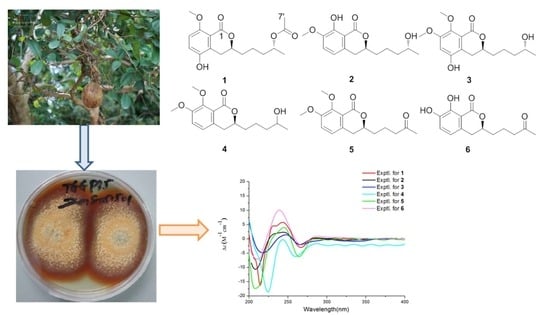
Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35
Abstract
:1. Introduction
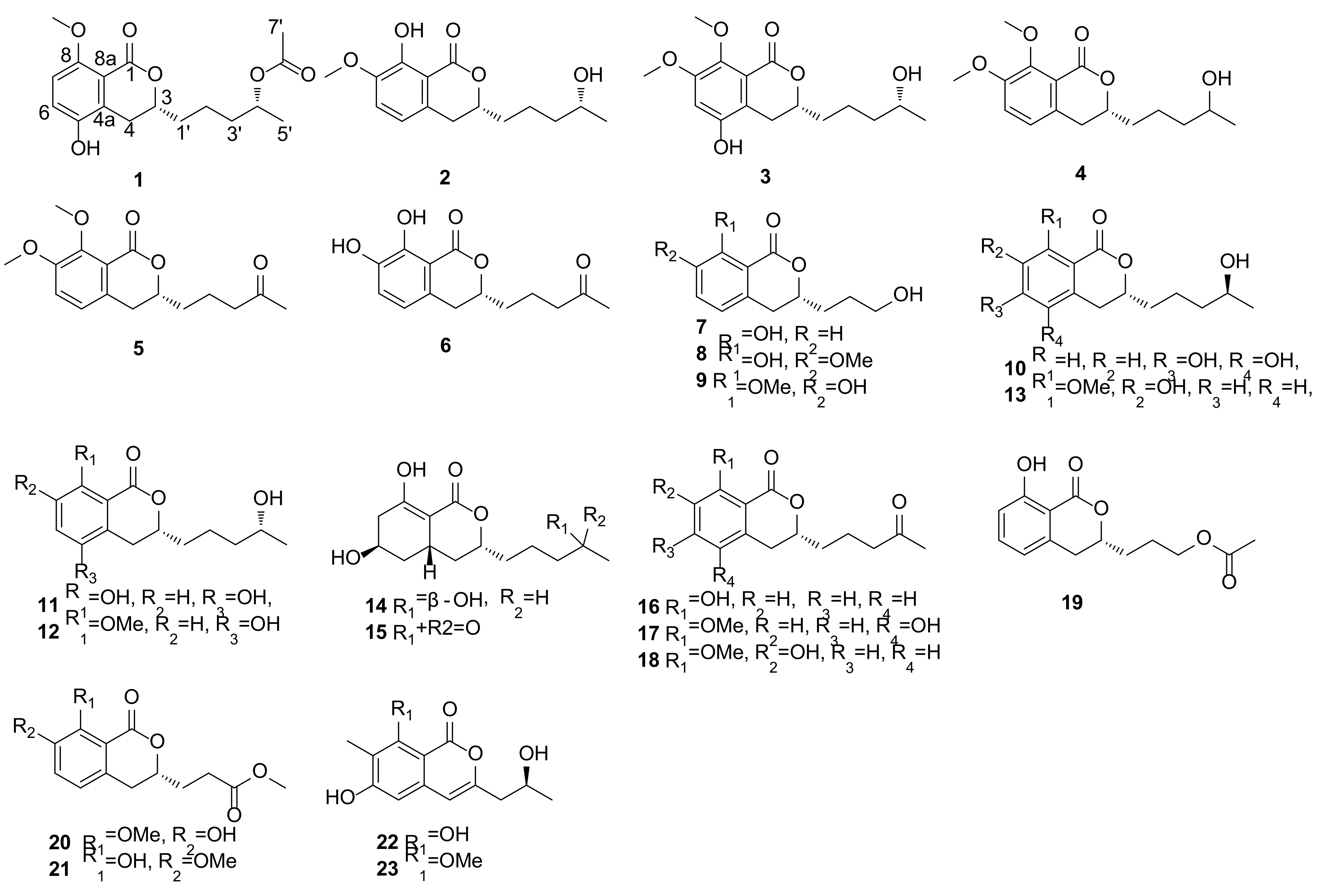
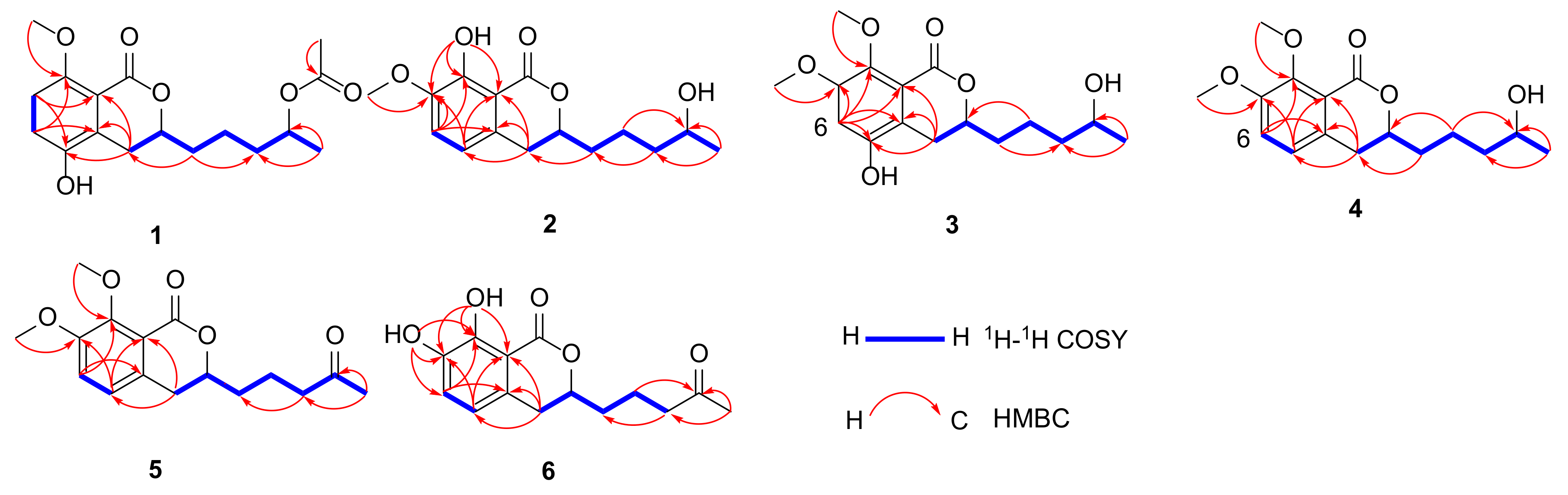
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Fermentation, Extraction and Isolation
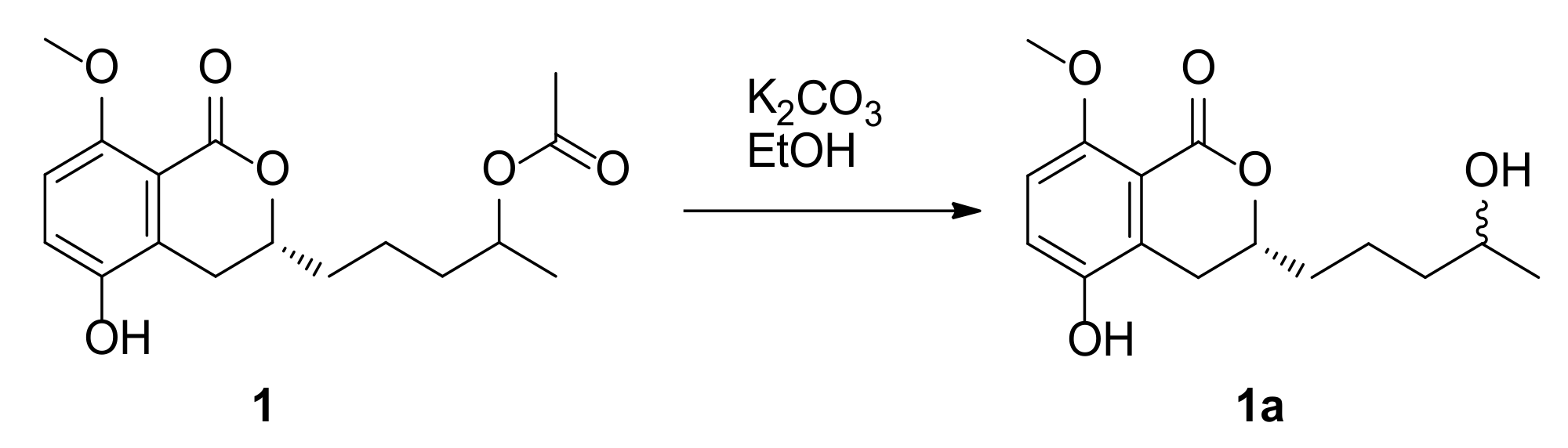
3.4. Preparations of the (S)- and (R)-MTPA Esters of Compounds 1, 2 and 3
3.5. Biological Assays
3.5.1. Antioxidant Activity
3.5.2. Antibacterial Activity
3.5.3. Anti-Phytopathogenic Activity
3.5.4. Inhibitory Activity against α-Glucosidase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 18, 1R–49R. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Wu, N.N.; Wu, Y.W.; Qi, Y.X.; Wei, M.Y.; Pineda, L.M.; Ng, M.G.; Spadafora, C.; Zheng, J.Y.; Lu, L.; et al. Structure modifcation, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022, 4, 88–97. [Google Scholar] [CrossRef]
- Chen, S.H.; Cai, R.L.; Liu, Z.M.; Gui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.M.; Cai, R.L.; Zang, Z.M.; Yang, W.C.; Wang, B.; Zhu, G.; Yuan, J.; She, Z.G. Azaphilone derivatives with anti-inflammatory activity from the mangrove endophytic fungus Penicillium sclerotiorum ZJHJJ-18. Bioorg. Chem. 2022, 122, 105721. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Y.M.; Che, Q.; Li, D.H.; Zhang, G.J.; Zhu, T.J. Citreobenzofurans D-F and phomenones A-B: Five novel sesquiterpenoids from the mangrove-derived fungus Penicillium sp. HDN13-494. Mar. Drugs 2022, 20, 137. [Google Scholar] [CrossRef]
- Qin, X.Y.; Huang, J.G.; Zhou, D.X.; Zhang, W.X.; Zhang, Y.J.; Li, J.; Yang, R.Y.; Huang, X.S. Polyketide derivatives, guhypoxylonols A-D from a mangrove endophytic fungus Aspergillus sp. GXNU-Y45 that inhibit nitric oxide production. Mar. Drugs 2022, 20, 5. [Google Scholar] [CrossRef]
- Su, J.H.; Wang, M.Q.; Li, Y.Z.; Lin, Y.S.; Gu, J.Y.; Zhu, L.P.; Yang, W.Q.; Jiang, S.Q.; Zhao, Z.X.; Sun, Z.H. Rare cytochalasans isolated from the mangrove endophytic fungus Xylaria arbuscular. Fitoterapia 2022, 157, 105124. [Google Scholar] [CrossRef]
- Zeng, W.N.; Huang, G.L.; Cai, J.; Zheng, C.J. Secondary metabolites and bioactivities of Penicillium sp. sourced from mangrove from 2007 to 2020. Chin. J. Org. Chem. 2021, 41, 4255–4278. [Google Scholar] [CrossRef]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef]
- Noor, A.O.; Almasri, D.M.; Bagalagel, A.A.; Abdallah, H.M.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 2020, 25, 395. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.L.; Li, J.P.; Zhang, K.; Wei, S.Z.; Lin, R.; Polyak, S.W.; Yang, N.; Song, F.H. New isocoumarin analogues from the marine-derived fungus Paraphoma sp. CUGBMF180003. Mar. Drugs 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Lu, Z.H.; Xia, G.R.; Song, W.M.; Guo, Z.Y.; Proksch, P. (+)-/(−)-Prunomarin A and (+)-pestalactone B, three new isocoumarin derivatives from the endophytic fungus Phomopsis prunorum. Tetrahedron. Lett. 2021, 75, 153205. [Google Scholar] [CrossRef]
- Ran, Y.Q.; Lan, W.J.; Qiu, Y.; Guo, Q.; Feng, G.K.; Deng, R.; Zhu, X.F.; Li, H.J.; Dong, J. Monarubins A-C from the marine shellfish-associated fungus Monascus ruber BB5. Mar. Drugs 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronado, L.; Zhang, X.Q.; Dorta, D.; Escala, N.; Pineda, L.M.; Ng, M.G.; Olmo, E.D.; Wang, C.Y.; Gu, Y.C.; Shao, C.L.; et al. Semisynthesis, antiplasmodial activity, and mechanism of action studies of isocoumarin derivatives. J. Nat. Prod. 2021, 84, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.N.; Cai, J.; Wang, B.; Chen, L.Y.; Pan, C.X.; Chen, S.J.; Huang, G.L.; Zheng, C.J. A new bioactive isocoumarin from the mangrove-derived fungus Penicillium sp. TGM112. J. Asian Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Huang, G.L.; Zhou, X.M.; Bai, M.; Liu, Y.X.; Zhao, Y.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y. Dihydroisocoumarins from the mangrove-derived fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef] [Green Version]
- Mei, R.Q.; Wang, B.; Zeng, W.N.; Huang, G.L.; Chen, G.Y.; Zheng, C.J. Bioactive isocoumarins isolated from a mangrove-derived fungus Penicillium sp. MGP11. Nat. Prod. Res. 2022, 36, 1260–1265. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New lactone and isocoumarin derivatives from the marine mangrove-derived endophytic fungus Penicillium coffeae MA-314. Phytochem. Lett. 2019, 32, 1–5. [Google Scholar] [CrossRef]
- Proksa, B. Talaromyces flavus and its metabolites. Chem. Pap. 2010, 64, 696–714. [Google Scholar] [CrossRef]
- Nicoletti, R.; Trincone, A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, R.; Salvatore, M.M.; Andolfi, A. Secondary metabolites of mangrove-associated strains of Talaromyces. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.H.; Teng, M.T.; Yun, Y.F.; Jiang, N.; Ma, L.; Sun, S.S.; Yuan, B.; Tang, J.; Wu, Q.Y.; Li, Q.; et al. Talarolactone A, an isocoumarin derivative fused with dihydrothiophene with selective antimigratory activity from the endolichenic fungus Talaromyces sp. J. Nat. Prod. 2020, 83, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Liu, Y.Y.; Liu, Z.M.; Cai, R.L.; Lu, Y.J.; Huang, X.S.; She, Z.G. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016, 6, 26412–26420. [Google Scholar] [CrossRef]
- Buttachon, S.; May, Z.; War, W.; Dethoup, T.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Kijjoa, A. Secondary metabolites from the culture of the marine sponge-associated fungi Talaromyces tratensis and Sporidesmium circinophorum. Planta Med. 2016, 82, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, F.; Yang, R.; Chen, D.D.; Wang, Y.; Qin, B.F.; Yang, X.J.; Zhou, L. Isolation, identification and antimicrobial activities of two secondary metabolites of Talaromyces verruculosus. Molecules 2012, 17, 14091–14098. [Google Scholar] [CrossRef] [Green Version]
- Duo-Chuan, L.I.; Chen, S.; Jing, L.U. Purification and partial characterization of two chitinases from the mycoparasitic fungus Talaromyces flavus. Mycopathologia 2005, 159, 223–229. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Chen, G. Austins-type meroterpenoids from a mangrove-derived Penicillium sp. J. Nat. Prod. 2021, 84, 2104–2110. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Zheng, C.; Niu, Z.G.; Chen, G.Y. Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef]
- Liao, H.X.; Shao, T.M.; Mei, R.Q.; Huang, G.L.; Zhou, X.M.; Zheng, C.J.; Wang, C.Y. Bioactive secondary metabolites from the culture of the mangrove-derived fungus Daldinia eschscholtzii HJ004. Mar. Drugs 2019, 17, 710. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Zheng, C.J.; Nong, X.H.; Zhou, X.M.; Luo, Y.P.; Chen, G.Y. Four new insecticidal xanthene derivatives from the mangrove-derived fungus Penicillium sp. JY246. Mar. Drugs 2019, 17, 649. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Nong, X.H.; Chen, G.Y.; Zheng, C.J. Bioactive lactones from the mangrove-derived fungus Penicillium sp. TGM112. Mar. Drugs 2019, 17, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.X.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Nong, X.H.; Shao, T.M.; Chen, G.; Wang, C.Y. Bioactive polyketide derivatives from the mangrove-derived fungus Daldinia eschscholtzii HJ004. J. Nat. Prod. 2019, 82, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Bai, M.; Zhou, X.M.; Huang, G.L.; Shao, T.M.; Luo, Y.P.; Niu, Y.Y.; Chen, G.Y.; Han, C.R. Penicilindoles A-C, cytotoxic indole diterpenes from the mangrove-derived fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Wen, S.; Ding, M.; Guo, H.X.; Huang, G.Y.; Zhu, X.T.; Huang, J.Y.; She, Z.G.; Long, Y.H. The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002. Mar. Drugs 2019, 17, 414. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhu, Z.X.; Song, Y.L.; Ren, Y.; Dong, D.; Zheng, J.; Liu, T.; Zhao, Y.F.; Tu, P.F.; Li, J. Anti-neuroinflammatory constituents from the fungus Penicillium purpurogenum MHZ 111. Nat. Prod. Res. 2017, 31, 562–567. [Google Scholar] [CrossRef]
- Qi, J.; Shao, C.L.; Li, Z.Y.; Gan, L.S.; Fu, X.M.; Bian, W.T.; Zhao, H.Y.; Wang, C.Y. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef]
- Li, S.D.; Wei, M.Y.; Chen, G.Y.; Lin, Y.C. Two new dihydroisocoumarins from the endophytic fungus Aspergillus sp. collected from the South China Sea. Chem. Nat. Compd. 2012, 48, 371–373. [Google Scholar] [CrossRef]
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inacio, A.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M.S.; et al. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Meroterpenoid, isocoumarin, and phenol derivatives from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194. Tetrahedron 2015, 71, 882–888. [Google Scholar] [CrossRef]
- He, J.; Yang, M.S.; Wang, W.X.; Li, Z.H.; Elkhateeb, W.A.M.; Wen, T.C.; Ai, H.L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef] [Green Version]
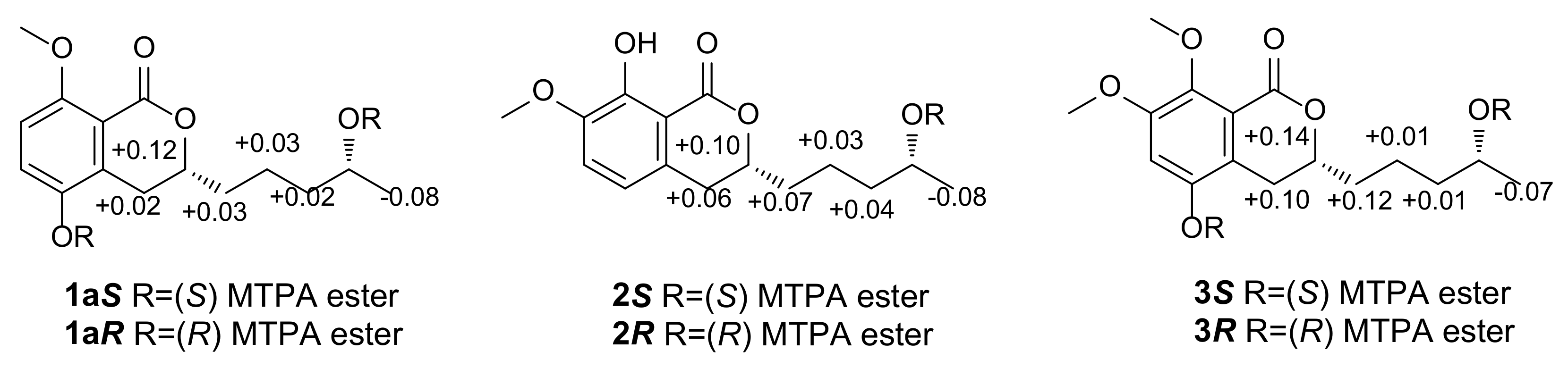

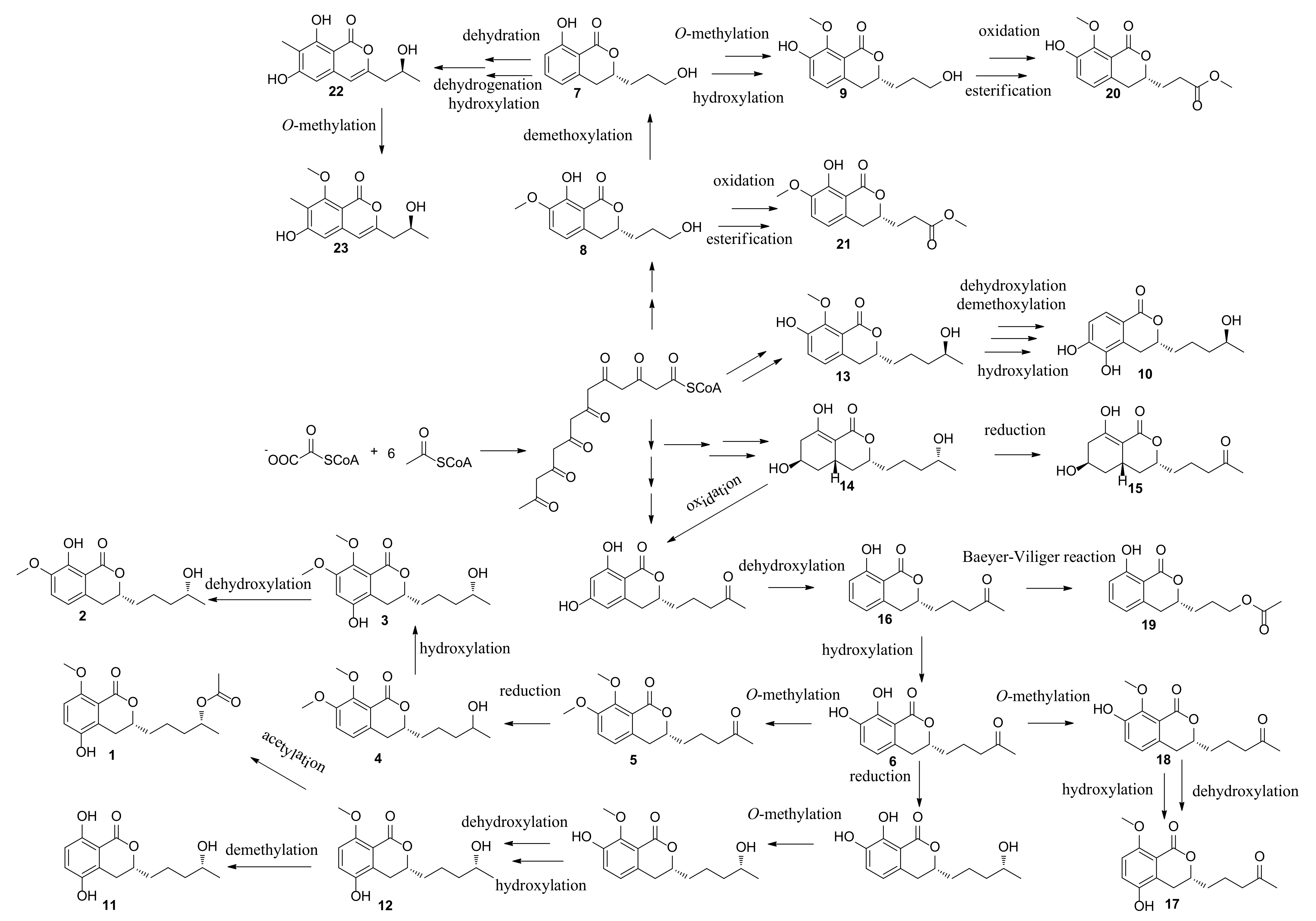
| Position | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 3 | 4.34, m | 4.54, m | 4.35, m | 4.36, m | 4.37, m | 4.58, m |
| 4 | 2.64, dd (16.4, 11.6) 3.08, dd (16.4, 2.8) | 2.86, m | 2.56, m 2.98, m | 2.82, m | 2.82, m | 2.87, m |
| 5 | 6.62, d (8.0) | 6.91, d (8.0) | 6.91, d (8.4) | 6.61, d (8.0) | ||
| 6 | 7.00, d (8.8) | 6.98, d (8.4) | 6.67, s | 7.06, d (8.0) | 7.06, d (8.0) | 7.07, d (8.0) |
| 7 | 6.78, d (8.8) | |||||
| 7-OH | 5.53, s | |||||
| 7-OMe | 3.87, s | 3.84, s | 3.88, s | 3.88, s | ||
| 8-OMe | 3.88, s | 3.89, s | 3.96, s | 3.95, s | ||
| 8-OH | 11.18, s | 11.00, s | ||||
| 1′ | 1.85, m | 1.87, m | 1.85, m | 1.88, m | 1.82, m | 1.78, m |
| 2′ | 1.57, m | 1.58, m | 1.60, m | 1.58, m | 1.78, m | 1.80, m |
| 3′ | 1.52, m | 1.47, m | 1.50, m | 1.51, m | 2.52, t (6.4) | 2.53, m |
| 4′ | 4.90, m | 3.80, m | 3.82, m | 3.83, m | ||
| 5′ | 1.22, d (6.4) | 1.19, d (6.4) | 1.21, d (6.0) | 1.21, d (4.0) | 2.16, s | 2.15, m |
| 7′ | 2.04, s |
| Position | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 163.1, C | 170.4, C | 162.7, C | 162.6, C | 162.5, C | 170.2, C |
| 3 | 77.4, CH | 80.4, CH | 78.3, CH | 78.5, CH | 78.3, CH | 80.6, CH |
| 4 | 28.0, CH2 | 32.4, CH2 | 27.4, CH2 | 33.8, CH2 | 33.7, CH2 | 32.2, CH2 |
| 4a | 128.4, C | 130.1, C | 119.9, C | 132.3, C | 132.2, C | 129.7, C |
| 5 | 145.3, C | 117.1, CH | 147.7, CH | 122.4, CH | 122.4, CH | 117.9, CH |
| 6 | 121.2, CH | 117.5, CH | 106.1, C | 117.5, CH | 117.4, CH | 120.8, CH |
| 7 | 111.5, CH | 147.4, C | 153.1, C | 153.0, C | 153.0, C | 143.9, C |
| 8 | 155.5, C | 152.5, C | 142.9, C | 151.6, C | 151.5, C | 149.1, C |
| 8a | 114.6, C | 108.6, C | 117.7, C | 119.6, C | 119.5, C | 108.4, C |
| 7-OMe | 56.4, CH3 | 56.6, CH3 | 56.5, CH3 | 56.5, CH3 | ||
| 8-OMe | 56.6, CH3 | 61.8, CH3 | 61.7, CH3 | 61.7, CH3 | ||
| 1′ | 34.7, CH2 | 34.8, CH2 | 34.9, CH2 | 34.8, CH2 | 34.0, CH2 | 34.1, CH2 |
| 2′ | 21.0, CH2 | 21.3, CH2 | 21.5, CH2 | 21.5, CH2 | 19.4, CH2 | 19.2, CH2 |
| 3′ | 35.7, CH2 | 38.9, CH2 | 39.1, CH2 | 39.1, CH2 | 43.2, CH2 | 43.0, CH2 |
| 4′ | 70.9, CH | 67.9, CH | 68.2, CH | 68.1, CH | 208.7, C | 208.6, C |
| 5′ | 20.1, CH3 | 23.7, CH3 | 23.8, CH3 | 23.8, CH3 | 30.1, CH3 | 30.1, CH3 |
| 6′ | 171.1, C | |||||
| 7′ | 21.6, CH3 |
| Compound | 2 | 6 | 7 | 8 | 9 | 10 | 11 | 17 | 18 | 19 | 21 | 22 | Trolox a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (mM) | 28.39 | 0.14 | 0.17 | 0.13 | 0.10 | 0.11 | 0.12 | 0.12 | 0.16 | 0.15 | 20.66 | 0.009 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Zhu, X.-C.; Zeng, W.-N.; Wang, B.; Luo, Y.-P.; Liu, J.; Chen, M.-J.; Li, G.-Y.; Huang, G.-L.; Chen, G.-Y.; et al. Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35. Mar. Drugs 2022, 20, 361. https://doi.org/10.3390/md20060361
Cai J, Zhu X-C, Zeng W-N, Wang B, Luo Y-P, Liu J, Chen M-J, Li G-Y, Huang G-L, Chen G-Y, et al. Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35. Marine Drugs. 2022; 20(6):361. https://doi.org/10.3390/md20060361
Chicago/Turabian StyleCai, Jin, Xiao-Chen Zhu, Wei-Nv Zeng, Bin Wang, You-Ping Luo, Jing Liu, Min-Jing Chen, Gao-Yu Li, Guo-Lei Huang, Guang-Ying Chen, and et al. 2022. "Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35" Marine Drugs 20, no. 6: 361. https://doi.org/10.3390/md20060361
APA StyleCai, J., Zhu, X.-C., Zeng, W.-N., Wang, B., Luo, Y.-P., Liu, J., Chen, M.-J., Li, G.-Y., Huang, G.-L., Chen, G.-Y., Xu, J., & Zheng, C.-J. (2022). Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35. Marine Drugs, 20(6), 361. https://doi.org/10.3390/md20060361