Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid
Abstract
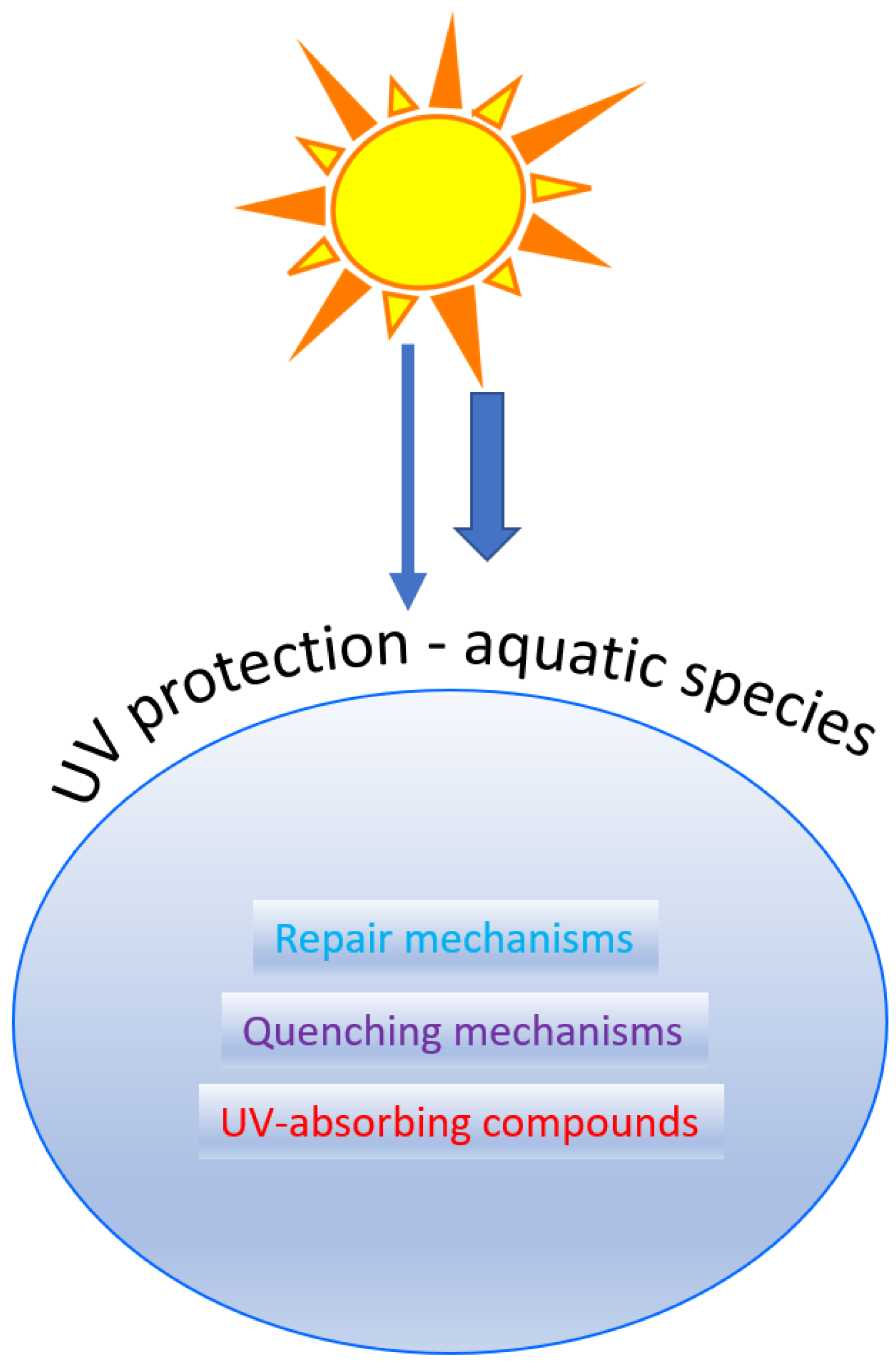
:1. Introduction
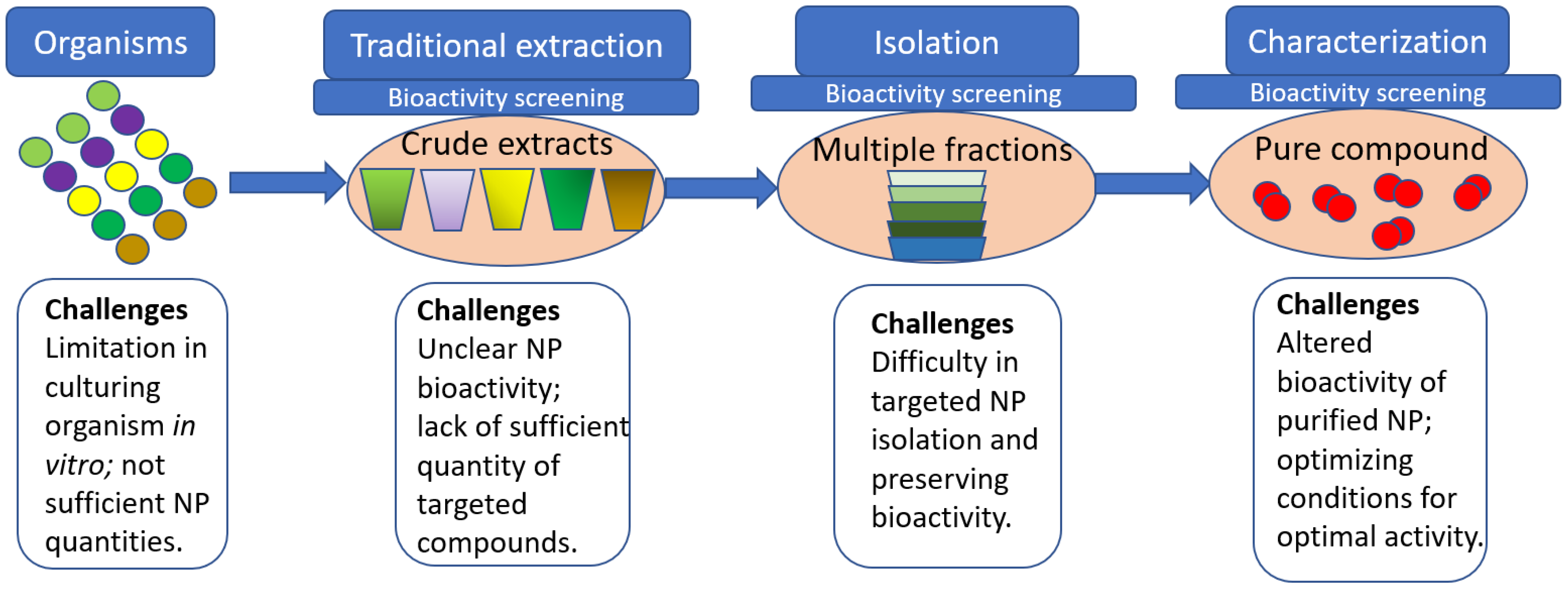
2. Traditional vs. New Ways of Natural Products Discovery
3. From Genes to Biotechnological Solutions
4. Genome-Mining Tools
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence Regarding the UV Sunscreen Role of a Mycosporine-Like Compound in the Cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and Analysis of Mycosporine-Like Amino Acids in Marine Algae. In Natural Products from Marine Algae; Methods in Molecular Biology; Stengel, D., Connan, S., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1308. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Malcolm, S.J. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biological and environmental perspective. J. Phycol. 1998, 34, 418–430. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-Like Amino Acids from Coral Dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, F.L. Mycosporine-Like Amino Acids from Marine Resource. Mar. Drugs 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.A.; Airs, R.L. Distribution and Abundance of MAAs in 33 Species of Microalgae across 13 Classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Häder, D.-P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46. [Google Scholar]
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Ngoc, H.N.; Karsten, U.; Ganzera, M. Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraldes, V.; de Medeiros, L.S.; Jacinavicius, F.R.; Long, P.F.; Pinto, E. Development and validation of a rapid LC-MS/MS method for the quantification of mycosporines and mycosporine-like amino acids (MAAs) from cyanobacteria. Algal Res. 2020, 46, 101796. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoennet, S.; Nishikawa, Y.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. A Method for the Isolation and Characterization of Mycosporine-Like Amino Acids from Cyanobacteria. Methods Protoc. 2018, 1, 46. [Google Scholar] [CrossRef] [Green Version]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory Effects of the Mycosporine-Like Amino Acids Shinorine and Porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-Y.; Lee, S.Y.; Kim, H.G.; Jeong, J.C.; Batara, D.C.; Kim, S.-H.; Cho, J.-Y. Shinorine and porphyra-334 isolated from laver (Porphyra dentata) inhibit adipogenesis in 3T3-L1 cells. Food Sci. Biotechnol. 2022, 31, 617–625. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Biol. 2016, 164, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2018, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Gacesa, R.; Long, P.; Young, A. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2017, 178, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and Antioxidant Activities and Mycosporine-Like Amino Acid Profiles of Wild-Harvested and Cultivated Edible Canadian Marine Red Macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of Collagenase by Mycosporine-like Amino Acids from Marine Sources. Planta Medica 2015, 81, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B Biol. 2014, 130, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N. Molecular Mechanisms of Stress Tolerance in Cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Rastogi, R.P., Ed.; Springer Nature: Singapore, 2021; p. 131. [Google Scholar]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pagès, C.; Gattuso, J.-P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef] [Green Version]
- Hernando, M.; Carreto, J.I.; Carignan, M.O.; Ferreyra, G.A.; Gross, C. Effects of solar radiation on growth and mycosporine-like amino acids content in Thalassiosira sp., an Antarctic diatom. In Ecological Studies in the Antarctic Sea Ice Zone; Springer: Berlin/Heidelberg, Germany, 2002; pp. 237–245. [Google Scholar] [CrossRef]
- Bertram, C.; Hass, R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008, 389, 211–220. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koltover, V.K. Antioxidant biomedicine: From free radical chemistry to systems biology mechanisms. Russ. Chem. Bull. 2010, 59, 37–42. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H.; Sopun, W.; Tanaka, Y.; Takabe, T. Identification and Upregulation of Biosynthetic Genes Required for Accumulation of Mycosporine-2-Glycine under Salt Stress Conditions in the Halotolerant Cyanobacterium Aphanothece halophytica. Appl. Environ. Microbiol. 2014, 80, 1763–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P.; Jeyakumar, R.B.; Bajhaiya, A.K. Microalgae as a Source of Mycosporine-like Amino Acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The Genetic and Molecular Basis for Sunscreen Biosynthesis in Cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of Gene Cluster for Mycosporine-Like Amino Acid Biosynthesis from Actinomycetales Microorganisms and Production of a Novel Mycosporine-Like Amino Acid by Heterologous Expression. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, P.M.; Woodhouse, J.N.; Liew, H.T.; Sehnal, L.; Pickford, R.; Wong, H.L.; Burns, B.P.; Neilan, B.A. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Env. Microbiol. 2019, 21, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl. Microbiol. Biotechnol. 2012, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Katoch, M.; Mazmouz, R.; Chau, R.; Pearson, L.A.; Pickford, R.; Neilan, B.A. Heterologous Production of Cyanobacterial Mycosporine-Like Amino Acids Mycosporine-Ornithine and Mycosporine-Lysine in Escherichia coli. Appl. Environ. Microbiol. 2016, 82, 6167–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milshteyn, A.; Schneider, J.S.; Brady, S.F. Mining the Metabiome: Identifying Novel Natural Products from Microbial Communities. Chem. Biol. 2014, 21, 1211–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and Preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Tewari, D.; Atanasov, A.G.; Semwal, P.; Wang, D. Natural products and their applications. Curr. Res. Biotechnol. 2021, 3, 82–83. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37. [Google Scholar]
- Gallo, C.; Barra, G.; Saponaro, M.; Manzo, E.; Fioretto, L.; Ziaco, M.; Nuzzo, G.; D’Ippolito, G.; Palma, R.; Fontana, A. A New Bioassay Platform Design for the Discovery of Small Molecules with Anticancer Immunotherapeutic Activity. Mar. Drugs 2020, 18, 604. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [Green Version]
- Rosic, N.N. DNA Shuffling of Cytochromes P450 for Indigoid Pigment Production. In Cytochrome P450 Protocols; Humana Press: Totowa, NJ, USA, 2013; Volume 987, pp. 205–224. [Google Scholar] [CrossRef]
- Gregori-Puigjané, E.; Setola, V.; Hert, J.; Crews, B.A.; Irwin, J.J.; Lounkine, E.; Marnett, L.; Roth, B.L.; Shoichet, B.K. Identifying mechanism-of-action targets for drugs and probes. Proc. Natl. Acad. Sci. USA 2012, 109, 11178–11183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumayer, E. Scarce or Abundant? The Economics of Natural Resource Availability. J. Econ. Surv. 2002, 14, 307–335. [Google Scholar] [CrossRef] [Green Version]
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampert, A. Over-exploitation of natural resources is followed by inevitable declines in economic growth and discount rate. Nat. Commun. 2019, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Rosic, N.; Bradbury, J.; Lee, M.; Baltrotsky, K.; Grace, S. The impact of pesticides on local waterways: A scoping review and method for identifying pesticides in local usage. Environ. Sci. Policy 2020, 106, 12–21. [Google Scholar] [CrossRef]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Kenshole, E.; Herisse, M.; Michael, M.; Pidot, S.J. Natural product discovery through microbial genome mining. Curr. Opin. Chem. Biol. 2020, 60, 47–54. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial genome mining for accelerated natural products discovery: Is a renaissance in the making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losantos, R.; Funes-Ardoiz, I.; Aguilera, J.; Herrera-Ceballos, E.; García-Iriepa, C.; Campos, P.J.; Sampedro, D. Rational Design and Synthesis of Efficient Sunscreens to Boost the Solar Protection Factor. Angew. Chem. Int. Ed. 2017, 56, 2632–2635. [Google Scholar] [CrossRef]
- Belknap, K.C.; Park, C.J.; Barth, B.M.; Andam, C.P. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 2020, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Pernice, M.; Dunn, S.; Dove, S.; Hoegh-Guldberg, O. Differential Regulation by Heat Stress of Novel Cytochrome P450 Genes from the Dinoflagellate Symbionts of Reef-Building Corals. Appl. Environ. Microbiol. 2010, 76, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- Rosic, N.N.; Leggat, W.; Kaniewska, P.; Dove, S.; Hoegh-Guldberg, O. New-old hemoglobin-like proteins of symbiotic dinoflagellates. Ecol. Evol. 2013, 3, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Pernice, M.; Dove, S.; Dunn, S.; Hoegh-Guldberg, O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: Possible implications for coral bleaching. Cell Stress Chaperon 2010, 16, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Rosic, N.N.; Pernice, M.; Rodriguez-Lanetty, M.; Hoegh-Guldberg, O. Validation of Housekeeping Genes for Gene Expression Studies in Symbiodinium Exposed to Thermal and Light Stress. Mar. Biotechnol. 2010, 13, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Příhoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Durcak, J.; Wurst, M.; Kotowski, J.; Chang, D.; et al. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 2019, 47, e110. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Versatile capacity of shuffled cytochrome P450s for dye production. Appl. Microbiol. Biotechnol. 2009, 82, 203–210. [Google Scholar] [CrossRef]
- Rosic, N.N.; Huang, W.; Johnston, W.A.; DeVoss, J.J.; Gillam, E.M. Extending the diversity of cytochrome P450 enzymes by DNA family shuffling. Gene 2007, 395, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 2018, 6, 7640128. [Google Scholar] [CrossRef]
- Kopp, J.; Slouka, C.; Spadiut, O.; Herwig, C. The Rocky Road from Fed-Batch to Continuous Processing With E. coli. Front. Bioeng. Biotechnol. 2019, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [Green Version]
- Porro, D.; Sauer, M.; Branduardi, P.; Mattanovich, D. Recombinant Protein Production in Yeasts. Mol. Biotechnol. 2005, 31, 245–260. [Google Scholar] [CrossRef]
- Chen, M.; Rubin, G.M.; Jiang, G.; Raad, Z.; Ding, Y. Biosynthesis and Heterologous Production of Mycosporine-Like Amino Acid Palythines. J. Org. Chem. 2021, 86, 11160–11168. [Google Scholar] [CrossRef]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes—A review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaniewska, P.; Chan, C.-K.K.; Kline, D.; Ling, E.Y.S.; Rosic, N.; Edwards, D.; Hoegh-Guldberg, O.; Dove, S. Transcriptomic Changes in Coral Holobionts Provide Insights into Physiological Challenges of Future Climate and Ocean Change. PLoS ONE 2015, 10, e0139223. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.; Kaniewska, P.; Chan, C.-K.K.; Ling, E.Y.S.; Edwards, D.; Dove, S.; Hoegh-Guldberg, O. Early transcriptional changes in the reef-building coral Acropora aspera in response to thermal and nutrient stress. BMC Genom. 2014, 15, 1052. [Google Scholar] [CrossRef] [Green Version]
- Sharpton, T.J. An introduction to the analysis of shotgun metagenomic data. Front. Plant Sci. 2014, 5, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voolstra, C.R.; Miller, D.J.; A Ragan, M.; Hoffmann, A.; Hoegh-Guldberg, O.; Bourne, D.; Ball, E.; Ying, H.; Foret, S.; Takahashi, S.; et al. The ReFuGe 2020 Consortium—using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front. Mar. Sci. 2015, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.N.; Sorout, N.; Cameron, A.J.; Stavrinides, J. The Integration of Genome Mining, Comparative Genomics, and Functional Genetics for Biosynthetic Gene Cluster Identification. Front. Genet. 2020, 11, 600116. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Larralde, M.; Fleck, J.S.; Ponnudurai, R.; Milanese, A.; Cappio, E.; Zeller, G. Accurate de novo identification of biosynthetic gene clusters with GECCO. bioRxiv 2021. [Google Scholar] [CrossRef]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Agarwala, R.; Bolton, E.E.; Brister, J.R.; Canese, K.; Clark, K.; Connor, R.; Fiorini, N.; Funk, K.; Hefferon, T.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2010, 39, D23–D28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, G.; Karsch-Mizrachi, I.; Takagi, T.; Sequence Database Collaboration, I.N. The international nucleotide sequence database collaboration. Nucleic Acids Res. 2018, 46, D48–D50. [Google Scholar]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Medema, M.H.; Kazempour, D.; Fischbach, M.A.; Breitling, R.; Takano, E.; Weber, T. antiSMASH 2.0—A versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013, 41, W204–W212. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Suarez Duran, H.G.; de los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. antiSMASH 4.0—improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. AntiSMASH 3.0-A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; van Hijum, S.A.; Bijlsma, J.J.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006, 34, W273–W279. [Google Scholar] [CrossRef]
- De Jong, A.; van Heel, A.J.; Kok, J.; Kuipers, O.P. BAGEL2: Mining for bacteriocins in genomic data. Nucleic Acids Res. 2010, 38, W647–W651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heel, A.J.; de Jong, A.; Montalban-Lopez, M.; Kok, J.; Kuipers, O.P. BAGEL3: Automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef]
- Van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef] [PubMed]
- Skinnider, M.; DeJong, C.A.; Rees, P.N.; Johnston, C.W.; Li, H.; Webster, A.L.H.; Wyatt, M.A.; Magarvey, N.A. Genomes to natural products PRediction Informatics for Secondary Metabolomes (PRISM). Nucleic Acids Res. 2015, 43, 9645–9662. [Google Scholar] [CrossRef] [Green Version]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res. 2017, 45, W49–W54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat. Commun. 2020, 11, 6058. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Rausch, C.; Lopez, P.; Hoof, I.; Gaykova, V.; Huson, D.; Wohlleben, W. CLUSEAN: A computer-based framework for the automated analysis of bacterial secondary metabolite biosynthetic gene clusters. J. Biotechnol. 2009, 140, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Khater, S.; Gupta, M.; Sain, N.; Mohanty, D. RiPPMiner: A bioinformatics resource for deciphering chemical structures of RiPPs based on prediction of cleavage and cross-links. Nucleic Acids Res. 2017, 45, W80–W88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Aberturas, J.; Chandra, G.; Frattaruolo, L.; Lacret, R.; Pham, T.H.; Vior, N.M.; Eyles, T.H.; Truman, A.W. Uncovering the unexplored diversity of thioamidated ribosomal peptides in Actinobacteria using the RiPPER genome mining tool. Nucleic Acids Res. 2019, 47, 4624–4637. [Google Scholar] [CrossRef]
- Moffat, A.D.; Santos-Aberturas, J.; Chandra, G.; Truman, A.W. A User Guide for the Identification of New RiPP Biosynthetic Gene Clusters Using a RiPPER-Based Workflow. In Antimicrobial Therapies: Methods and Protocols; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2021; p. 227. [Google Scholar]
- Tietz, J.I.; Schwalen, C.J.; Patel, P.S.; Maxson, T.; Blair, P.M.; Tai, H.-C.; Zakai, U.I.; Mitchell, D.A. A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat. Chem. Biol. 2017, 13, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Suarez Duran, H.G.; Blin, K.; Osbourn, A.; Medema, M.H. plantiSMASH: Automated identification, annotation and expression analysis of plant biosynthetic gene clusters. Nucleic Acids Res. 2017, 45, W55–W63. [Google Scholar] [CrossRef] [Green Version]
- Töpfer, N.; Fuchs, L.-M.; Aharoni, A. The PhytoClust tool for metabolic gene clusters discovery in plant genomes. Nucleic Acids Res. 2017, 45, 7049–7063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Brown, L.C.W.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, B.-J. Hidden Markov Models and their Applications in Biological Sequence Analysis. Curr. Genom. 2009, 10, 402–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.H.; Philmus, B.; Ziemert, N. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucleic Acids Res. 2017, 45, W42–W48. [Google Scholar] [CrossRef] [PubMed]

| MAA Name (Molecular Formula; Mw) | Chemical Structure | Key Features (ʎmax)/Bioactive Properties [Reference] |
|---|---|---|
| 4-deoxygadusol (C8H12O5; 188 g/mol) |  | Direct MAA precursor UV-absorbing property (268 nm) |
| Mycosporine-glycine (C10H15NO6; 245 g/mol) |  | UV-absorbing property (310 nm) Antioxidative [17,18,19,20] Anti-inflammatory [18] Antiaging [18] |
| Shinorine (C13H20N2O8; 332 g/mol) |  | UV-absorbing property (333 nm) Antioxidative [17,19,21] Anti-inflammatory [18,22] Antiaging [18] Anti-adipogenic [23] |
| Porphyra-334 (C14H22N2O8; 346 g/mol) |  | UV-absorbing property (334 nm) Antioxidative [17,19,21] Anti-inflammatory [22] Antiaging [24] Anti-adipogenic [23] |
| Mycosporine-2-glycine (C12H18N2O7; 302 g/mol) |  | UV-absorbing property (332 nm) Antioxidative [19,25,26] Anti-inflammatory [26] Antiaging [26] |
| Palythine (C13H20N2O5; 284 g/mol) |  | UV-absorbing property (320 nm) Antioxidative [25,27] Anti-proliferative [28] Antiaging [29] |
| Software Name (Key Features) | Website Availability | Application and Improvements | Reference |
|---|---|---|---|
| AntiSMASH (ANTIbiotics & Secondary Metabolite Analysis Shell; BCGs discovery in bacteria and fungi genome sequences) | Bacteria: antiSMASH bacterial version (secondarymetabolites.org/, accessed on 12 June 2022) Fungi: antiSMASH fungal version (secondarymetabolites.org, accessed on 12 June 2022) | Release of software | [95] |
| Improved versions (2–5) | [96,97,98,99] | ||
| The latest version (6) with improved BCGs detection | [100] | ||
| BAGEL (Automated identification of genes encoding ribosomally synthesised and post-translationally modified peptides -RiPPs) | Bacteria: http://bagel.molgenrug.nl http://bagel4.molgenrug.nl/, accessed on 12 June 2022 | Release of software | [101] |
| BAGEL2 | [102] | ||
| BAGEL3 | [103] | ||
| BAGEL4 | [104] | ||
| PRISM (PRediction Informatics for Secondary Metabolomes; prediction of chemical structures of NPs) | Microbe genomes: http://magarveylab.ca/prism/, accessed on 12 June 2022 | Release of software | [105] |
| PRISM 3 | [106] | ||
| PRISM 4 | [107] | ||
| CLUSEAN (CLUster SEquence Analyzer; bacterial secondary metabolite BCGs, automated analyses; Bioperl-based annotation pipeline) | Bacteria: https://bitbucket.org/tilmweber/clusean, accessed on 12 June 2022 | Release of software | [108] |
| RiPPMiner (Automated Prediction of BGCs and Crosslinked Chemical Structures of -RiPPs) | http://www.nii.ac.in/rippminer.html, accessed on 12 June 2022 | Release of software | [109] |
| RiPPER (for detection of BGCs of– RiPPs) | Actinobacteria: streptomyces/ripdock—Docker Image|Docker Hub | Release of software (Specific application for thioamidated ribosomal peptides) Updated version | [110] [111] |
| RODEO (Rapid ORF Description and Evaluation Online; for detection of BGCs for RiPPs) | http://www.ripp.rodeo/, accessed on 12 June 2022 | Release of tool (AntiSMASH combined with Pfam * domain prediction) | [112] |
| BiG-SCAPE (The Biosynthetic Gene Similarity Clustering and Prospecting Engine; build sequence similarity report for new BGCs; using metabolomic data) | Multigenomes: BiG-SCAPE CORASON|July 2018 (secondarymetabolites.org, accessed on 12 June 2022) | Release of software | [113] |
| plantiSMASH (antiSMASH for plant genomes) | Plant genomes: http://plantismash.secondarymetabolites.org, accessed on 12 June 2022 | Release of tool | [114] |
| PhytoClust (discovery of Metabolic Gene Clusters (MGCs) in plant genomes) | http://phytoclust.weizmann.ac.il/, accessed on 12 June 2022 | Release of tool | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosic, N. Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid. Mar. Drugs 2022, 20, 478. https://doi.org/10.3390/md20080478
Rosic N. Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid. Marine Drugs. 2022; 20(8):478. https://doi.org/10.3390/md20080478
Chicago/Turabian StyleRosic, Nedeljka. 2022. "Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid" Marine Drugs 20, no. 8: 478. https://doi.org/10.3390/md20080478
APA StyleRosic, N. (2022). Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid. Marine Drugs, 20(8), 478. https://doi.org/10.3390/md20080478






