Acid Derivatives from the Deep-Sea-Derived Fungus Penicillium brevicompactum DFFSCS025. Mar. Drugs 2017, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lou, Q.; He, L.; Wen, C.; Lin, M.; Zhu, Z.; Wang, F.; Huang, L.; Lan, W.; Iwamoto, A.; et al. Reduced-gliotoxin induces ROS-mediated anoikis in human colorectal cancer cells. Int. J. Oncol. 2018, 52, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Shaala, L.A.; Youssef, D.T.A. Hemimycalins C-E.; Cytotoxic and Antimicrobial Alkaloids with Hydantoin and 2-Iminoimidazolidin-4-one Backbones from the Red Sea Marine Sponge Hemimycale sp. Marine Drugs 2021, 19, 691. [Google Scholar] [CrossRef]
- Lin, L.-C.; Kuo, T.-T.; Chang, H.-Y.; Liu, W.-S.; Hsia, S.-M.; Huang, T.-C. Manzamine A Exerts Anticancer Activity against Human Colorectal Cancer Cells. Marine Drugs 2018, 16, 252. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, H.; Dong, M.; Wu, Z.; Shen, Z.; Xie, Y.; Kong, Z.; Dai, X.; Xu, B. Metabolic reprogramming and AMPKα1 pathway activation by caulerpin in colorectal cancer cells. Int. J. Oncol. 2017, 50, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Shanmugathasan, M.; Jothy, S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol. Int. 2000, 50, 273–279. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Yu, G.; Qi, X.; Zhang, A.; Lu, Z.; Li, D.; Li, J. Identification of a novel non-ATP-competitive protein kinase inhibitor of PGK1 from marine nature products. Biochem. Pharmacol. 2021, 183, 114343. [Google Scholar] [CrossRef]
- Karan, D.; Dubey, S.; Pirisi, L.; Nagel, A.; Pina, I.; Choo, Y.M.; Hamann, M.T. The Marine Natural Product Manzamine A Inhibits Cervical Cancer by Targeting the SIX1 Protein. J. Nat. Prod. 2020, 83, 286–295. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alamri, S.A.; Alfaifi, M.Y.; Alrumman, S.A.; Elbehairi, S.E.I.; Taha, T.H.; Hashem, M. L-Glutaminase Synthesis by Marine Halomonas meridiana Isolated from the Red Sea and Its Efficiency against Colorectal Cancer Cell Lines. Molecules 2021, 26, 1963. [Google Scholar] [CrossRef]
- Byun, W.S.; Kim, S.; Shin, Y.-H.; Kim, W.K.; Oh, D.-C.; Lee, S.K. Antitumor Activity of Ohmyungsamycin A through the Regulation of the Skp2-p27 Axis and MCM4 in Human Colorectal Cancer Cells. J. Nat. Prod. 2020, 83, 118–126. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, E.; Ruan, H.; Ma, J.; Zhao, X.; Zhu, Y.; Xie, X.; Han, N.; Li, J.; Zhang, H.; et al. Actinomycin V Induces Apoptosis Associated with Mitochondrial and PI3K/AKT Pathways in Human CRC Cells. Mar. Drugs 2021, 19, 599. [Google Scholar] [CrossRef]
- Lee, J.; Gamage, C.D.B.; Kim, G.J.; Hillman, P.F.; Lee, C.; Lee, E.Y.; Choi, H.; Kim, H.; Nam, S.-J.; Fenical, W. Androsamide, a Cyclic Tetrapeptide from a Marine Nocardiopsis sp., Suppresses Motility of Colorectal Cancer Cells. J. Nat. Prod. 2020, 83, 3166–3172. [Google Scholar] [CrossRef]
- Cai, W.; Matthew, S.; Chen, Q.-Y.; Paul, V.J.; Luesch, H. Discovery of new A- and B-type laxaphycins with synergistic anticancer activity. Bioorg. Med. Chem. 2018, 26, 2310–2319. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Zhu, J.; Huang, W.; Luo, Y.; Shi, H.; Yu, D.; Chen, L.; Song, L.; Yu, R. A Novel Peptide Derived from Arca inflata Induces Apoptosis in Colorectal Cancer Cells through Mitochondria and the p38 MAPK Pathway. Mar. Drugs 2022, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Varier, K.M.; Chinnasamy, A.; Gajendran, B.; Nagarathnam, R. Isolation and characterization of a novel anticancer muscle protein from edible marine catfish tachysurus dussumeiri. Int. J. Pharm. Sci. Res. 2018, 9, 2720–2730. [Google Scholar] [CrossRef]
- Tarhouni-Jabberi, S.; Zakraoui, O.; Ioannou, E.; Riahi-Chebbi, I.; Haoues, M.; Roussis, V.; Kharrat, R.; Essafi-Benkhadir, K. Mertensene, a halogenated monoterpene, induces G2/M cell cycle arrest and caspase dependent apoptosis of human colon adenocarcinoma HT29 cell line through the modulation of ERK-1/-2, AKT and NF-κB signaling. Mar. Drugs 2017, 15, 221. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Shang, R.-Y.; Sun, M.; Li, Y.-X.; Liu, H.-Y.; Lin, H.-W.; Jiao, W.-H. Trichodermaloids A-C, Cadinane Sesquiterpenes from a Marine Sponge Symbiotic Trichoderma sp. SM16 Fungus. Chem. Biodivers. 2020, 17, e2000036. [Google Scholar] [CrossRef]
- Jiso, A.; Demuth, P.; Bachowsky, M.; Haas, M.; Seiwert, N.; Heylmann, D.; Rasenberger, B.; Christmann, M.; Dietrich, L.; Brunner, T.; et al. Natural Merosesquiterpenes Activate the DNA Damage Response via DNA Strand Break Formation and Trigger Apoptotic Cell Death in p53-Wild-type and Mutant Colorectal Cancer. Cancers 2021, 13, 3282. [Google Scholar] [CrossRef]
- Chikamatsu, S.; Saijo, K.; Imai, H.; Narita, K.; Kawamura, Y.; Katoh, T.; Ishioka, C. In Vitro and in Vivo antitumor activity and the mechanism of siphonodictyal B in human colon cancer cells. Cancer Med. 2019, 8, 5662–5672. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-C.; Peng, B.-R.; Hsu, K.-C.; El-Shazly, M.; Shih, S.-P.; Lin, T.E.; Kuo, F.-W.; Chou, Y.-C.; Lin, H.-Y.; Lu, M.-C. 13-Acetoxysarcocrassolide Exhibits Cytotoxic Activity against Oral Cancer Cells through the Interruption of the Keap1/Nrf2/p62/SQSTM1 Pathway: The Need to Move beyond Classical Concepts. Mar. Drugs 2020, 18, 382. [Google Scholar] [CrossRef]
- Tseng, W.-R.; Ahmed, A.E.; Huang, C.-Y.; Tsai, Y.-Y.; Tai, C.-J.; Orfali, R.S.; Hwang, T.-L.; Wang, Y.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive Capnosanes and Cembranes from the Soft Coral Klyxum flaccidum. Mar. Drugs 2019, 17, 461. [Google Scholar] [CrossRef] [Green Version]
- Sobahi, T.R.A.; Ayyad, S.-E.N.; Abdel-Lateff, A.; Algandaby, M.M.; Alorfi, H.S.; Abdel-Naim, A.B. Cytotoxic Metabolites from Callyspongia siphonella Display Antiproliferative Activity by Inducing Apoptosis in HCT-116 Cells. Pharmacogn. Mag. 2017, 13, S37–S40. [Google Scholar] [CrossRef]
- Park, H.B.; Tuan, N.Q.; Oh, J.; Son, Y.; Hamann, M.T.; Stone, R.; Kelly, M.; Oh, S.; Na, M. Sesterterpenoid and steroid metabolites from a deep-water Alaska sponge inhibit Wnt/β-catenin signaling in colon cancer cells. Mar. Drugs 2018, 16, 297. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Hu, B.; Cao, Q.; Xing, M.; Song, S.; Ji, A. Fucoidan Induces Apoptosis of HT-29 Cells via the Activation of DR4 and Mitochondrial Pathway. Mar. Drugs 2020, 18, 220. [Google Scholar] [CrossRef]
- Han, Y.-s.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.P.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Park, S.-H.; Jeong, J.-W.; Yoon, D.; Han, M.H.; Lee, D.-S.; Choi, G.; Yim, M.-J.; Lee, J.M.; Kim, D.-H.; et al. Induction of p53-Independent Apoptosis and G1 Cell Cycle Arrest by Fucoidan in HCT116 Human Colorectal Carcinoma Cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcinogenesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Kim, M.-H.; Joo, H.-G. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol. Lett. 2008, 115, 138–143. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Yuan, P.; Cai, S.; Bao, J.; Zhao, Y.; Aimaier, A.; Aipire, A.; Lu, J.; Li, J. In Vitro and In Vivo Dendritic Cell Immune Stimulation Effect of Low Molecular Weight Fucoidan from New Zealand Undaria pinnatifida. Mar. Drugs 2022, 20, 197. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Joo, H.-G. Evaluation of adjuvant effects of fucoidan for improving vaccine efficacy. J. Vet. Sci. 2015, 16, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Anastyuk, S.D.; Kasprik, A.E.; Zvyagintsev, N.V.; Ermakova, S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 2019, 221, 157–165. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Zdobnova, E.V.; Silchenko, A.S.; Kusaykin, M.I.; Ermakova, S.P. Radiosensitizing effect of the fucoidan from brown alga Fucus evanescens and its derivative in human cancer cells. Carbohydr. Polym. 2019, 205, 465–471. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R., Jr.; Neufeld, M.J.; Sun, C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021, 500, 208–219. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Kuo, T.-H. O-carboxymethyl chitosan/fucoidan nanoparticles increase cellular curcumin uptake. Food Hydrocoll. 2016, 53, 261–269. [Google Scholar] [CrossRef]
- Chen, L.-M.; Liu, P.-Y.; Chen, Y.-A.; Tseng, H.-Y.; Shen, P.-C.; Hwang, P.-A.; Hsu, H.-L. Oligo-Fucoidan prevents IL-6 and CCL2 production and cooperates with p53 to suppress ATM signaling and tumor progression. Sci. Rep. 2017, 7, 11864. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.W.; Yun, S.; Lee, J.H.; Han, Y.-S.; Yoon, Y.M.; An, D.; Lee, S.H. Silencing Prion Protein in HT29 Human Colorectal Cancer Cells Enhances Anticancer Response to Fucoidan. Anticancer. Res. 2016, 36, 4449–4458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Surits, V.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line. Mar. Drugs 2021, 19, 540. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Yamashita, K.; Sakai, S.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Induction of apoptosis in DLD-1 human colon cancer cells by peridinin isolated from the dinoflagellate, Heterocapsa triquetra. Biosci. Biotechnol. Biochem. 2007, 71, 1069–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Araki, S.; Kim, D.J.; Park, C.B.; Takasuka, N.; Baba-Toriyama, H.; Ota, T.; Nir, Z.; Khachik, F.; Shimidzu, N.; et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.H.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-A(y) mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Mar. Drugs 2018, 16, 275. [Google Scholar] [CrossRef] [Green Version]
- Kotake-Nara, E.; Sugawara, T.; Nagao, A. Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish. Sci. 2005, 71, 459–461. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G(0)/G(1) phase in human colon carcinoma cells through up-regulation of p21(WAF1/Cip1). Biochim. Biophys. Acta-Gen. Subj. 2005, 1726, 328–335. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Mutoh, M. Induction of Anoikis in Human Colorectal Cancer Cells by Fucoxanthinol. Nutr. Cancer Int. J. 2017, 69, 1043–1052. [Google Scholar] [CrossRef]
- Yokoyama, R.; Kojima, H.; Takai, R.; Ohta, T.; Maeda, H.; Miyashita, K.; Mutoh, M.; Terasaki, M. Effects of CLIC4 on Fucoxanthinol-Induced Apoptosis in Human Colorectal Cancer Cells. Nutr. Cancer Int. J. 2021, 73, 889–898. [Google Scholar] [CrossRef]
- Terasaki, M.; Mima, M.; Kudoh, S.; Endo, T.; Maeda, H.; Hamada, J.; Osada, K.; Miyashita, K.; Mutoh, M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol. Rep. 2018, 40, 414–424. [Google Scholar] [CrossRef]
- Tamura, S.; Narita, T.; Fujii, G.; Miyamoto, S.; Hamoya, T.; Kurokawa, Y.; Takahashi, M.; Miki, K.; Matsuzawa, Y.; Komiya, M.; et al. Inhibition of NF-kappaB transcriptional activity enhances fucoxanthinol-induced apoptosis in colorectal cancer cells. Genes Environ. 2019, 41, 1. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Masaka, S.; Fukada, C.; Houzaki, M.; Endo, T.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Salivary Glycine Is a Significant Predictor for the Attenuation of Polyp and Tumor Microenvironment Formation by Fucoxanthin in AOM/DSS Mice. Vivo 2019, 33, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2021, 42, 210–219. [Google Scholar] [CrossRef]
- Terasaki, M.; Ikuta, M.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Dietary Fucoxanthin Induces Anoikis in Colorectal Adenocarcinoma by Suppressing Integrin Signaling in a Murine Colorectal Cancer Model. J. Clin. Med. 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Liddle, D.M.; Hutchinson, A.L.; Monk, J.M.; Power, K.A.; Robinson, L.E. Dietary omega-3 polyunsaturated fatty acids modulate CD4+ T-cell subset markers, adipocyte antigen-presentation potential, and NLRP3 inflammasome activity in a coculture model of obese adipose tissue. Nutrition 2021, 91, 111388. [Google Scholar] [CrossRef]
- Kang, K.W.; Kim, S.; Cho, Y.-B.; Ryu, S.R.; Seo, Y.-J.; Lee, S.-M. Endogenous n-3 Polyunsaturated Fatty Acids Are Beneficial to Dampen CD8+ T Cell-Mediated Inflammatory Response upon the Viral Infection in Mice. Int. J. Mol. Sci. 2019, 20, 4510. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-M.; Jeong, M.; Park, J.-M.; Kim, M.-Y.; Go, E.-J.; Cha, J.Y.; Kim, K.J.; Hahm, K.B. The omega-3 polyunsaturated fatty acids prevented colitis-associated carcinogenesis through blocking dissociation of beta-catenin complex, inhibiting COX-2 through repressing NF-kappa B, and inducing 15-prostaglandin dehydrogenase. Oncotarget 2016, 7, 63583–63595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skender, B.; Hofmanova, J.; Slavik, J.; Jelinkova, I.; Machala, M.; Moyer, M.P.; Kozubik, A.; Vaculova, A.H. DHA-mediated enhancement of TRAIL-induced apoptosis in colon cancer cells is associated with engagement of mitochondria and specific alterations in sphingolipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hu, Z.; Qi, H.; Shi, Z.; Chang, Y.; Yao, Q.; Cui, H.; Zheng, L.; Han, Y.; Han, X.; et al. G-protein-coupled receptors mediate omega-3 PUFAs-inhibited colorectal cancer by activating the Hippo pathway. Oncotarget 2016, 7, 58315–58330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshie, Y.; Wang, W.; Hsieh, Y.-P.; Suzuki, T. Compositional difference of phenolic compounds between two seaweeds, Halimeda spp. J. Tokyo Univ. Fish. 2002, 88, 21–24. [Google Scholar]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Connan, S.; Delisle, F.; Deslandes, E.; Gall, E.A. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006, 49, 39–46. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lanteri, P.; Clement, Y.; Leonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic Fraction Obtained From Thalassia testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020, 11, 1939. [Google Scholar] [CrossRef]
- Hernández-Balmaseda, I.; Guerra, I.R.; Declerck, K.; Herrera Isidrón, J.A.; Pérez-Novo, C.; Van Camp, G.; De Wever, O.; González, K.; Labrada, M.; Carr, A.; et al. Marine Seagrass Extract of Thalassia testudinum Suppresses Colorectal Tumor Growth, Motility and Angiogenesis by Autophagic Stress and Immunogenic Cell Death Pathways. Mar. Drugs 2021, 19, 52. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 1982, 104, 6846–6848. [Google Scholar] [CrossRef]
- Kortmansky, J.; Schwartz, G.K. Bryostatin-1: A novel PKC inhibitor in clinical development. Cancer Investig. 2003, 21, 924–936. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Uranchimeg, B.; Cardellina, J.H.; Meragelman, T.L.; Matsunaga, S.; Fusetani, N.; Del Bufalo, D.; Shoemaker, R.H.; Melillo, G. Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J. Natl. Cancer Inst. 2008, 100, 1233–1246. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Iglesias, O.; Carrera, I.; Naidoo, V.; Cacabelos, R. AntiGan: An Epinutraceutical Bioproduct with Antitumor Properties in Cultured Cell Lines. Life 2022, 12, 97. [Google Scholar] [CrossRef]
- Lombardi, V.R.M.; Carrera, I.; Cacabelos, R. In vitro and in vivo cytotoxic effect of AntiGan against tumor cells. Exp. Ther. Med. 2018, 15, 2547–2556. [Google Scholar] [CrossRef]
- Kamiya, H.; Muramoto, K.; Goto, R. Purification and properties of agglutinins from conger eel, conger-myriaster (brevoort), skin mucus. Dev. Comp. Immunol. 1988, 12, 309–318. [Google Scholar] [CrossRef]
- Nakamura, O.; Matsuoka, H.; Ogawa, T.; Muramoto, K.; Kamiya, H.; Watanabe, T. Opsonic effect of congerin, a mucosal galectin of the Japanese conger, Conger myriaster (Brevoort). Fish Shellfish. Immunol. 2006, 20, 433–435. [Google Scholar] [CrossRef]
- Ogawa, T.; Watanabe, M.; Naganuma, T.; Muramoto, K. Diversified carbohydrate-binding lectins from marine resources. J. Amino Acids 2011, 2011, 838914. [Google Scholar] [CrossRef] [Green Version]
- Corzo, L.; Fernandez-Novoa, L.; Carrera, I.; Martinez, O.; Rodriguez, S.; Alejo, R.; Cacabelos, R. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, E.; Yukawa, M.; Ueno, M.; Kimura, K.-I.; Takahashi, H. A Novel Method of Screening Cell-Cycle Blockers as Candidates for Anti-Tumor Reagents Using Yeast as a Screening Tool. Biosci. Biotechnol. Biochem. 2010, 74, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Naffouje, R.; Grover, P.; Yu, H.; Sendilnathan, A.; Wolfe, K.; Majd, N.; Smith, E.P.; Takeuchi, K.; Senda, T.; Kofuji, S.; et al. Anti-Tumor Potential of IMP Dehydrogenase Inhibitors: A Century-Long Story. Cancers 2019, 11, 1346. [Google Scholar] [CrossRef] [Green Version]
- Peled, Y.; Ram, E.; Lavee, J.; Sternik, L.; Segev, A.; Wieder-Finesod, A.; Mandelboim, M.; Indenbaum, V.; Levy, I.; Raanani, E.; et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J. Heart Lung Transplant. 2021, 40, 759–762. [Google Scholar] [CrossRef]
- Allison, A.C.; Eugui, E.M. Immunosuppressive and long-acting anti-inflammatory activity of mycophenolic acid and derivative, RS-61443. Br. J. Rheumatol. 1991, 30, 57–61. [Google Scholar]
- Chen, L.; Zhu, T.; Zhu, G.; Liu, Y.; Wang, C.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Bioactive Natural Products from the Marine-Derived Penicillium brevicompactum OUCMDZ-4920. Chin. J. Org. Chem. 2017, 37, 2752–2762. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zheng, Z.; Lu, X.; Zhang, X.; Dong, A.; Duan, B.; Zhao, B. Induction Effect of Mycophenolic Acid on Apoptosis of Human Colon Cancer Cell Line SW620 and Its Mechanism. J. Hebei Norm. Univ. (Nat. Sci. Ed.) 2013, 37, 519–523. [Google Scholar]
- Franklin, T.J.; Jacobs, V.; Jones, G.; Ple, P.; Bruneau, P. Glucuronidation associated with intrinsic resistance to mycophenolic acid in human colorectal carcinoma cells. Cancer Res. 1996, 56, 984–987. [Google Scholar] [PubMed]
- Kim, M.C.; Machado, H.; Jang, K.H.; Trzoss, L.; Jensen, P.R.; Fenical, W. Integration of Genomic Data with NMR Analysis Enables Assignment of the Full Stereostructure of Neaumycin B, a Potent Inhibitor of Glioblastoma from a Marine-Derived Micromonospora. J. Am. Chem. Soc. 2018, 140, 10775–10784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Salcedo, R.; Olano, C.; Fernandez, R.; Brana, A.F.; Mendez, C.; de la Calle, F.; Salas, J.A. Elucidation of the glycosylation steps during biosynthesis of antitumor macrolides PM100117 and PM100118 and engineering for novel derivatives. Microb. Cell Factories 2016, 15, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.-H.; Peng, B.-R.; Su, C.-H.; El-Shazly, M.; Sun, Y.-L.; Shih, M.-C.; Huang, Y.-T.; Yen, P.-T.; Wang, L.-S.; Su, J.-H. Anti-Proliferative Potential of Secondary Metabolites from the Marine Sponge Theonella sp.: Moving from Correlation toward Causation. Metabolites 2021, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Li, X.Q.; Zheng, L.; Zhang, Y.H.; Dai, J.J.; Shang, E.L.; Yu, Y.Y.; Zhang, Y.T.; Hu, W.P.; Shi, D.Y. Sesquiterpenoids From the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11. Front. Microbiol. 2021, 12, 1388. [Google Scholar] [CrossRef]
- Qi, X.; Liu, B.; Jiang, Z. A new cytotoxic phenalenone derivative from Penicillium oxalicum. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef]
- Cao Van, A.; Kang, J.S.; Choi, B.-K.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. Polyketides and Meroterpenes from the Marine-Derived Fungi Aspergillus unguis 158SC-067 and A. flocculosus 01NT-1.1.5 and Their Cytotoxic and Antioxidant Activities. Mar. Drugs 2021, 19, 415. [Google Scholar] [CrossRef]
- Qi, X.; Li, M.; Zhang, X.M.; Dai, X.F.; Cui, J.; Li, D.H.; Gu, Q.Q.; Lv, Z.H.; Li, J. Trichothecin Inhibits Cancer-Related Features in Colorectal Cancer Development by Targeting STAT3. Molecules 2020, 25, 2306. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Yang, Y.; Chen, H. Shellmycin A-D, Novel Bioactive Tetrahydroanthra-gamma-Pyrone Antibiotics from Marine Streptomyces sp. Shell-016. Mar. Drugs 2020, 18, 58. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.Y.; Liao, L.; Park, S.H.; Kim, W.K.; Shin, J.; Lee, S.K. Antitumor Activity of Asperphenin A, a Lipopeptidyl Benzophenone from Marine-Derived Aspergillus sp. Fungus, by Inhibiting Tubulin Polymerization in Colon Cancer Cells. Mar. Drugs 2020, 18, 110. [Google Scholar] [CrossRef] [Green Version]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides C4, D1, D2, M, M1, M2, N and Q, new triterpene glycosides with diverse carbohydrate chains from sea cucumber Cladolabes schmeltzii. An uncommon 20,21,22,23,24,25,26,27-okta-nor-lanostane aglycone. The synergism of inhibitory action of non-toxic dose of the glycosides and radioactive irradiation on colony formation of HT-29 cancer cells. Carbohydr. Res. 2018, 468, 36–44. [Google Scholar] [CrossRef]
- Khan, N.; Yilmaz, S.; Aksoy, S.; Uzel, A.; Tosun, C.; Kirmizibayrak, P.B.; Bedir, E. Polyethers isolated from the marine actinobacterium Streptomyces cacaoi inhibit autophagy and induce apoptosis in cancer cells. Chem.-Biol. Interact. 2019, 307, 167–178. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic psammaplysin analogues from the verongid red sea sponge aplysinella species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Kurniasari, K.D.W.I.; Arsianti, A.; Nugrahayning Aziza, Y.A.; Dyahningrum Mandasari, B.K.; Masita, R.; Ruhama Zulfa, F.; Kusumaning Dewi, M.; Zahira Zagloel, C.R.; Azizah, N.N.U.R.; Putrianingsih, R. Phytochemical analysis and anticancer activity of seaweed gracilaria verrucosa against colorectal HCT-116 cells. Orient. J. Chem. 2018, 34, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Malyarenko, T.V.; Malyarenko, O.S.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. In vitro anticancer and proapoptotic activities of steroidal glycosides from the starfish anthenea aspera. Mar. Drugs 2018, 16, 420. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Nuzzo, G.; Galasso, C.; Casotti, R.; Fontana, A.; Romano, G.; Ianora, A. The Marine Dinoflagellate Alexandrium andersoni Induces Cell Death in Lung and Colorectal Tumor Cell Lines. Mar. Biotechnol. 2018, 20, 343–352. [Google Scholar] [CrossRef]
- Subroto, P.A.M.; Arsianti, A.; Putri, T.; Lesmana, E. Phytochemical analysis and anticancer activity of seaweed Eucheuma Sp. against colon HCT-116 cells. In Proceedings of the 3rd International Symposium of Biomedical Engineering’s Recent Progress in Biomaterials, Drugs Development, and Medical Devices, ISBE 2018, Jakarta, Indonesia, 6–8 August 2018. [Google Scholar]
- Guner, A.; Nalbantsoy, A.; Sukatar, A.; Yavasoglu, N.U.K. Apoptosis-inducing activities of Halopteris scoparia L. Sauvageau (Brown algae) on cancer cells and its biosafety and antioxidant properties. Cytotechnology 2019, 71, 687–704. [Google Scholar] [CrossRef]
- Li, F.; Peifer, C.; Janussen, D.; Tasdemir, D. New Discorhabdin Alkaloids from the Antarctic Deep-Sea Sponge Latrunculia biformis. Mar. Drugs 2019, 17, 439. [Google Scholar] [CrossRef] [Green Version]
- Nursid, M.; Marraskuranto, E.; Chasanah, E. Cytotoxicity and Apoptosis Induction of Sea Cucumber Holothuria atra Extracts. Pharmacogn. Res. 2019, 11, 41–46. [Google Scholar] [CrossRef]
- Syed, C.S.; Sairam, M.; Audipudi, A.V. Exploration of antibacterial and antiproliferative secondary metabolites from marine bacillus. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 628–633. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Forsythe, N.; Pérez-Sánchez, A.; Van Schaeybroeck, S.; Barrajón-Catalán, E.; Micol, V. A Nudibranch Marine Extract Selectively Chemosensitizes Colorectal Cancer Cells by Inducing ROS-Mediated Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 261. [Google Scholar] [CrossRef]
- Musa, A.; Elmaidomy, A.H.; Sayed, A.M.; Alzarea, S.I.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Abdelgawad, M.A.; Youssif, K.A.; Refaat, H.; et al. Cytotoxic Potential, Metabolic Profiling, and Liposomes of Coscinoderma sp. Crude Extract Supported by in silico Analysis. Int. J. Nanomed. 2021, 16, 3861–3874. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, Z.; Emtyazjoo, M.; Mostafavi, G.P.; Bonakdar, S. Research Article Antiproliferative activity of Portunus segnis muscle extract on apoptosis of colon cancer cell line (HT-29). Iran. J. Fish. Sci. 2022, 21, 157–173. [Google Scholar] [CrossRef]
- Su, F.; Song, Q.; Zhang, C.; Xu, X.; Li, M.; Yao, D.; Wu, L.; Qu, X.; Guan, H.; Yu, G.; et al. A beta-1,3/1,6-glucan from Durvillaea Antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydr. Polym. 2019, 222, 114993. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Ono, S.; Hashimoto, S.; Kubota, A.; Kojima, H.; Ohta, T.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Suppression of C-C chemokine receptor 1 is a key regulation for colon cancer chemoprevention in AOM/DSS mice by fucoxanthin. J. Nutr. Biochem. 2022, 99, 108871. [Google Scholar] [CrossRef]
- Lynch, H.T.; Lynch, J.F.; Lynch, P.M.; Attard, T. Hereditary colorectal cancer syndromes: Molecular genetics, genetic counseling, diagnosis and management. Fam. Cancer 2008, 7, 27–39. [Google Scholar] [CrossRef]
- Su, L.K.; Kinzler, K.W.; Vogelstein, B.; Preisinger, A.C.; Moser, A.R.; Luongo, C.; Gould, K.A.; Dove, W.F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the apc gene. Science 1992, 256, 668–670. [Google Scholar] [CrossRef]
- Yamada, Y.; Mori, H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007, 98, 6–10. [Google Scholar] [CrossRef]
- Janssen, K.-P.; Alberici, P.; Fsihi, H.; Gaspar, C.; Breukel, C.; Franken, P.; Rosty, C.; Abal, M.; El Marjou, F.; Smits, R.; et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006, 131, 1096–1109. [Google Scholar] [CrossRef]
- Halberg, R.B.; Katzung, D.S.; Hoff, P.D.; Moser, A.R.; Cole, C.E.; Lubet, R.A.; Donehower, L.A.; Jacoby, R.F.; Dove, W.F. Tumorigenesis in the multiple intestinal neoplasia mouse: Redundancy of negative regulators and specificity of modifiers. Proc. Natl. Acad. Sci. USA 2000, 97, 3461–3466. [Google Scholar] [CrossRef]
- Terasaki, M.; Matsumoto, N.; Hashimoto, R.; Endo, T.; Maeda, H.; Hamada, J.; Osada, K.; Miyashita, K.; Mutoh, M. Fucoxanthin administration delays occurrence of tumors in xenograft mice by colonospheres, with an anti-tumor predictor of glycine. J. Clin. Biochem. Nutr. 2019, 64, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Kwok, H.F. Development of Marine-Derived Compounds for Cancer Therapy. Mar. Drugs 2021, 19, 342. [Google Scholar] [CrossRef]
- Costales-Carrera, A.; Fernandez-Barral, A.; Bustamante-Madrid, P.; Guerra, L.; Cantero, R.; Barbachano, A.; Munoz, A. Plocabulin Displays Strong Cytotoxic Activity in a Personalized Colon Cancer Patient-Derived 3D Organoid Assay. Mar. Drugs 2019, 17, 648. [Google Scholar] [CrossRef] [Green Version]
- Elez, E.; Gomez-Roca, C.; Soto Matos-Pita, A.; Argiles, G.; Valentin, T.; Coronado, C.; Iglesias, J.; Macarulla, T.; Betrian, S.; Fudio, S.; et al. First-in-human phase I study of the microtubule inhibitor plocabulin in patients with advanced solid tumors. Investig. New Drugs 2019, 37, 674–683. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Associations among dietary seaweed intake, c-MYCrs6983267 polymorphism, and risk of colorectal cancer in a Korean population: A case-control study. Eur. J. Nutr. 2020, 59, 1963–1974. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Ye, X.; Zhang, S.; Song, M.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Marine omega-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol. Biomark. Prev. 2018, 27, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Ou, F.-S.; Zemla, T.J.; Hull, M.A.; Shi, Q.; Limburg, P.J.; Alberts, S.R.; Sinicrope, F.A.; Giovannucci, E.L.; Van Blarigan, E.L.; et al. Marine omega-3 fatty acid intake and survival of stage III colon cancer according to tumor molecular markers in NCCTG Phase III trial N0147 (Alliance). Int. J. Cancer 2019, 145, 380–389. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.; Kim, S.C.; You, S.; Lee, C.W.; Shin, J.; Park, Y.I. Glucuronorhamnoxylan from Capsosiphon fulvescens inhibits the growth of HT-29 human colon cancer cells in vitro and in vivo via induction of apoptotic cell death. Int. J. Biol. Macromol. 2019, 124, 1060–1068. [Google Scholar] [CrossRef]
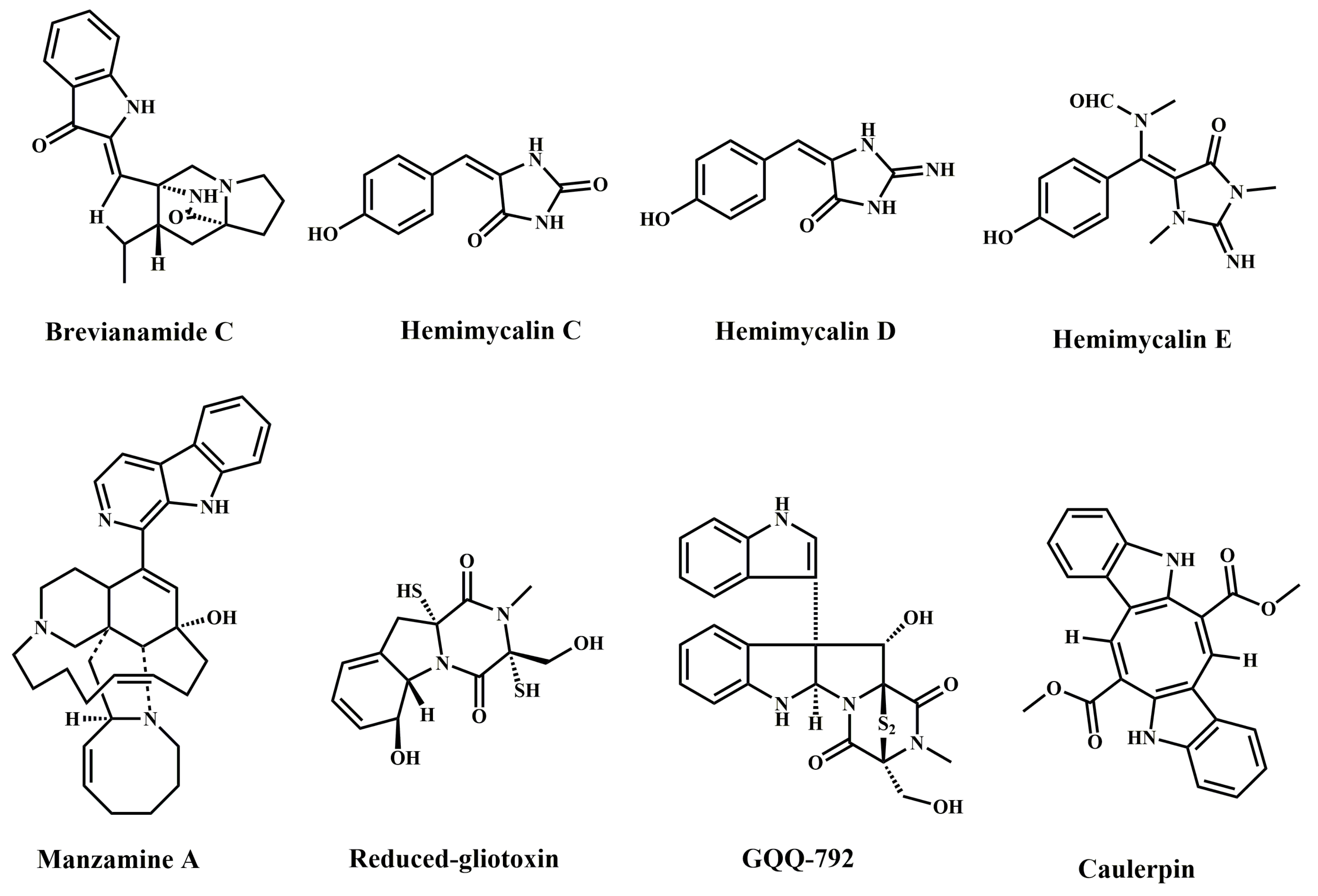





| Compound Name | Marine Organism | Species Name | Cell Lines | IC50 | Mechanism | References |
|---|---|---|---|---|---|---|
| Brevianamide C | Marine fungi | Penicillium brevicompactum | HCT-116 | 15.6 µM, 72 h | Anti-proliferation | [8] |
| Hemimycalin C | Red Sea sponge | Hemimycale sp. | HCT-116 | 18.6 ± 0.12 µM, 72 h | Anti-proliferation | [10] |
| Hemimycalin D | Red Sea sponge | Hemimycale sp. | HCT-116 | 17.1 ± 0.09 µM, 72 h | Anti-proliferation | [10] |
| Hemimycalin E | Red Sea sponge | Hemimycale sp. | HCT-116 | 8.6 ± 0.06 µM, 72 h | Anti-proliferation | [10] |
| Manzamine A | Marine sponge | Haliclona sp. | HCT-116 | 4.5 ± 1.7 µM, 24 h | Anti-proliferation; apoptosis; G0/G1 cell cycle arrest; block EMT | [11,16] |
| Reduced-gliotoxin | Marine fungi | Neosartorya pseudofischeri | HCT-116 | ≈5 µM, 24 h | Anti-proliferation; apoptosis; anoikis | [9] |
| HT-29 | ≈7 µM, 24 h | Anti-proliferation; apoptosis; anoikis | ||||
| GQQ-792 | Mangrove endophytic fungi | Tilachlidium sp. | HCT-116 | ≈1.19 µM, 72 h | Anti-proliferation | [15] |
| Caulerpin | Green algae | Caulerpa cylindracea | LoVo | 20 µM, 48 h | Anti-proliferation Apoptosis; AMPK/mTOR pathway | [12] |
| Compound Name | Marine Organism | Species Name | Cell Lines | IC50 | Mechanism | References |
|---|---|---|---|---|---|---|
| L-Glutaminase | Marine bacterium | Halomonas meridian | LS-174-T | 7 µg/mL, 72 h | Anti-proliferation; apoptosis | [17] |
| HCT-116 | 13.2 µg/mL, 72 h | Anti-proliferation; apoptosis | ||||
| Ohmyungsamycin A | Marine actinomycetes | Streptomyces sp. | HCT-116 | 7.61 µM, 72 h | Anti-proliferation; apoptosis; G0/G1 cell cycle arrest | [18] |
| Actinomycin V | Marine actinomycetes | Streptomyces sp. | HCT-116 | 2.85 ± 0.10 nmol/L, 48 h | anti-proliferation; apoptosis; PI3K/AKT pathway | [19] |
| HT-29 | 6.38 ± 0.46 nmol/L, 48 h | Anti-proliferation | ||||
| SW620 | 6.43 ± 0.16 nmol/L, 48 h | Anti-proliferation | ||||
| SW480 | 8.65 ± 0.31 nmol/L, 48 h | Anti-proliferation | ||||
| Androsamide | Marine actinomycetes | Nocardiopsis sp. | Caco-2 | 13 µM, 48 h | Anti-proliferation; block EMT | [20] |
| HCT-116 | 21 µM, 48 h | Anti-proliferation | ||||
| Laxaphycin B4 | Marine cyanobacterium | Hormothamnionen teromorphoides | HCT-116 | 1.7 µM, 48 h | Anti-proliferation | [21] |
| Laxaphycin A2 | Marine cyanobacterium | Hormothamnionen teromorphoides | HCT-116 | 23 µM, 48 h | Anti-proliferation | [21] |
| Peptide, (P6) | Bvalve mollusk | Arca inflata | DLD-1 | 2.14 ± 0.28 µg/mL, 48 h | Anti-proliferation; apoptosis; S/G2 cell cycle arrest; p38 MAPK pathway | [22] |
| HT-29 | 4.43 ± 0.15 µg/mL, 48 h | Anti-proliferation | ||||
| HCT-116 | 10.88 ± 0.72 µg/mL, 48 h | Anti-proliferation | ||||
| Catfish muscle | Marine catfish | Tachysaurus dussumieri | HT-29 | 20 µg/mL, 24 h | Anti-proliferation | [24] |
| Compound Name | Marine Organism | Species Name | Cell Lines | IC50 | Mechanism | References |
|---|---|---|---|---|---|---|
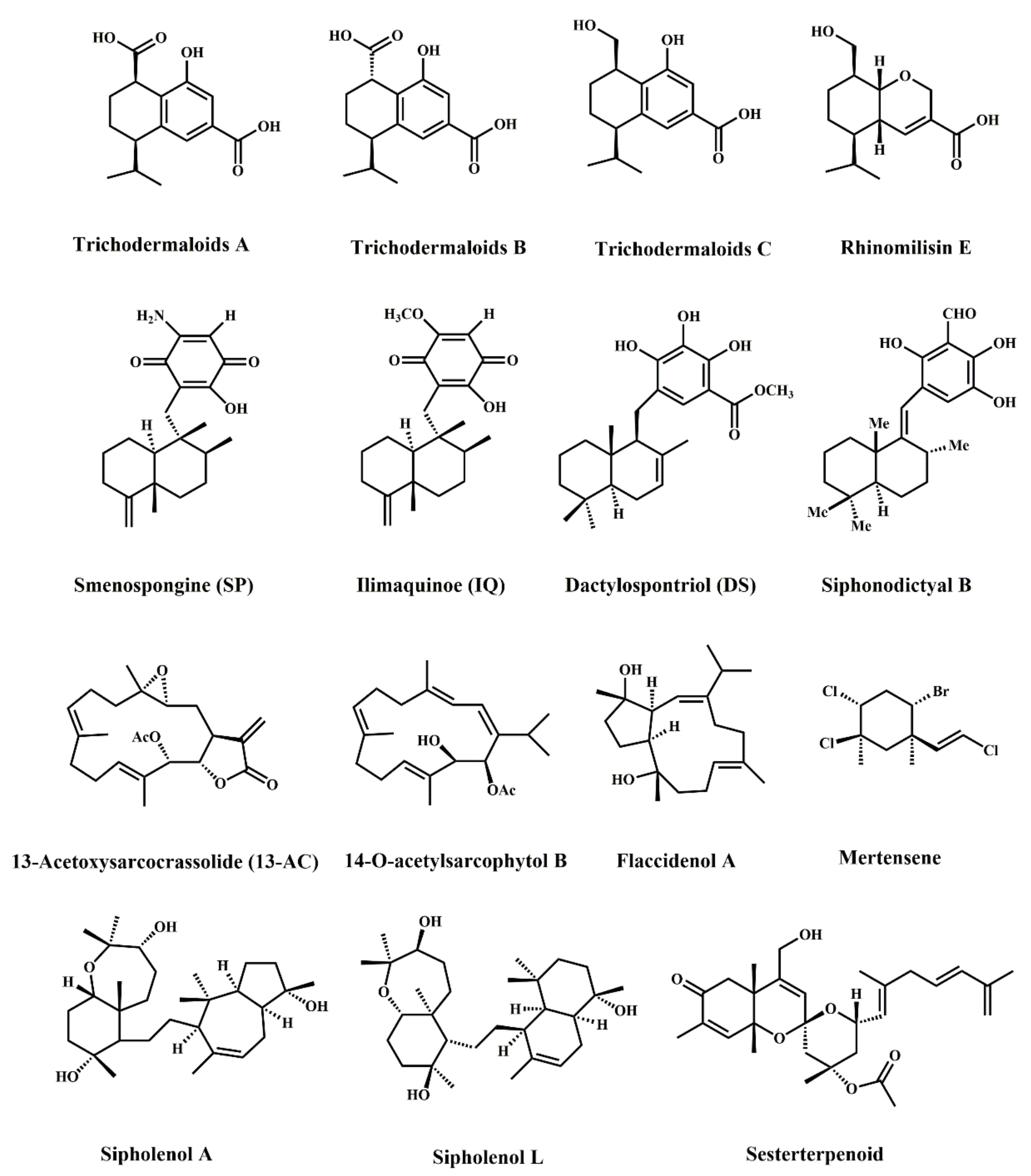
| Trichodermaloids A | Marine sponge symbiotic fungi | Dysidea sp. and Trichoderma sp. | SW620 | 9.3 ± 2.2 µM, \ | Anti-proliferation | [26] |
| Trichodermaloids B | Marine sponge symbiotic fungi | Dysidea sp. and Trichoderma sp. | SW620 | 8.6 ± 1.9 µM, \ | Anti-proliferation | [26] |
| Trichodermaloids C | Marine sponge symbiotic fungi | Dysidea sp. and Trichoderma sp. | SW620 | 12.7 ± 0.6 µM, \ | Anti-proliferation | [26] |
| Rhinomilisin E | Marine sponge symbiotic fungi | Dysidea sp. and Trichoderma sp. | SW620 | 22.7 ± 2.3 µM, \ | Anti-proliferation | [26] |
| Smenospongine | Marine sponge | Haliclona sp. | HCT-116 | 8 µM, 72 h | Anti-proliferation; apoptosis; G2/M and G1 cell cycle arrest; DNA damage | [27] |
| RKO | 15 µM, 72 h | Anti-proliferation | ||||
| HT-29 | 10 µM, 72 h | Anti-proliferation; apoptosis; G1 cell cycle arrest | ||||
| Ilimaquinone | Marine sponge | Verongula rigida | HT-29 | 13 µM, 72 h | Anti-proliferation; apoptosis; G1 cell cycle arrest; DNA damage | [27] |
| Dactylospontriol | Marine sponge | Verongula rigida | HCT-116 | 19 µM, 72 h | Anti-proliferation; G1 cell cycle arrest | [27] |
| Siphonodictyal B | Marine sponge | Aka coralliphaga | HCT-116 | 1 µM, 24 h | Apoptosis; G1 cell cycle arrest; PI3K inhibitor; p38 MAPK pathway | [28] |
| 13-Acetoxysarcocrassolide | Alcyonacean coral | Lobophytum crassum | HCT-116 | 1.36 ± 0.27 µg/mL, 72 h | Anti-proliferation | [29] |
| LoVo | 1.38 ± 0.37 µg/mL, 72 h | Anti-proliferation | ||||
| DLD-1 | 1.64 ± 0.36 µg/mL, 72 h | Anti-proliferation | ||||
| 14-O-acetylsarcophytol B | Marine soft coral | Klyxum flaccidum | DLD-1 | 11.7 ± 4.8 µg/mL, \ | Cytotoxicity | [30] |
| Flaccidenol A | Marine soft coral | Klyxum flaccidum | DLD-1 | 6.0 ± 0.4 µg/mL, \ | Cytotoxicity | [30] |
| Mertensene | Red alga | Pterocladiella capillacea | HT-29 | 56.50 ± 8.68 µg/mL, 72 h | Anti-proliferation; apoptosis; G2/M cell cycle arrest; ERK-1/-2, AKT and NF-κB activation | [25] |
| LS-174-T | 49.77 ± 4.51 µg/mL, 72 h | Anti-proliferation | ||||
| Sipholenol A | Red Sea sponge | Callyspongia siphonella | HCT-116 | 14.8 ± 2.33 µM, 72 h | Anti-proliferation; apoptosis; G2/M and S cell cycle arrest | [31] |
| Sipholenol L | Red Sea sponge | Callyspongia siphonella | HCT-116 | 19.8 ± 3.78 µM, 72 h | Anti-proliferation; apoptosis; G2/M and S cell cycle arrest | [31] |
| Sesterterpenoid | Marine sponge | Monanchora pulchra | HCT-116 | 43.5 µM, 48 h | Anti-proliferation; Wnt/β-Catenin pathway | [32] |
| Compound Name | Marine Organism | Chemical Class | Molecular Target | Cancer Type | Year of FDA-Approval |
|---|---|---|---|---|---|
| Crytarabine | Marine sponge | Nucleoside | DNA polymerase | Leukemia | 1969 |
| Eribulin mesylate | Marine sponge | Macrolide | Microtubules | Metastatic breast cancer | 2010 |
| Brentuximab vedotin | Mollusk/ cyanobacterium | ADC (MMAE) 1 | CD30 and microtubules | Anaplastic large T-cell systemic malignant lymphoma, Hodgkin disease | 2011 |
| Trabectedin | Tunicate | Alkaloid | Minor groove of DNA | Soft tissue sarcoma and ovarian cancer | 2015 |
| Panobinostat | Marine sponge | Hydroxamic acid | Histone | Multiple myeloma | 2015 |
| Plitidepsin | Tunicate | Dipsipetide | eEF1A2 | Multiple myeloma, leukemia, lymphoma | 2018 (Australia) 2 |
| Polatuzumabvedotin | Mollusk/ cyanobacterium | ADC (MMAF) 1 | CD76b and microtubules | Non-Hodgkin lymphoma, chronic lymphocytic leukemia, lymphoma, B-cell lymphoma, folicular | 2019 |
| Enfortumabvedotin | Mollusk/ cyanobacterium | ADC (MMAE) | Nectin-4 | Metastatic urothelial cancer | 2019 |
| Belantamabmafodotin | Mollusk/ cyanobacterium | ADC (MMAF) | BCMA | Relapsed/refractory multiple myeloma | 2020 |
| Lurbinectedin | Tunicate | Alkaloid | RNA polymerase II | Metastatic small-cell lung cancer | 2020; 2021 (Australia) |
| Disitamab vedotin | Mollusk/ cyanobacterium | ADC (MMAE) | HER2 | Urothelial carcinoma advanced cancer, gastric cancer, HER2 overexpressing gastric carcinoma, advanced breast cancer, solid tumors | 2021 |
| Compound Name | Marine Organism | Chemical Class | Molecular Target | Cancer Type | Clinical phase |
|---|---|---|---|---|---|
| Plinabulin | Marine fungi | Diketopiperazine | Microtubules | Non-small-cell lung cancer, Brain tumor | Phase III |
| Marizomib | Marine bacterium | Bata-lactone-gamma lactam | 20S proteasome | Non-small-cell lung cancer, Pancreatic cancer, Melanoma, Lymphoma, Multiple myeloma | Phase III |
| Plocabulin (PM184) | Marine sponge | Polyketide | Microtubule | Solid tumors | Phase II |
| Tisotumab vedotin | Mollusk/ cyanobacterium | ADC (MMAE) | Tissue factor and microtubules | Ovary cancer, Cervix cancer, Endometrium cancer, Bladder cancer, Prostate cancer, Head and neck cancer, Esophagus cancer, Lung cancer | Phase II |
| Ladiratuzumabvedotin (SGNLIV1A) | Mollusk/ cyanobacterium | ADC (MMAE) | LIV-1 and microtubules | Breast cancer | Phase II |
| Telisotuzumabvedotin (ABBV-399) | Mollusk/ cyanobacterium | ADC (MMAE) | c-Met | Solid tumors | Phase II |
| CAB-ROR2 (BA-3021) | Mollusk/ cyanobacterium | ADC (MMAE) | ROR2 | Solid tumor, non-small-cell lung cancer, triple-negative breast cancer, soft tissue sarcoma | Phase II |
| CX-2029 (ABBV-2029) | Mollusk/ cyanobacterium | ADC (MMAE) | CD71 | Solid tumor, head and neck cancer, non-small-cell lung, pancreatic cancer, diffuse large B-cell lymphoma | Phase II |
| W0101 | Mollusk/ cyanobacterium | ADC (MMAE) | IGF-R1 | Advanced or metastatic solid tumors | Phase II |
| ARX-788 | Mollusk/ cyanobacterium | Amberstatin269 | HER2 and microtubules | Breast cancer, gastric cancer | Phase I |
| XMT-1536 | Mollusk/ cyanobacterium | ADC (Dolaflexin) | NaPi2b and microtubules | Solid tumors | Phase I |
| ALT-P7 | Mollusk/ cyanobacterium | ADC (MMAE) | HER2 and microtubules | Breast cancer, gastric cancer | Phase I |
| MORAb-202 | Marine sponge | ADC (Macrolide) | Microtubules | Solid tumors | Phase I |
| PF-06804103 | Mollusk/ cyanobacterium | ADC (Auristatin variant) | HER2 | Breast neoplasms, stomach neoplasms, esophagogastric junction neoplasm, carcinoma, Non-small-cell lung | Phase I |
| ZW-49 | Mollusk/ cyanobacterium | ADC (Auristatin variant) | HER2 | HER2-expressing cancers | Phase I |
| MRG003 | Mollusk/ cyanobacterium | ADC (MMAE) | EGFR | Non-small-cell lung | Phase I |
| STRO-002 | Marine sponge | Taltobulin | Folate receptor alpha (FolRa) | Ovarian, endometrial | Phase I |
| RC-88 | Mollusk/ cyanobacterium | ADC (MMAE) | Mesothelin | Solid tumors | Phase I |
| SGN-B6A | Mollusk/ cyanobacterium | ADC (MMAE) | Integrin beta-6 | Solid tumors | Phase I |
| SGN-CD228A | Mollusk/ cyanobacterium | ADC (MMAE) | CD228 | Solid tumors | Phase I |
| FOR-46 | Mollusk/ cyanobacterium | ADC (MMAF) | CD46 | Multiple myeloma, prostate | Phase I |
| A-166 | Mollusk/ cyanobacterium | Duostatin 5 | HER2 | HER2-expressing cancers | Phase I |
| Cofetuzumabpelidotin (ABBV-647) | Mollusk/ cyanobacterium | ADC (Auristatin variant) | PTK7 | Non-small-cell lung | Phase I |
| Compound Name | Marine Organism | Chemical Class | Effect on Colorectal Cancer | Phase | References |
|---|---|---|---|---|---|
| Fucoxanthin\fucoxanthinol | Brown algae | Carotenoid | Prevention of colorectal cancer | \ | [132] |
| Marine omega-3 fatty acid | Marine fish | Polyunsaturated fatty acids | Prevention and improve colon cancer survival | NCCTG Phase III | [131,139,133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.; Li, J.; Li, X. Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo. Mar. Drugs 2022, 20, 349. https://doi.org/10.3390/md20060349
Han N, Li J, Li X. Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo. Marine Drugs. 2022; 20(6):349. https://doi.org/10.3390/md20060349
Chicago/Turabian StyleHan, Ningning, Jianjiang Li, and Xia Li. 2022. "Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo" Marine Drugs 20, no. 6: 349. https://doi.org/10.3390/md20060349
APA StyleHan, N., Li, J., & Li, X. (2022). Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo. Marine Drugs, 20(6), 349. https://doi.org/10.3390/md20060349





