Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges †
Abstract
:1. Introduction
2. Health Impact of Current Treatments for Sleep and Depressed Mood
3. Therapeutic Potential of Microalgae in Sleep and Mood Regulation
3.1. Whole Microalgal Biomass
3.2. β-Phenylethylamine (PEA)
3.3. Apigenin
3.4. Ferulic Acid
3.5. Quercetin
3.6. Hesperidin
3.7. Fucosterol
3.8. Carotenoids
3.9. Omega-3 and Other Polyunsaturated Fatty Acids (PUFAs)
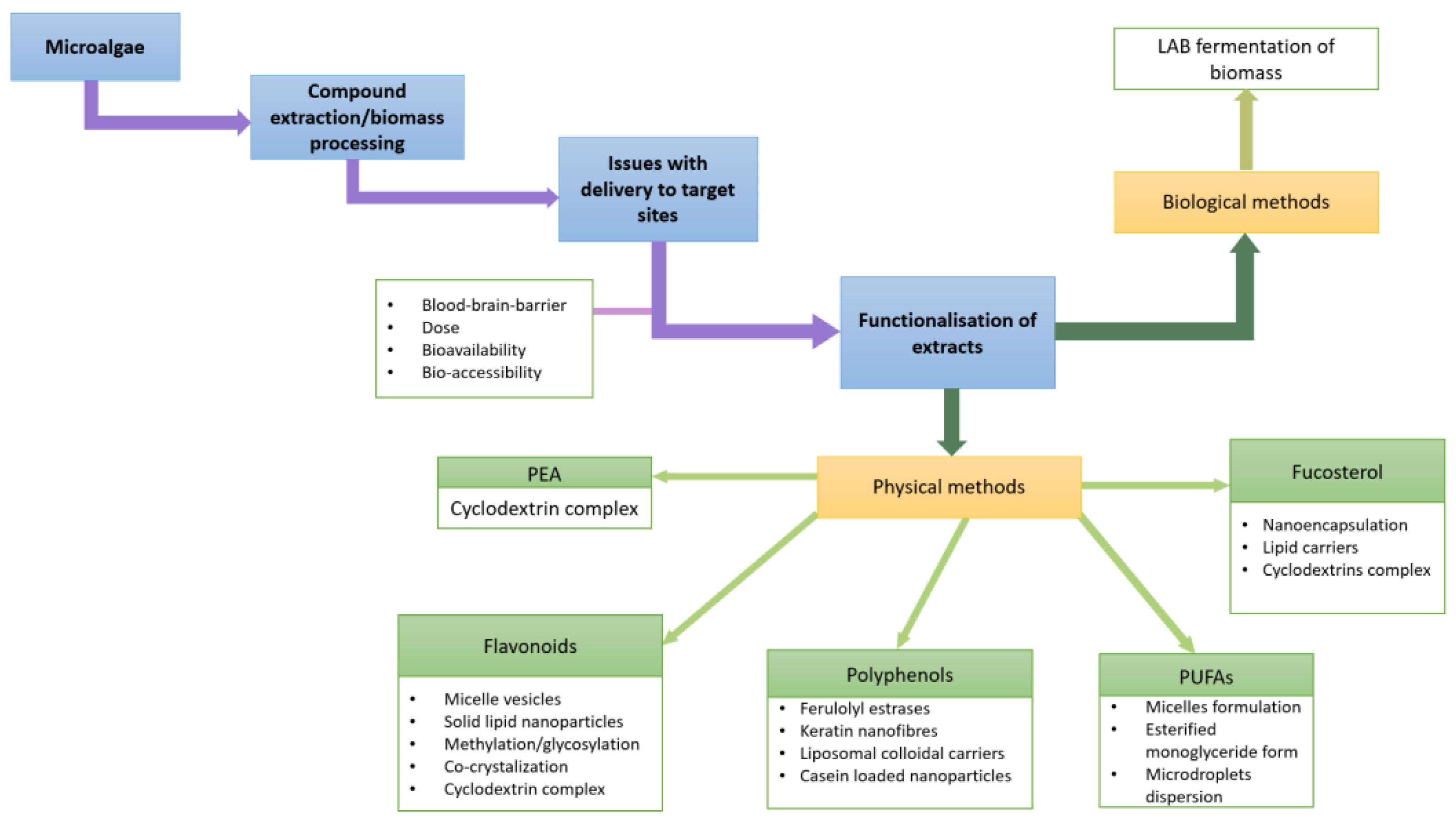
4. Bioavailability and Delivery of Microalgae Extracts to the Target Sites
4.1. β-Phenylethylamine (PEA)
4.2. Flavonoids
Strategies for Enhanced Delivery of Flavonoids
4.3. Polyphenols
Functionalisation of Polyphenols for Improved Absorption
4.4. Fucosterol
Novel Approaches for Phytosterol Delivery
4.5. Omega-3 PUFAs
Transformations of Omega-3 PUFAs for Increased Bioavailability
4.6. Fermentation as a Biological Functionalization of Microalgal Biomass
5. Methodology
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bhaskar, S.; Hemavathy, D.; Prasad, S. Prevalence of Chronic Insomnia in Adult Patients and Its Correlation with Medical Comorbidities. J. Fam. Med. Prim. Care 2016, 5, 780. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.; Manzar, M.; Kumary, S.; Burman, D.; Spence, D.; Pandi-Perumal, S. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, R.J.; Sheehan, C.M.; Nguyen, A.W.; Johnson, D. COVID-19 Hardships and Self-Reported Sleep Quality among American Adults in March and April 2020: Results from a Nationally Representative Panel Study. Sleep Health 2022, 8, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.A.; Santos, L.P.; Manosso, L.M.; Quadra, M.R.; Meller, F.O. Relationship between Sleep Duration and Quality and Mental Health before and during COVID-19 Pandemic: Results of Population-Based Studies in Brazil. J. Psychosom. Res. 2022, 158, 110910. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, V.F.; Kim, J.R.; Grillakis, A.A.; Taylor, M.R.; York, C.M. Insomnia in the Military: Application and Effectiveness of Cognitive and Pharmacologic Therapies. Curr. Psychiatry Rep. 2015, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.T.; Yang, H.-J.; Lee, G.T.; Nguyen, L.T.K.; Kuo, S.-Y. Relationships of Excessive Internet Use with Depression, Anxiety, and Sleep Quality among High School Students in Northern Vietnam. J. Pediatr. Nurs. 2022, 62, e91–e97. [Google Scholar] [CrossRef]
- Carney, C.E.; Harris, A.L.; Falco, A.; Edinger, J.D. The Relation between Insomnia Symptoms, Mood, and Rumination about Insomnia Symptoms. J. Clin. Sleep Med. 2013, 9, 567–575. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M. Short- and Long-Term Health Consequences of Sleep Disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.J.; Webb, T.L.; Martyn-St James, M.; Rowse, G.; Weich, S. Improving Sleep Quality Leads to Better Mental Health: A Meta-Analysis of Randomised Controlled Trials. Sleep Med. Rev. 2021, 60, 101556. [Google Scholar] [CrossRef] [PubMed]
- Bröer, C.; Besseling, B. Sadness or Depression: Making Sense of Low Mood and the Medicalization of Everyday Life. Soc. Sci. Med. 2017, 183, 28–36. [Google Scholar] [CrossRef]
- Radwan, B.; Jansen, G.; Chaudhury, D. Sleep-Wake Dynamics Pre- and Post-Exposure to Chronic Social Stress. iScience 2021, 24, 103204. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xie, Y.; Zou, X. Association between Sleep Duration and Depression in US Adults: A Cross-Sectional Study. J. Affect. Disord. 2022, 296, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as Versatile Cellular Factories for Valued Products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Barreira, L.; Pereira, H.; Gangadhar, K.N.; Custódio, L.; Varela, J. Medicinal Effects of Microalgae-Derived Fatty Acids. In Handbook of Marine Microalgae; Kim, S.K., Ed.; Elsevier: London, UK, 2015; pp. 209–231. [Google Scholar] [CrossRef]
- Bollu, P.C.; Kaur, H. Sleep Medicine: Insomnia and Sleep. Mo. Med. 2019, 116, 68–75. [Google Scholar]
- Aljarallah, S.; Al-Hussain, F. Acute Fatal Posthypoxic Leukoencephalopathy Following Benzodiazepine Overdose: A Case Report and Review of the Literature. BMC Neurol. 2015, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Afzal, A.; Kiyatkin, E.A. Interactions of Benzodiazepines with Heroin: Respiratory Depression, Temperature Effects, and Behavior. Neuropharmacology 2019, 158, 107677. [Google Scholar] [CrossRef]
- Brett, J.; Murnion, B. Management of Benzodiazepine Misuse and Dependence. Aust. Prescr. 2015, 38, 152–155. [Google Scholar] [CrossRef] [Green Version]
- Maust, D.T.; Lin, L.A.; Blow, F.C. Benzodiazepine Use and Misuse Among Adults in the United States. Psychiatr. Serv. 2019, 70, 97–106. [Google Scholar] [CrossRef]
- Bi, K.; Chen, S. Sleep Profiles as a Longitudinal Predictor for Depression Magnitude and Variability Following the Onset of COVID-19. J. Psychiatr. Res. 2022, 147, 159–165. [Google Scholar] [CrossRef]
- Ferguson, J.M. SSRI Antidepressant Medications. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ringdahl, E.N.; Pereira, S.L.; Delzell, J.E. Treatment of Primary Insomnia. J. Am. Board Fam. Med. 2004, 17, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfarth, T.D.; van Zwieten, B.J.; Lekkerkerker, F.J.; Gispen-de Wied, C.C.; Ruis, J.R.; Elferink, A.J.A.; Storosum, J.G. Antidepressants Use in Children and Adolescents and the Risk of Suicide. Eur. Neuropsychopharmacol. 2006, 16, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Liebert, R.; Gavey, N. “There Are Always Two Sides to These Things”: Managing the Dilemma of Serious Adverse Effects from SSRIs. Soc. Sci. Med. 2009, 68, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, S.; Pick, C.G. Trazodone and Mirtazapine: A Possible Opioid Involvement in Their Use (at Low Dose) for Sleep? Med. Hypotheses 2020, 136, 109501. [Google Scholar] [CrossRef]
- Fluyau, D.; Cook, S.C.; Chima, A.; Kailasam, V.K.; Revadigar, N. Pharmacological Management of Psychoactive Substance Withdrawal Syndrome. Drugs Ther. Perspect. 2021, 37, 519–535. [Google Scholar] [CrossRef]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohsawa, I.; Konishi, F.; Hasegawa, T.; Kumamoto, S.; Suzuki, Y.; Ohta, S. Preventive Effects of Chlorella on Cognitive Decline in Age-Dependent Dementia Model Mice. Neurosci. Lett. 2009, 464, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Voss, L.J.; Plouviez, M.; Whittle, N. Microalgae-Based Photosynthetic Strategy for Oxygenating Avascularised Mouse Brain Tissue—An in Vitro Proof of Concept Study. Brain Res. 2021, 1768, 147585. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yasuda, K.; Murata, A.; Suzuki, K.; Miura, N. Effects of Euglena Gracilis Intake on Mood and Autonomic Activity under Mental Workload, and Subjective Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 3243. [Google Scholar] [CrossRef]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M. Effects of the Aphanizomenon Flos-Aquae Extract (Klamin®) on a Neurodegeneration Cellular Model. Oxid. Med. Cell. Longev. 2018, 2018, 9089016. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.; De Cooman, L. Detection of Flavonoids in Microalgae from Different Evolutionary Lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The Role of Natural Polyphenols in Cell Signaling and Cytoprotection against Cancer Development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; Oulad El Majdoub, Y.; Kounnoun, A.; Miceli, N.; Fernanda Taviano, M.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [Green Version]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting Neuroinflammation by Polyphenols: A Promising Therapeutic Approach against Inflammation-Associated Depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel Application of Brain-Targeting Polyphenol Compounds in Sleep Deprivation-Induced Cognitive Dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellahcen, T.O.; AAmiri, A.; Touam, I.; Hmimid, F.; Amrani, A.E.; Cherif, A.; Cherki, M. Evaluation of Moroccan Microalgae: Spirulina Platensis as a Potential Source of Natural Antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, L.; Tong, Y.; Li, Y.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Xiao, D.; Wen, K.; et al. The Antidepressant Effects of Hesperidin on Chronic Unpredictable Mild Stress-Induced Mice. Eur. J. Pharmacol. 2019, 853, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V. Hesperidin Alleviates Chronic Restraint Stress and Lipopolysaccharide-Induced Hippocampus and Frontal Cortex Damage in Mice: Role of TLR4/NF-ΚB, P38 MAPK/JNK, Nrf2/ARE Signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef] [PubMed]
- Machado Sierra, E.; Serrano, M.C.; Manares, A.; Guerra, A.; Aranguren Díaz, Y. Microalgae: Potential for Bioeconomy in Food Systems. Appl. Sci. 2021, 11, 11316. [Google Scholar] [CrossRef]
- De Medeiros, V.P.B.; de Souza, E.L.; de Albuquerque, T.M.R.; da Costa Sassi, C.F.; dos Santos Lima, M.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater Microalgae Biomasses Exert a Prebiotic Effect on Human Colonic Microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health Benefit of Fucosterol from Marine Algae: A Review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- Zhen, X.-H.; Quan, Y.-C.; Jiang, H.-Y.; Wen, Z.-S.; Qu, Y.-L.; Guan, L.-P. Fucosterol, a Sterol Extracted from Sargassum Fusiforme, Shows Antidepressant and Anticonvulsant Effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of Marine Algae: Insights into the Potential Health Benefits and Molecular Pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Corrigendum: Edible Seaweed-Derived Constituents: An Undisclosed Source of Neuroprotective Compounds. Neural Regen. Res. 2021, 16, 2564–2568. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Rabinovitz, S.; Mostofsk, D.I. Essential Fatty Acids and Sleep: Mini-Review and Hypothesis. Med. Hypotheses 1998, 50, 139–145. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Muylaert, K.; Foubert, I. Microalgae as an Alternative Source of Omega-3 Long Chain Polyunsaturated Fatty Acids. Lipid Technol. 2012, 24, 128–130. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Mayyas, F.; Abu Zamzam, H.I. Omega-3 Fatty Acids Protects against Chronic Sleep-Deprivation Induced Memory Impairment. Life Sci. 2019, 227, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as Sources of Omega-3 Polyunsaturated Fatty Acids: Biotechnological Aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Reigada, L.C.; Buchanan, E.M.; Hazeltine, D.B.; Shakil, H.; Polokowski, A.R. A Pilot Randomized Controlled Trial Testing Supplements of Omega-3 Fatty Acids, Probiotics, Combination or Placebo on Symptoms of Depression, Anxiety and Stress. J. Affect. Disord. Rep. 2021, 5, 100141. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena Gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.-K. Stress, the Autonomic Nervous System, and the Immune-Kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Sabelli, H. Phenylethylamine Deficit and Replacement in Depressive Illness. In Naural Medications for Psychiatric Disorders; Mischoulon, D., Rosenbaum, J., Eds.; Lippencott Williams and Wilkins: Baltimore, MD, USA, 2002; pp. 83–110. [Google Scholar]
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-Phenylethylamine, a Small Molecule with a Large Impact. Webmedcentral 2013, 4, 4409. [Google Scholar]
- Borah, A.; Paul, R.; Mazumder, M.K.; Bhattacharjee, N. Contribution of β-Phenethylamine, a Component of Chocolate and Wine, to Dopaminergic Neurodegeneration: Implications for the Pathogenesis of Parkinson’s Disease. Neurosci. Bull. 2013, 29, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in Marine Algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajakumar, P.D. Psychoactive Properties of Microalgae. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–334. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Chierchia, E.; Lanzoni, C.; Santagni, S.; Veltri, F.; Ricchieri, F.; Rattighieri, E.; Nappi, R.E. Effects of Klamath Algae Extract on Psychological Disorders and Depression in Menopausal Women: A Pilot Study. Minerva Ginecol. 2010, 62, 381–388. [Google Scholar] [PubMed]
- Cremonte, M.; Sisti, D.; Maraucci, I.; Giribone, S.; Colombo, E.; Rocchi, M.B.L.; Scoglio, S. The Effect of Experimental Supplementation with the Klamath Algae Extract Klamin on Attention-Deficit/Hyperactivity Disorder. J. Med. Food 2017, 20, 1233–1239. [Google Scholar] [CrossRef]
- Sabelli, H.; Fink, P.; Fawcett, J.; Tom, C. Sustained Antidepressant Effect of PEA Replacement. J. Neuropsychiatry Clin. Neurosci. 1996, 8, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.A.; O’reilly, R.L.; Placatka, C.L.; Paterson, I.A.; Yu, P.H.; Durden, D.A. Effect of Dietary Phenylalanine on the Plasma Concentrations of Phenylalanine, Phenylethylamine and Phenylacetic Acid in Healthy Volunteers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1991, 15, 611–623. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Sui, Y.; Vlaeminck, S.E. Dunaliella Microalgae for Nutritional Protein: An Undervalued Asset. Trends Biotechnol. 2020, 38, 10–12. [Google Scholar] [CrossRef]
- Eltanahy, E.; Torky, A. CHAPTER 1. Microalgae as Cell Factories: Food and Feed-Grade High-Value Metabolites. In Microalgal Biotechnology; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–35. [Google Scholar] [CrossRef]
- Araújo, A.C.M.F.; Araújo, W.M.C.; Marquez, U.M.L.; Akutsu, R.; Nakano, E.Y. Table of Phenylalanine Content of Foods: Comparative Analysis of Data Compiled in Food Composition Tables. JIMD Rep. 2017, 34, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Lupatini Menegotto, A.L.; de Souza, L.E.S.; Colla, L.M.; Costa, J.A.V.; Sehn, E.; Bittencourt, P.R.S.; de Moraes Flores, É.L.; Canan, C.; Colla, E. Investigation of Techno-Functional and Physicochemical Properties of Spirulina Platensis Protein Concentrate for Food Enrichment. LWT 2019, 114, 108267. [Google Scholar] [CrossRef]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate Concentration-Shift Cultivation to Enhance Protein Content of Heterotrophic Microalga Chlorella Vulgaris: Over-Compensation Strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Silva, J.; Shao, A.S.; Liang, J.; Wallner, M.; Shao, X.M.; Li, M.; Olsen, R.W. Flavonoid Compounds Isolated from Tibetan Herbs, Binding to GABAA Receptor with Anxiolytic Property. J. Ethnopharmacol. 2021, 267, 113630. [Google Scholar] [CrossRef] [PubMed]
- Alsadat, A.M.; Nikbakht, F.; Hossein Nia, H.; Golab, F.; Khadem, Y.; Barati, M.; Vazifekhah, S. GSK-3β as a Target for Apigenin-Induced Neuroprotection against Aβ 25–35 in a Rat Model of Alzheimer’s Disease. Neuropeptides 2021, 90, 102200. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, H.A.; Weinshenker, D. Good Night and Good Luck: Norepinephrine in Sleep Pharmacology. Biochem. Pharmacol. 2010, 79, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Gazola, A.C.; Costa, G.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; de Lima, T.C.M.; Schenkel, E.P. Involvement of GABAergic Pathway in the Sedative Activity of Apigenin, the Main Flavonoid from Passiflora Quadrangularis Pericarp. Rev. Bras. Farmacogn. 2015, 25, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to Enhance the Production of Photosynthetic Pigments and Lipids in Chlorophycae Species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Gutiérrez-del-Río, I.; Yagüe, P.; Manteca, Á.; Villar, C.J.; Lombó, F. De Novo Biosynthesis of Apigenin, Luteolin, and Eriodictyol in the Actinomycete Streptomyces Albus and Production Improvement by Feeding and Spore Conditioning. Front. Microbiol. 2017, 8, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassier-Chauvat, C.; Veaudor, T.; Chauvat, F. Comparative Genomics of DNA Recombination and Repair in Cyanobacteria: Biotechnological Implications. Front. Microbiol. 2016, 7, 1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victor, M.M.; David, J.M.; Sakukuma, M.C.K.; França, E.L.; Nunes, A.V.J. A Simple and Efficient Process for the Extraction of Naringin from Grapefruit Peel Waste. Green Process. Synth. 2018, 7, 524–529. [Google Scholar] [CrossRef]
- Żyszka, B.; Anioł, M.; Lipok, J. Modulation of the Growth and Metabolic Response of Cyanobacteria by the Multifaceted Activity of Naringenin. PLoS ONE 2017, 12, e0177631. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Leite, I.B.; Martins, A.B.N.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba Berry: A Comprehensive Review on Its Polyphenol Composition, Health Effects, Metabolism, and the Development of Food Products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Yang, H.-J. The Release and Catabolism of Ferulic Acid in Plant Cell Wall by Rumen Microbes: A Review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential Applications of Ferulic Acid from Natural Sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic Acid Inhibits Neuro-Inflammation in Mice Exposed to Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Shao, T.; Ruan, L.; Wang, L.; Sun, J.; Li, J.; Zhu, X.; O’Donnell, J.M.; Pan, J. Ferulic Acid Increases Pain Threshold and Ameliorates Depression-like Behaviors in Reserpine-Treated Mice: Behavioral and Neurobiological Analyses. Metab. Brain Dis. 2013, 28, 571–583. [Google Scholar] [CrossRef]
- Brighenti, E.; Casagrande, K.; Cardoso, P.Z.; Pasa, M.; Ciotta, M.N.; Brighenti, A.F. Total Polyphenols Contents in Different Grapevine Varieties in Highlands of Southern Brazil. BIO Web Conf. 2017, 9, 01024. [Google Scholar] [CrossRef] [Green Version]
- Strejckova, A.; Dvorak, M.; Klejdus, B.; Krystofova, O.; Hedbavny, J.; Adam, V.; Huska, D. The Strong Reaction of Simple Phenolic Acids during Oxidative Stress Caused by Nickel, Cadmium and Copper in the Microalga Scenedesmus Quadricauda. New Biotechnol. 2019, 48, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Betterle, N.; Hidalgo Martinez, D.; Melis, A. Recombinant Protein Stability in Cyanobacteria. ACS Synth. Biol. 2021, 10, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Gmur, D.; Kochanowicz, E.; Górka, K.; Skrzypek, T. Inhibitory Effect of a Combination of Baicalein and Quercetin Flavonoids against Candida Albicans Strains Isolated from the Female Reproductive System. Fungal Biol. 2022, 126, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, N.; He, F.; Duan, C. Reactivity Comparison of Three Malvidin-Type Anthocyanins Forming Derived Pigments in Model Wine Solutions. Food Chem. 2022, 384, 132534. [Google Scholar] [CrossRef] [PubMed]
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning from Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nakamura, T.; Kikuchi, H.; Sasaki, T.; Yamamoto, Y. Co-Variation of Depressive Mood and Locomotor Dynamics Evaluated by Ecological Momentary Assessment in Healthy Humans. PLoS ONE 2013, 8, e74979. [Google Scholar] [CrossRef] [Green Version]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Sun, X.; Yan, Y.; Yuan, Q.; Wang, J.; Shen, X. Metabolic Engineering of Microorganisms for the Production of Flavonoids. Front. Bioeng. Biotechnol. 2020, 8, 589069. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Bayav, İ.; Genç, A.; Kandemir, Ö. Neuromodulatory Effects of Hesperidin against Sodium Fluoride-Induced Neurotoxicity in Rats: Involvement of Neuroinflammation, Endoplasmic Reticulum Stress, Apoptosis and Autophagy. Neurotoxicology 2022, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bandyopadhyay, J.; Chakraborty, S.; Basu, S. Multi-Target Screening Mines Hesperidin as a Multi-Potent Inhibitor: Implication in Alzheimer’s Disease Therapeutics. Eur. J. Med. Chem. 2016, 121, 810–822. [Google Scholar] [CrossRef]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Guthrie, G.; Thijs, C.; Te Velde, A.A.; Vreugdenhil, A.C.E.; et al. Plant-Based Sterols and Stanols in Health & Disease: “Consequences of Human Development in a Plant-Based Environment?”. Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Valentão, P.; Andrade, P.B. Sterols in Algae and Health. In Bioactive Compounds from Marine Foods; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 173–191. [Google Scholar] [CrossRef]
- Bouzidi, N.; Viano, Y.; Ortalo-Magné, A.; Seridi, H.; Alliche, Z.; Daghbouche, Y.; Culioli, G.; El Hattab, M. Sterols from the Brown Alga Cystoseira Foeniculacea: Degradation of Fucosterol into Saringosterol Epimers. Arab. J. Chem. 2019, 12, 1474–1478. [Google Scholar] [CrossRef] [Green Version]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Yang, T.; Sun, J.; Zhang, D. Associations between Dietary Carotenoid Intakes and the Risk of Depressive Symptoms. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Moskalev, A.; Shaposhnikov, M.; Zemskaya, N.; Belyi, A.; Dobrovolskaya, E.; Patova, A.; Guvatova, Z.; Lukyanova, E.; Snezhkina, A.; Kudryavtseva, A. Transcriptome analysis reveals mechanisms of geroprotective effects of fucoxanthin in Drosophila. BMC Genom. 2018, 19, 77. [Google Scholar] [CrossRef] [Green Version]
- Culver, M.; Bowman, J.; Juturu, V. Lutein and Zeaxanthin Isomers Effect on Sleep Quality: A Randomized Placebo-Controlled Trial. Biomed. J. Sci. Tech. Res. 2018, 9, 7018–7024. [Google Scholar] [CrossRef] [Green Version]
- Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Suárez-Montenegro, Z.J.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Study of the potential neuroprotective effect of Dunaliella salina extract in SH-SY5Y cell model. Anal. Bioanal. Chem. 2021, 414, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzajew, J.; Radzik, T.; Rebas, E. Calcium-Involved Action of Phytochemicals: Carotenoids and Monoterpenes in the Brain. Int. J. Mol. Sci. 2020, 21, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.J.; Vishwanathan, R.; Scott, T.M.; Schalch, W.; Wittwer, J.; Hausman, D.B.; Davey, A.; Johnson, M.A.; Green, R.C.; Gearing, M.; et al. Serum carotenoids as a biomarker for carotenoid concentrations in the brain. FASEB J. 2011, 25, 344.2. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from Marine Microalgae: A Promising Bioactive Compound for Industrial Production and Food Application. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Petit, H.V.; Dewhurst, R.J.; Scollan, N.D.; Proulx, J.G.; Khalid, M.; Haresign, W.; Twagiramungu, H.; Mann, G.E. Milk Production and Composition, Ovarian Function, and Prostaglandin Secretion of Dairy Cows Fed Omega-3 Fats. J. Dairy Sci. 2002, 85, 889–899. [Google Scholar] [CrossRef]
- Fagioli, I.; Baroncini, P.; Ricour, C.; Salzarulo, P. Decrease of Slow-Wave Sleep in Children with Prolonged Absence of Essential Lipids Intake. Sleep 1989, 12, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Rabinovtz, S.; Carasso, R.L.; Mostofsky, D.I. Essential Fatty Acids Preparation (Sr-3) Improves Alzheimer’s Patients Quality of Life. Int. J. Neurosci. 1996, 87, 141–149. [Google Scholar] [CrossRef]
- Malikova, N.A.; Krzhechkovskaia, V.V.; Marokko, I.N.; Mazo, V.K. [The Effect of Fatty Compounds with Different Ratios of the Polyunsaturated Fatty Acids of the Omega-3 and Omega-6 Families on the Expression of Food-Related Anaphylaxis, on the Liver Cytochrome P-450 System and on 17-Hydroxycorticosteroid Metabolism in Guinea Pigs]. Vopr. Pitan. 1995, 4, 13–16. [Google Scholar]
- Wang, T.; Niu, K.; Fan, A.; Bi, N.; Tao, H.; Chen, X.-T.; Wang, H.-L. Dietary Intake of Polyunsaturated Fatty Acids Alleviates Cognition Deficits and Depression-like Behaviour via Cannabinoid System in Sleep Deprivation Rats. Behav. Brain Res. 2020, 384, 112545. [Google Scholar] [CrossRef]
- Lotrich, F.E.; Sears, B.; McNamara, R.K. Polyunsaturated Fatty Acids Moderate the Effect of Poor Sleep on Depression Risk. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangard, L.; Sadeghi, A.; Ahmadpanah, M.; Holsboer-Trachsler, E.; Sadeghi Bahmani, D.; Haghighi, M.; Brand, S. Influence of Adjuvant Omega-3-Polyunsaturated Fatty Acids on Depression, Sleep, and Emotion Regulation among Outpatients with Major Depressive Disorders—Results from a Double-Blind, Randomized and Placebo-Controlled Clinical Trial. J. Psychiatr. Res. 2018, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M.; Malik, I.; Babay, S. Dietary Omega-3 Polyunsaturated Fatty Acid Supplementation in an Animal Model of Anxiety. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 17–20. [Google Scholar] [CrossRef]
- Charles, C.N.; Msagati, T.; Swai, H.; Chacha, M. Microalgae: An Alternative Natural Source of Bioavailable Omega-3 DHA for Promotion of Mental Health in East Africa. Sci. Afr. 2019, 6, e00187. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 Fatty Acids and Mental Health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2017, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Prather, A.A.; Vogelzangs, N.; Penninx, B.W. Sleep duration, insomnia, and markers of systemic inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA). J. Psychiatr. Res. 2014, 60, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Lacerda-Pinheiro, S.F.; Junior, R.F.F.P.; de Lima, M.A.P.; da Silva, C.G.L.; dos Santos, M.D.S.V.; Júnior, A.G.T.; de Oliveira, P.N.L.; Ribeiro, K.D.B.; Rolim-Neto, M.L.; Bianco, B.A.V. Are there depression and anxiety genetic markers and mutations? A systematic review. J. Affect. Disord. 2014, 168, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The Effects of Different Extraction Methods of Lipids from Nannochloropsis Oceanica on the Contents of Omega-3 Fatty Acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum Tricornutum Microalgae as a Rich Source of Omega-3 Oil: Progress in Lipid Induction Techniques towards Industry Adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Magoni, C.; Bertacchi, S.; Giustra, C.M.; Guzzetti, L.; Cozza, R.; Ferrari, M.; Torelli, A.; Marieschi, M.; Porro, D.; Branduardi, P.; et al. Could Microalgae Be a Strategic Choice for Responding to the Demand for Omega-3 Fatty Acids? A European Perspective. Trends Food Sci. Technol. 2022, 121, 142–155. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of Compounds That Cross the Blood-Brain Barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef] [Green Version]
- Mosnaim, A.D.; Callaghan, O.H.; Hudzik, T.; Wolf, M.E. Rat Brain-Uptake Index for Phenylethylamine and Various Monomethylated Derivatives. Neurochem. Res. 2013, 38, 842–846. [Google Scholar] [CrossRef]
- Distinto, S.; Meleddu, R.; Yanez, M.; Cirilli, R.; Bianco, G.; Sanna, M.L.; Arridu, A.; Cossu, P.; Cottiglia, F.; Faggi, C.; et al. Drug Design, Synthesis, in Vitro and in Silico Evaluation of Selective Monoaminoxidase B Inhibitors Based on 3-Acetyl-2-Dichlorophenyl-5-Aryl-2,3-Dihydro-1,3,4-Oxadiazole Chemical Scaffold. Eur. J. Med. Chem. 2016, 108, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin–Phenylethylamine Inclusion Complex: A Ternary System for Photothermal Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 15177–15188. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of Food Matrix on the Content and Bioavailability of Flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical Delivery through Nanocarriers: A Review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef] [PubMed]
- Trakooncharoenvit, A.; Tanaka, S.; Mizuta, E.; Hira, T.; Hara, H. Water-Soluble Dietary Fibers Enhance Bioavailability of Quercetin and a Fiber Derived from Soybean Is Most Effective after Long-Term Feeding in Rats. Eur. J. Nutr. 2020, 59, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Combination of Lipids and Emulsifiers Enhances the Absorption of Orally Administered Quercetin in Rats. J. Agric. Food Chem. 2002, 50, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Chee, W.S.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.-N.; Williamson, G. Bioavailability Is Improved by Enzymatic Modification of the Citrus Flavonoid Hesperidin in Humans: A Randomized, Double-Blind, Crossover Trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-Brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, D.; Biswasroy, P.; Kar, B.; Ghosh, G.; Rath, G. Recent Advances in Colloidal Technology for the Improved Bioavailability of the Nutraceuticals. J. Drug Deliv. Sci. Technol. 2021, 65, 102693. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.-A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The Bioavailability of Ferulic Acid Is Governed Primarily by the Food Matrix Rather than Its Metabolism in Intestine and Liver in Rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Swamy, S.K.P.; Govindaswamy, V. Therapeutical Properties of Ferulic Acid and Bioavailability Enhancement through Feruloyl Esterase. J. Funct. Foods 2015, 17, 657–666. [Google Scholar] [CrossRef]
- Babazadeh, A.; Mohammadi Vahed, F.; Jafari, S.M. Nanocarrier-Mediated Brain Delivery of Bioactives for Treatment/Prevention of Neurodegenerative Diseases. J. Control. Release 2020, 321, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential Metabolic Modulators in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jafari, S.M.; Hamishehkar, H.; Ghanbarzadeh, B. Phytosterols as the Core or Stabilizing Agent in Different Nanocarriers. Trends Food Sci. Technol. 2020, 101, 73–88. [Google Scholar] [CrossRef]
- Méndez, L.; Medina, I. Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects. Molecules 2021, 26, 2438. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. New Insights into the Molecular Mechanism of Intestinal Fatty Acid Absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, L.; Vachon, A.; Plourde, M.D.S. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J. Nutr. 2021, 151, 1111–1118. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R. Strategies to Improve Bioavailability of Omega-3 Fatty Acids from Ethyl Ester Concentrates. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic Acid Fermentation of Arthrospira Platensis (Spirulina) Biomass for Probiotic-Based Products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira Platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
| Effect | Microalgal Biomass/Bioactive Compound | Microalgae | Reference |
|---|---|---|---|
| Neuroprotection | Whole biomass | Arthrospira platensis | [29] |
| Prevention of cellular oxidative damage | |||
| Decreased inflammation | |||
| Neuroprotection | Whole biomass | Chlorella | [30] |
| Prevention of cellular oxidative damage | |||
| Inflammation lowering activity | |||
| Oxygen generation in CNS | Cultures | Chlamydomonas reinhardtii | [31] |
| Shorter sleep latency | Whole biomass | Euglena gracilis | [32] |
| Mood improvement | |||
| Ability to wind down | |||
| Calming of the sympathetic nervous system | |||
| Improvement in social interaction, focus and concentration | |||
| Sleep cycle regulation | β-Phenylethylamine | Aphanizomenon flos-aquae (Klamin® extract) | [33] |
| Anti-depressive effect | |||
| Neuron regeneration | |||
| Antioxidative and anti-inflammatory action | |||
| Norepinephrine elevating action | Apigenin | Arthrospira platensis | [34,35] |
| Chlorella vulgaris | |||
| Anti-depressive effect | Diacronema lutheri | ||
| Haematococcus lacustris | |||
| Sleep cycle regulation | Leptolyngbya sp. | ||
| Phaeodactylum tricornutum | |||
| Sedative effect | Porphyridium purpureum | ||
| Tetraselmis suecica | |||
| Mood regulation | Ferulic acid | Arthrospira sp., | [34,36,37,38,39,40] |
| Chlorella sp. | |||
| Anti-depressive action | Desmodesmus sp. | ||
| Diacronema sp. | |||
| Stress lowering activity | Dunaliella salina | ||
| Haematococcus sp. | |||
| Anti-inflammatory action | Nannochloropsis sp. | ||
| Phaeodactylum sp. | |||
| Increased norepinephrine concentration | Porphyridium sp. | ||
| Tetraselmis sp. | |||
| Improvement in cognitive function, locomotor activity and mood regulation | Quercetin | Arthrospira platensis | [34,35,40,41,42] |
| Calothrix brevissima | |||
| Diacronemalutheri | |||
| Haematococcus pluvialis | |||
| Hapalosiphon fontinalis | |||
| Limnothrix obliqueacuminata | |||
| Microchaete tenera | |||
| Nostoc ellipsosporum | |||
| Phormidium tenue | |||
| Porphyridiumpurpureum | |||
| Scenedesmus quadricauda | |||
| Westiellopsis prolifica | |||
| Anti-depressive action | Hesperidin | Arthrospira platensis | [40,43,44,45,46] |
| Anabaena sp. | |||
| Reduced inflammation and anxiety | Chlorella vulgaris | ||
| Chlamydomonas sp. | |||
| Improvement memory and learning ability | Chlorococcum hypnosporum | ||
| Tolypothrix sp. | |||
| Anti-depressive, neuro-modulatory and neuroprotective action | Fucosterol | Chrysoderma sp. | [47,48,49,50,51,52,53] |
| Chrysomeris sp. | |||
| Anti-inflammatory and anticholinergic action | Chrysowaernella sp. | ||
| Ecklonia cava subsp. stolonifera extract | |||
| Elevated serotonin and norepinephrine concentrations | Isochrysis galbana | ||
| Nannochloropsis sp. BR2 | |||
| Improvement of cognitive function | Olisthodiscus luteus | ||
| Diacronema lutheri | |||
| Promotion of cell longevity | Phaeodactylumtricornutum | ||
| Tetraselmis sp. M8 | |||
| Correct functioning of membranes | PUFAs including omega-3 fatty acids | Cryptomonas sp. | [15,54,55,56,57,58] |
| Improvement in synaptic activity | Dunaliella sp. | ||
| Neuroprotective action | Nephroselmis sp. | ||
| Decreased neuroinflammation | Rhodomonas sp. | ||
| Sleep promoting action | Tetraselmis viridis | ||
| Improvement of cognitive ability | |||
| Mood regulation | |||
| Anti-depressive action | |||
| Anxiety lowering effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarthy, B.; O’Neill, G.; Abu-Ghannam, N. Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges. Mar. Drugs 2022, 20, 493. https://doi.org/10.3390/md20080493
McCarthy B, O’Neill G, Abu-Ghannam N. Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges. Marine Drugs. 2022; 20(8):493. https://doi.org/10.3390/md20080493
Chicago/Turabian StyleMcCarthy, Bozena, Graham O’Neill, and Nissreen Abu-Ghannam. 2022. "Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges" Marine Drugs 20, no. 8: 493. https://doi.org/10.3390/md20080493
APA StyleMcCarthy, B., O’Neill, G., & Abu-Ghannam, N. (2022). Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges. Marine Drugs, 20(8), 493. https://doi.org/10.3390/md20080493







