Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster
Abstract
:1. Introduction
2. Results
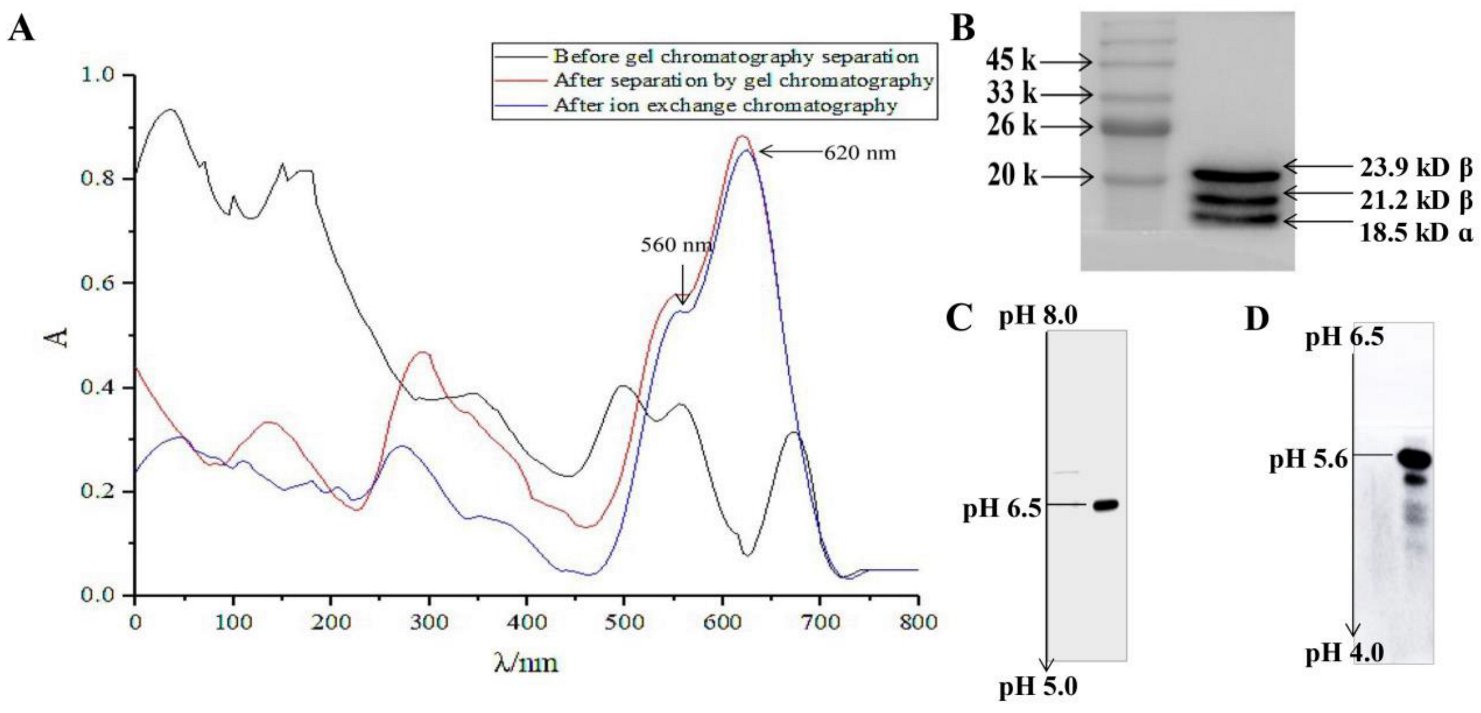
2.1. Isolation and Purification of R-PC
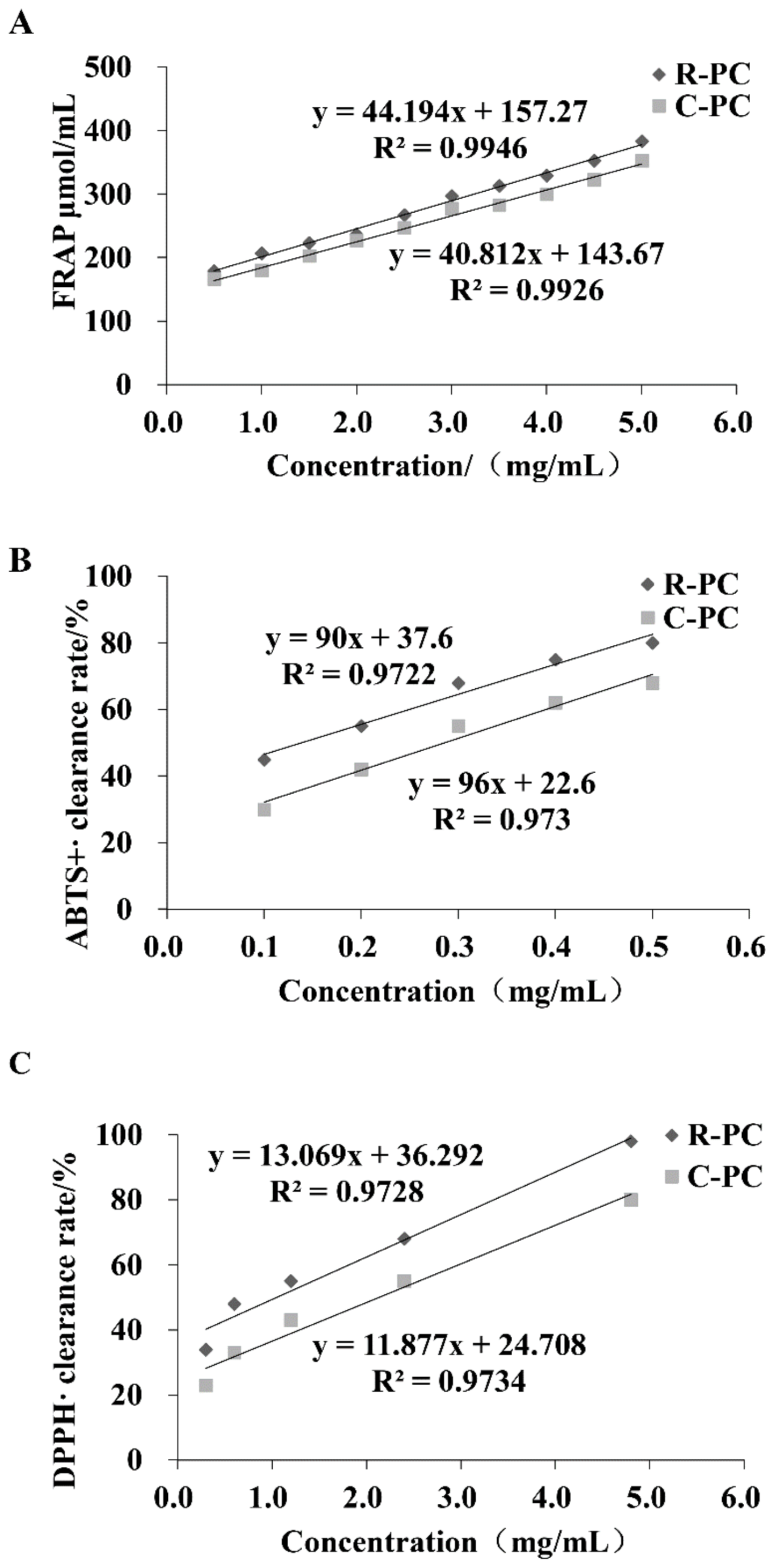
2.2. Antioxidant Capacity In Vitro
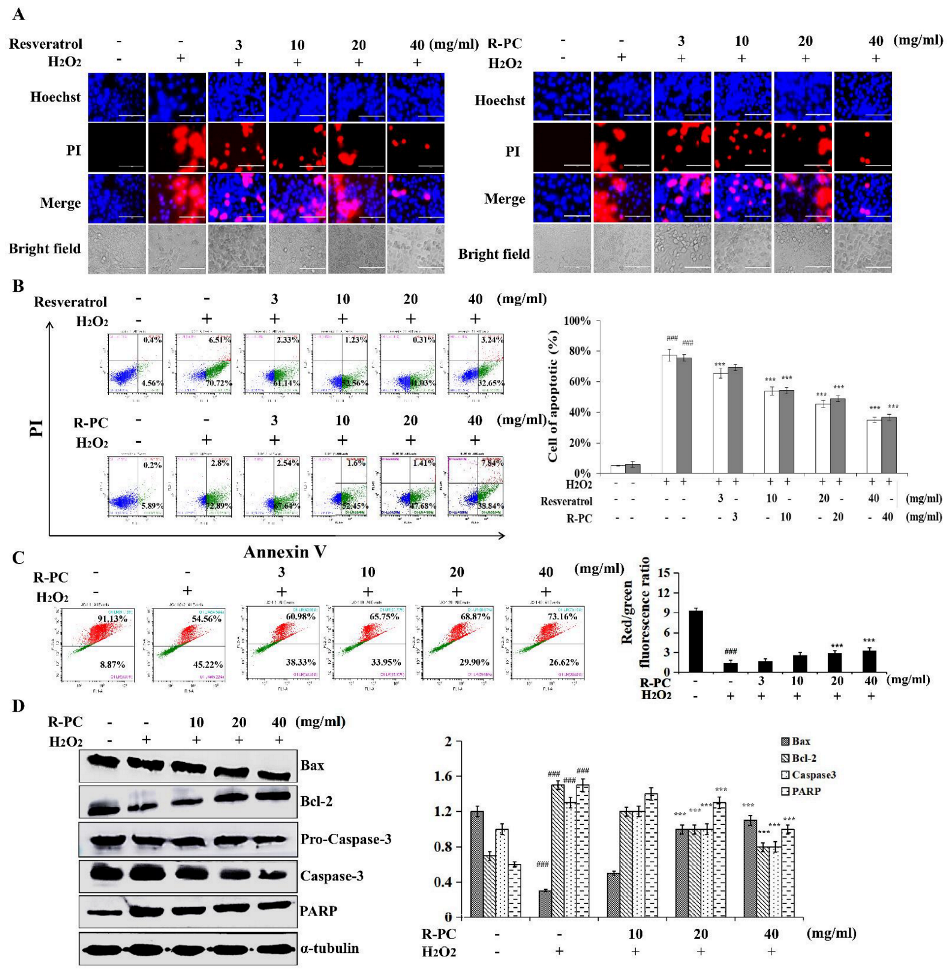
2.3. R-PC Inhibits H2O2-Induced Cytotoxicity in HUVEC Cells
2.4. P-RC Protects against H2O2-Induced Apoptotic Cell Death in HUVEC Cells
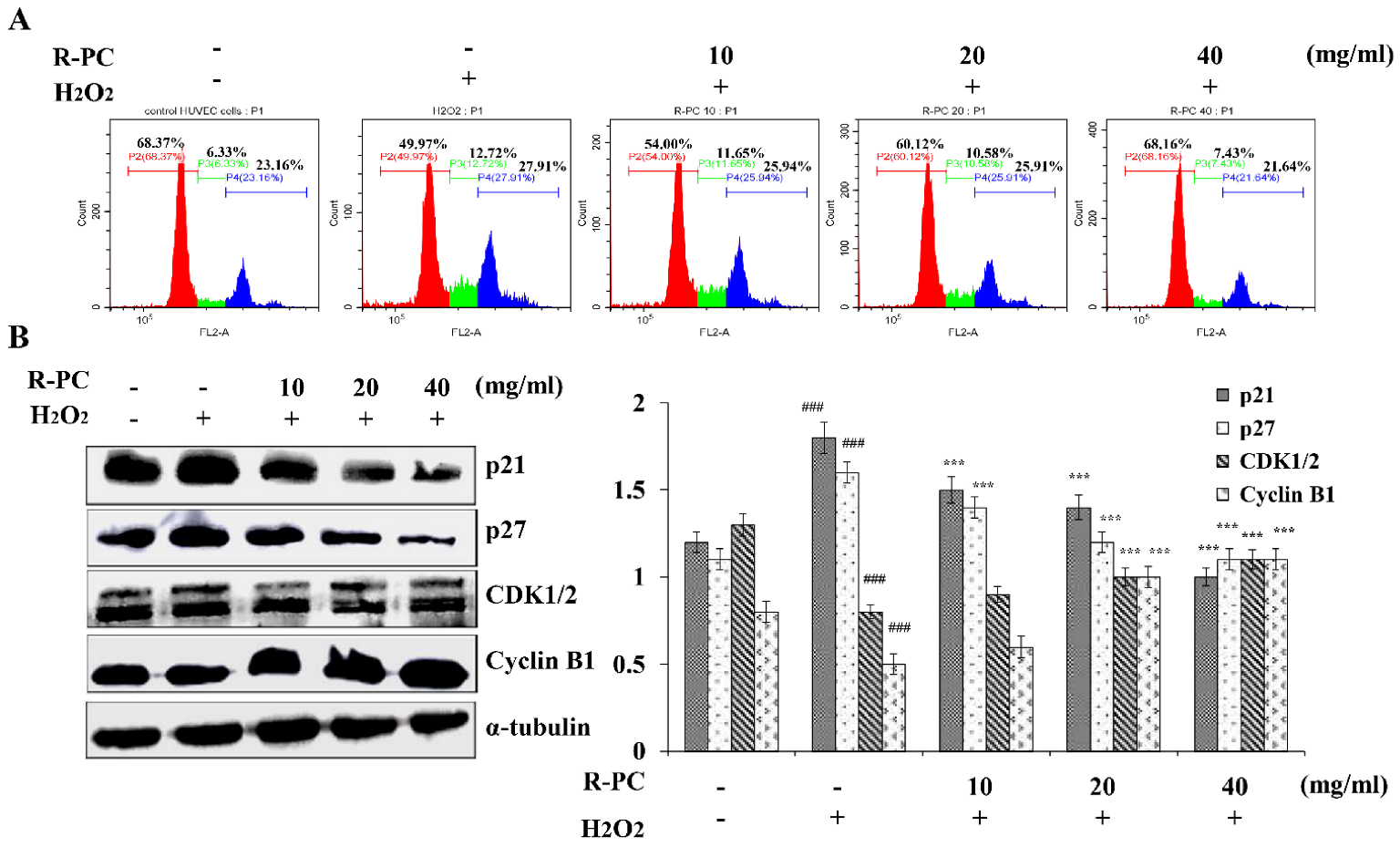
2.5. R-PC Prevents H2O2-Induced Cell Cycle Progression at the G2/M Phase in HUVEC Cells
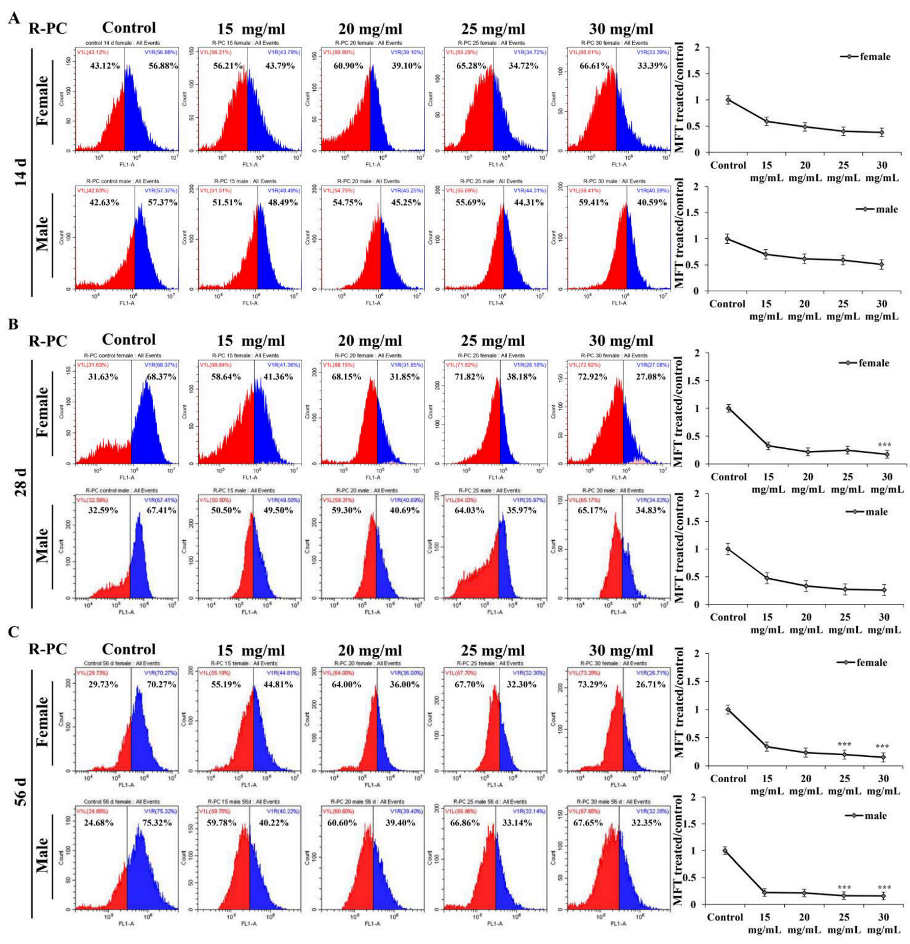
2.6. Effect of R-PC on the Lifespan of Drosophila melanogaster and the Climbing Ability of Drosophila melanogaster
2.7. Effect of R-PC on ROS Levels and Antioxidant-Related Gene Expression in Drosophila melanogaster
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Isolation and Purification of R-PC
4.3. Antioxidant Capacity In Vitro
4.4. Cell Cultures
4.5. CCK-8 Assay
4.6. SA β-Gal Staining
4.7. Hoechst and PI Staining Kit
4.8. Annexin V-Fluorescein Isothiocyanate (FITC)/PI Double Staining and Flow Cytometry
4.9. Mitochondrial Membrane Potential Analysis
4.10. Cell Cycle Analysis
4.11. Measurement of Intracellular ROS Levels and Flow Cytometry
4.12. Survival and Life Span Studies
4.13. Climbing Ability Assays
4.14. Western Blot Assay
4.15. Quantitative Polymerase Chain Reaction (q-PCR) Assay
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckman, G.A.; Mckelvie, R.S. Cardiovascular aging and exercise in healthy older adults. Clin. J. Sport Med. 2008, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Back, P.; Braeckman, B.P.; Matthijssens, F. ROS in Aging Caenorhabditis elegans: Damage or Signaling? Oxidative Med. Cell. Longev. 2012, 2012, 608478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gojon-Romanillos, G. Systemic Treatment of Pathological Conditions Resulting From Oxidative Stress and/or Redox Imbalance. U.S. Patent 20,090,181,081, 16 March 2009. [Google Scholar]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Mammucari, C.; Rizzuto, R. Signaling pathways in mitochondrial dysfunction and aging. Mech. Ageing Dev. 2010, 131, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Weidong, W.; Yuee, S. In vitro and in vivo antioxidant activities of polyphenol extracted from black garlic. Food Sci. Technol. 2017, 37, 681–685. [Google Scholar]
- Wang, X.; Wang, P.; Zhang, Z.; Farré, J.-C.; Li, X.; Wang, R.; Xia, Z.; Subramani, S.; Ma, C. The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway. Autophagy 2019, 16, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef]
- Liu, Q.M.; Xu, S.S.; Li, L.; Pan, T.-M.; Shi, C.-L.; Liu, H.; Cao, M.-J.; Su, W.-J.; Liu, G.-M. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]
- Yan, X.H.; Lv, F.; Liu, C.J.; Zheng, Y.-F. Selection and characterization of a high-temperature tolerant strain of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J. Appl. Phycol. 2010, 22, 511–516. [Google Scholar] [CrossRef]
- Duan, J.; Duan, J.; Zhang, Z.; Tong, T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int. J. Biochem. Cell Biol. 2005, 37, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, O.; Salmon, M.; Pascal, T.; Magalhaes, J.P.; Chainiaux, F. Stress-induced Premature Senescence (SIPS); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Jamal, J.; Mustafa, M.R.; Wong, P.F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Young, I.C.; Chuang, S.T.; Hsu, C.H.; Sun, Y.-J.; Lin, F.-H. C-phycocyanin alleviates osteoarthritic injury in chondrocytes stimulated with H2O2 and compressive stress. Int. J. Biol. Macromol. 2016, 93 Pt A, 852–859. [Google Scholar] [CrossRef]
- Soojin, L.; Min, B.S.; Woo, L.J.; Cho, K.S. Evaluation of Traditional Medicines for Neurodegenerative Diseases Using Drosophila Models. Evid.-Based Complement. Altern. Med. 2014, 2014, 967462. [Google Scholar]
- Newman, T.; Sinadinos, C.; Johnston, A.; Sealey, M.; Mudher, A. Using Drosophila models of neurodegenerative diseases for drug discovery. Expert Opin. Drug Discov. 2011, 6, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Cohen, A. Drosophila Models of Aging. In Handbook of Models for Human Aging; Academic Press: Cambridge, MA, USA, 2006; pp. 253–265. [Google Scholar]
- Chen, X.Q.; Gong, X.G.; Feng, S.; Pan, Y.J. Chromatographic Purification and Characteristic of R-phycoerythrin from Porphyra haitanensis. Anal. Lab. 2004, 23, 5–9. [Google Scholar]
- Ma, Y.; Xie, J.; Zhang, R.; Hu, C.; Zhao, J. Molecular properties of R-phycocyanin subunits from Polysiphonia urceolata in potassium phosphate buffer. Photochem. Photobiol. Sci. 2008, 7, 263–268. [Google Scholar] [CrossRef]
- Ma, S.-Y.; Wang, G.-C.; Sun, H.-B.; Zeng, C.-K. Characterization of the artificially covalent conjugate of B-phycoerythrin and R-phycocyanin and the phycobilisome from Porphyridium cruentum. Plant Sci. 2003, 164, 253–257. [Google Scholar] [CrossRef]
- Farooq, S.M.; Boppana, N.B.; Devarajan, A.; Sekaran, S.D.; Shankar, E.M.; Li, C.; Gopal, K.; Bakar, S.A.; Karthik, H.S.; Ebrahim, A.S. C-Phycocyanin Confers Protection against Oxalate-Mediated Oxidative Stress and Mitochondrial Dysfunctions in MDCK Cells. PLoS ONE 2014, 9, e93056. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, R.; Ortiz-Butron, R.; Blas-Valdivia, V.; Hernández-García, A.; Cano-Europa, E. Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem. 2012, 135, 2359–2365. [Google Scholar] [CrossRef]
- Niu, Y.J.; Zhou, W.; Guo, J.; Nie, Z.; Shin, K.; Kim, N.; Lv, W.; Cui, X. C-phycocyanin protects against mitochondrial dysfunction and oxidative stress in parthenogenetic porcine embryos. Sci. Rep. 2017, 7, 16992. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, L.; Wang, J.; Zhang, L.; Zhou, D.; Qu, J.; Wang, H.; Yin, M.; Hong, J.; Zhao, W. C-phycocyanin Ameliorates Mitochondrial Fission and Fusion Dynamics in Ischemic Cardiomyocyte Damage. Front. Pharmacol. 2019, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Luo, H.; Lai, Z.; Zou, L.; Zhu, L.; Mao, J.; Jacob, T.; Ye, W.; Wang, L.; Chen, L. ClC-3 Chloride Channel Proteins Regulate the Cell Cycle by Up-regulating cyclin D1-CDK4/6 through Suppressing p21/p27 Expression in Nasopharyngeal Carcinoma Cells. Sci. Rep. 2016, 6, 30276. [Google Scholar] [CrossRef] [Green Version]
- Pattarayan, D.; Rajarajan, D.; Ayyanar, S.; Palanichamy, R.; Subbiah, R. C-phycocyanin suppresses transforming growth factor-β1-induced epithelial mesenchymal transition in human epithelial cells. Pharmacol. Rep. 2017, 69, 426–431. [Google Scholar] [CrossRef]
- Ratana, S. Aging Interventions and Therapies; World Scientific: Singapore, 2005. [Google Scholar]
- Humily, F.; Farrant, G.K.; Marie, D.; Partensky, F.; Mazard, S.; Perennou, M.; Labadie, K.; Aury, J.M.; Wincker, P.; Segui, A.N.; et al. Synechococcus sp. RS9909 C-Phycocyanin Beta Subunit (rpcB) and C-Phycocyanin Alpha Subunit (rpcA) Genes, Complete Cds. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KF528823 (accessed on 23 June 2022).
- Huadraksasat, S.; Chittapun, S.; Charoenrat, T.; Dedvisitsakul, P.; Piyapittayanun, C. Identification and Analysis of a C-phycocyanin Beta Subunit Gene from Thermosynechococcus sp. TUBT-T01. Burapha Sci. J. 2017, 22, 222–227. [Google Scholar]
- Cherdkiatikul, T.; Suwanwong, Y. Production of the α and β Subunits of Spirulina Allophycocyanin and C-Phycocyanin in Escherichia coli: A Comparative Study of Their Antioxidant Activities. J. Biomol. Screen. 2014, 19, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.Y.; Zhang, W.J.; Zhang, X.W.; Li, Y.; Wang, J.; Lin, H.; Tang, X.; Qin, S. A potent anti-oxidant property: Fluorescent recombinant alpha-phycocyanin of Spirulina. J. Appl. Microbiol. 2009, 106, 1093–1100. [Google Scholar] [CrossRef]
- Yu, P.; Li, P.; Chen, X.; Chao, X. Combinatorial biosynthesis of Synechocystis PCC6803 phycocyanin holo-alpha-subunit (CpcA) in Escherichia coli and its activities. Appl. Microbiol. Biotechnol. 2016, 100, 1–14. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Sarian, F.D.; Van Wijk, A.; Martinez-Garcia, M.; Van Der Maarel, M.J.E.C. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. J. Appl. Phycol. 2017, 29, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Kondo, S.; Kagami, S.; Shimizu, M. System of reactive oxygen spiecies (ROS) generation in the development of progressive glomerular disease in animal models. Nihon Shoni Jinzobyo Gakkai Zasshi 2003, 16, 69–72. [Google Scholar] [CrossRef]
- Wong, W.L.; Puthalakath, H. Bcl-2 family proteins: The sentinels of the mitochondrial apoptosis pathway. IUBMB Life 2010, 60, 390–397. [Google Scholar] [CrossRef]
- Gupta, S.; Kass, G.; Szegezdi, E.; Joseph, B. The mitochondrial death pathway: A promising therapeutic target in diseases. J. Cell. Mol. Med. 2010, 13, 1004–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, P.; Padala, M.K.; Cox, J.; Guntaka, R.V. Cycle Arrest in G2/M Phase by Binding to Cyclin D1. Int. J. Cell Biol. 2009, 2009, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Benavente, C.A.; Jacobson, E.L. Niacin restriction upregulates NADPH oxidase and ROS in human keratinocytes. Free. Radic. Biol. Med. 2011, 44, 527–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-J.; Han, Z.; Ge, L.; Zhou, C.-J.; Zhao, Y.-F.; Wang, D.-H.; Ren, J.; Niu, X.-X.; Liang, C.-G. C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget 2016, 7, 17393–17409. [Google Scholar] [CrossRef] [Green Version]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Antioxidative mechanisms of whole-apple antioxidants employing different varieties from Luxembourg. J. Med. Food 2011, 14, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Zunino, S. Resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide reduce DNA strand breakage but not apoptosis in Jurkat T cells treated with camptothecin. Oncol. Lett. 2017, 14, 2517. [Google Scholar] [CrossRef] [Green Version]
- Kopp, T.I.; Vogel, U.; Dragsted, L.O.; Tjonneland, A.; Ravn-Haren, G. Association between single nucleotide polymorphisms in the antioxidant genes CAT, GR and SOD1, erythrocyte enzyme activities, dietary and life style factors and breast cancer risk in a Danish, prospective cohort study. Oncotarget 2017, 8, 62984–62997. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Lu, H.; Hu, J.; Zheng, B.; Zhang, Y. Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster. Mar. Drugs 2022, 20, 468. https://doi.org/10.3390/md20080468
Feng Y, Lu H, Hu J, Zheng B, Zhang Y. Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster. Marine Drugs. 2022; 20(8):468. https://doi.org/10.3390/md20080468
Chicago/Turabian StyleFeng, Yanyu, Hanjin Lu, Jiamiao Hu, Baodong Zheng, and Yi Zhang. 2022. "Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster" Marine Drugs 20, no. 8: 468. https://doi.org/10.3390/md20080468
APA StyleFeng, Y., Lu, H., Hu, J., Zheng, B., & Zhang, Y. (2022). Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster. Marine Drugs, 20(8), 468. https://doi.org/10.3390/md20080468







