Single-Disulfide Conopeptide Czon1107, an Allosteric Antagonist of the Human α3β4 Nicotinic Acetylcholine Receptor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of α-conotoxin Czon1107 and Analogues
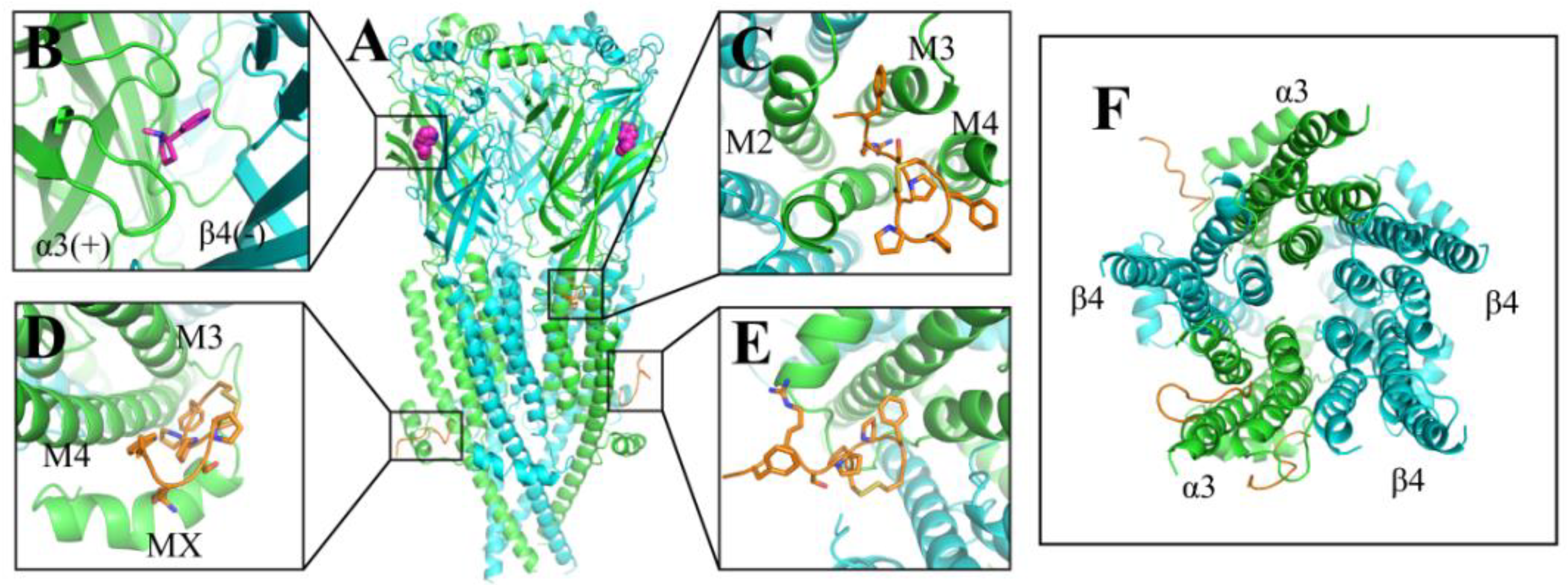
2.2. Molecular Dynamics (MD) Simulations of hα3β4 nAChR Bound with Czon1107
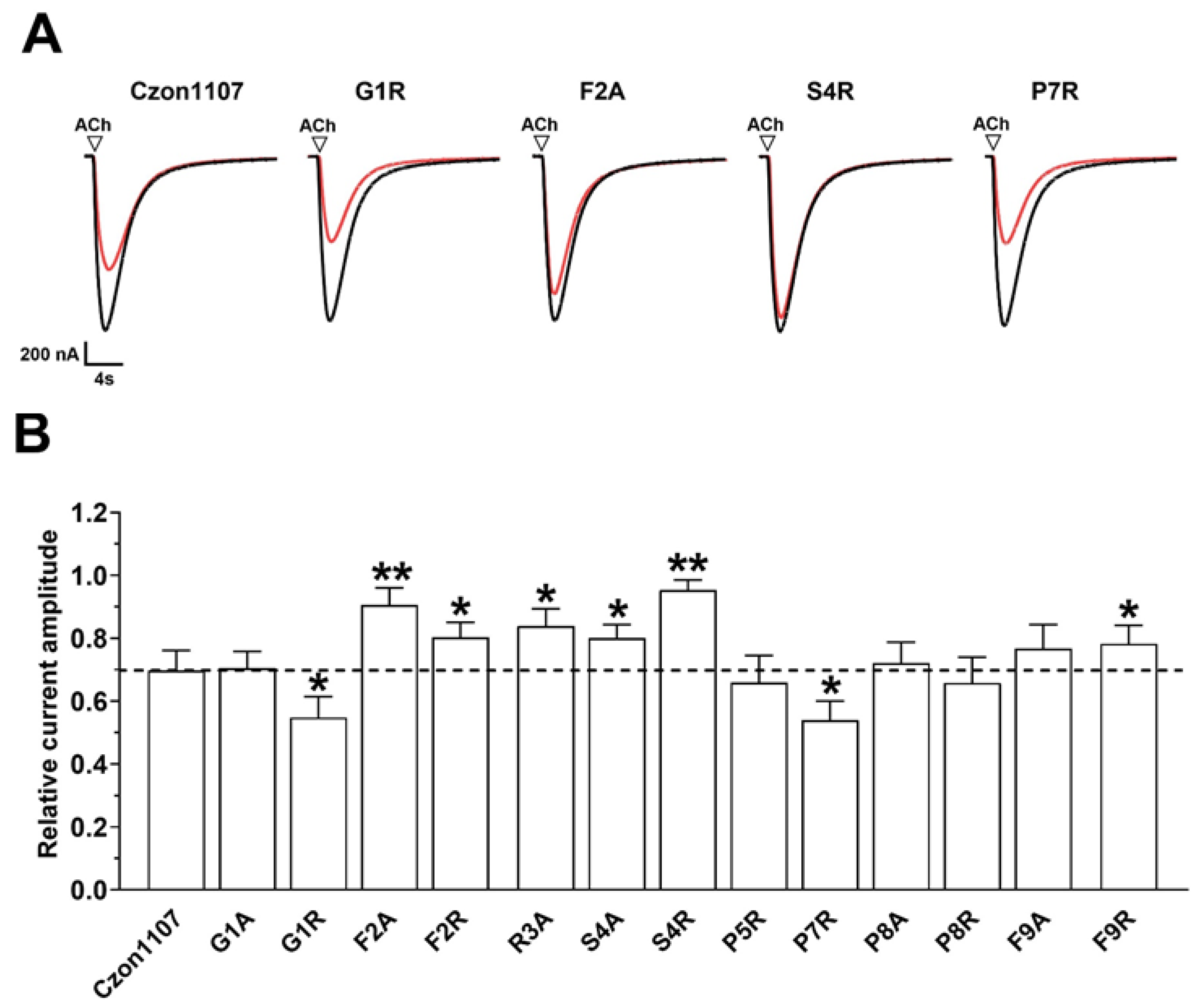
2.3. Activity of Czon1107 Analogues at hα3β4 nAChRs
3. Materials and Methods
3.1. Synthesis of α-Conotoxin Czon1107 and Its Analogues
3.2. Xenopus laevis Oocyte Preparation and Microinjection
3.3. Two-Electrode Voltage Clamp Recording of Oocytes and Data Analysis
3.4. Docking and Molecular Dynamics (MD) Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, S.; Sudweeks, S.; Yakel, J.L. Nicotinic receptors in the brain: Correlating physiology with function. Trends Neurosci. 1999, 22, 555–561. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [Green Version]
- Corringer, P.J.; Le Novère, N.; Changeux, J.P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. Nicotinic receptors as therapeutic targets for drug addictive disorders. CNS Neurol. Disord.-Drug Targets 2013, 12, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.R.; Sweeney, A.; Topf, M.; Millar, N.S. Stoichiometry-selective antagonism of α4β2 nicotinic acetylcholine receptors by fluoroquinolone antibiotics. ACS Chem. Neurosci. 2022, 13, 1805–1817. [Google Scholar] [CrossRef]
- Kalamida, D.; Poulas, K.; Avramopoulou, V.; Fostieri, E.; Lagoumintzis, G.; Lazaridis, K.; Sideri, A.; Zouridakis, M.; Tzartos, S.J. Muscle and neuronal nicotinic acetylcholine receptors: Structure, function and pathogenicity. FEBS J. 2007, 274, 3799–3845. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ren, J.; Li, R.; Li, X.; Zhangsun, D.; Wu, Y.; Luo, S. Synthesis and evaluation of disulfide-rich cyclic α-conotoxin [S9A] TxID analogues as novel α3β4 nAChR antagonists. Bioorganic Chem. 2021, 112, 104875. [Google Scholar] [CrossRef]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [Green Version]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis, and structure activity relationships of conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Tosti, E.; Boni, R.; Gallo, A. Pathophysiological responses to conotoxin modulation of voltage-gated ion currents. Mar. Drugs 2022, 20, 282. [Google Scholar] [CrossRef]
- Lewis, R.J. Conotoxins as selective inhibitors of neuronal ion channels, receptors and transporters. IUBMB Life 2004, 56, 89–93. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Armishaw, C.J.; Colgrave, M.L.; Wahlstrom, M.E.; Alewood, P.F.; Daly, N.L.; Craik, D.J. Cyclic MrIA: A stable and potent cyclic conotoxin with a novel topological fold that targets the norepinephrine transporter. J. Med. Chem. 2006, 49, 6561–6568. [Google Scholar] [CrossRef]
- Ramírez, D.; Gonzalez, W.; Fissore, R.A.; Carvacho, I. Conotoxins as tools to understand the physiological function of voltage-gated calcium (CaV) channels. Mar. Drugs 2017, 15, 313. [Google Scholar] [CrossRef] [Green Version]
- Halai, R.; Craik, D.J. Conotoxins: Natural product drug leads. Nat. Prod. Rep. 2009, 26, 526–536. [Google Scholar] [CrossRef]
- Woodward, S.R.; Cruz, L.J.; Olivera, B.M.; Hillyard, D.R. Constant and hypervariable regions in conotoxin propeptides. EMBO J. 1990, 9, 1015–1020. [Google Scholar] [CrossRef]
- Santos, A.D.; McIntosh, J.M.; Hillyard, D.R.; Cruz, L.J.; Olivera, B.M. The A-superfamily of conotoxins: Structural and functional divergence. J. Biol. Chem. 2004, 279, 17596–17606. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.S.; Steel, D.; Jacobsen, R.B.; Lirazan, M.B.; Cruz, L.J.; Hooper, D.; Shetty, R.; DelaCruz, R.C.; Nielsen, J.S.; Zhou, L.M.; et al. The T-superfamily of conotoxins. J. Biol. Chem. 1999, 274, 30664–30671. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, E.C.; Shetty, R.P.; Lirazan, M.; Rivier, J.; Walker, C.; Abogadie, F.C.; Yoshikami, D.; Cruz, L.J.; Olivera, B.M. Novel excitatory Conus peptides define a new conotoxin superfamily. J. Neurochem. 2003, 85, 610–621. [Google Scholar] [CrossRef]
- Sandall, D.W.; Satkunanathan, N.; Keays, D.A.; Polidano, M.A.; Liping, X.; Pham, V.; Down, J.G.; Khalil, Z.; Livett, B.G.; Gayler, K.R. A novel α-conotoxin identified by gene sequencing is active in suppressing the vascular response to selective stimulation of sensory nerves in vivo. Biochemistry 2003, 42, 6904–6911. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.; Haberlandt, C.; Gomez-Casati, M.E.; Watkins, M.; Elgoyhen, A.B.; McIntosh, J.M.; Olivera, B.M. α-RgIA: A novel conotoxin that specifically and potently blocks the α9α10 nAChR. Biochemistry 2006, 45, 1511–1517. [Google Scholar] [CrossRef]
- Grishin, A.A.; Wang, C.I.; Muttenthaler, M.; Alewood, P.F.; Lewis, R.J.; Adams, D.J. α-Conotoxin AuIB isomers exhibit distinct inhibitory mechanisms and differential sensitivity to stoichiometry of α3β4 nicotinic acetylcholine receptors. J. Biol. Chem. 2010, 285, 22254–22263. [Google Scholar] [CrossRef] [Green Version]
- Tae, H.S.; Gao, B.; Jin, A.H.; Alewood, P.F.; Adams, D.J. Globular and ribbon isomers of Conus geographus α-conotoxins antagonize human nicotinic acetylcholine receptors. Biochem. Pharmacol. 2021, 190, 114638. [Google Scholar] [CrossRef]
- Mohan, M.K.; Abraham, N.; Rajesh, R.P.; Jayaseelan, B.F.; Ragnarsson, L.; Lewis, R.J.; Sarma, S.P. Structure and allosteric activity of a single-disulfide conopeptide from Conus zonatus at human α3β4 and α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2020, 295, 7096–7112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Kompella, S.N.; Adams, D.J.; Craik, D.J.; Kaas, Q. Determination of the α-conotoxin Vc1. 1 binding site on the α9α10 nicotinic acetylcholine receptor. J. Med. Chem. 2013, 56, 3557–3567. [Google Scholar] [CrossRef]
- Kong, R.; Liu, R.R.; Xu, X.M.; Zhang, D.W.; Xu, X.S.; Shi, H.; Chang, S. Template-based modeling and ab-initio docking using CoDock in CAPRI. Proteins Struct. Funct. Bioinform. 2020, 88, 1100–1109. [Google Scholar] [CrossRef]
- Voronina, L.; Scutelnic, V.; Masellis, C.; Rizzo, T.R. Can mutational analysis be used to assist structure determination of peptides? J. Am. Chem. Soc. 2018, 140, 2401–2404. [Google Scholar] [CrossRef] [PubMed]
- Cuny, H.; Kompella, S.N.; Tae, H.S.; Yu, R.; Adams, D.J. Key Structural Determinants in the Agonist Binding Loops of Human beta2 and beta4 Nicotinic Acetylcholine Receptor Subunits Contribute to alpha3beta4 Subtype Selectivity of alpha-Conotoxins. J. Biol. Chem. 2016, 291, 23779–23792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, C.; Kasavajhala, K.; Belfon, K.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef]
- Grossfield, A.; Pitman, M.C.; Feller, S.E.; Soubias, O.; Gawrisch, K. Internal hydration increases during activation of the G-protein-coupled receptor rhodopsin. J. Mol. Biol. 2008, 381, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Dutton, J.L.; Bansal, P.S.; Hogg, R.C.; Adams, D.J.; Alewood, P.F.; Craik, D.J. A new level of conotoxin diversity, a non-native disulfide bond connectivity in α-conotoxin AuIB reduces structural definition but increases biological activity. J. Biol. Chem. 2002, 277, 48849–48857. [Google Scholar] [CrossRef] [Green Version]
- Kompella, S.N.; Hung, A.; Clark, R.J.; Mari, F.; Adams, D.J. Alanine scan of α-conotoxin RegIIA reveals a selective α3β4 nicotinic acetylcholine receptor antagonist. J. Biol. Chem. 2015, 290, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Zhangsun, D.; Harvey, P.J.; Kaas, Q.; Wu, Y.; Zhu, X.; Hu, Y.; Li, X.; Tsetlin, V.I.; Christensen, S.; et al. Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4026–E4035. [Google Scholar] [CrossRef] [Green Version]
- Ellison, M.; McIntosh, J.M.; Olivera, B.M. α-Conotoxins ImI and ImII. Similar α7 nicotinic receptor antagonists act at different sites. J. Biol. Chem. 2003, 278, 757–764. [Google Scholar] [CrossRef] [Green Version]


| No. | Name | Sequence |
|---|---|---|
| 1 | Czon1107 | GFRSPCPPFC# |
| 2 | Czon1107-G1A | AFRSPCPPFC# |
| 3 | Czon1107-F2A | GARSPCPPFC# |
| 4 | Czon1107-R3A | GFASPCPPFC# |
| 5 | Czon1107-S4A | GFRAPCPPFC# |
| 6 | Czon1107-P8A | GFRSPCPAFC# |
| 7 | Czon1107-F9A | GFRSPCPPAC# |
| 8 | Czon1107-G1R | RFRSPCPPFC# |
| 9 | Czon1107-F2R | GRRSPCPPFC# |
| 10 | Czon1107-S4R | GFRRPCPPFC# |
| 11 | Czon1107-P5R | GFRSRCPPFC# |
| 12 | Czon1107-P7R | GFRSPCRPFC# |
| 13 | Czon1107-P8R | GFRSPCPRFC# |
| 14 | Czon1107-F9R | GFRSPCPPRC# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Cao, Q.; Yang, M.; Gao, Y.; Fu, S.; Du, W.; Adams, D.J.; Jiang, T.; Tae, H.-S.; Yu, R. Single-Disulfide Conopeptide Czon1107, an Allosteric Antagonist of the Human α3β4 Nicotinic Acetylcholine Receptor. Mar. Drugs 2022, 20, 497. https://doi.org/10.3390/md20080497
Ma Y, Cao Q, Yang M, Gao Y, Fu S, Du W, Adams DJ, Jiang T, Tae H-S, Yu R. Single-Disulfide Conopeptide Czon1107, an Allosteric Antagonist of the Human α3β4 Nicotinic Acetylcholine Receptor. Marine Drugs. 2022; 20(8):497. https://doi.org/10.3390/md20080497
Chicago/Turabian StyleMa, Yuan, Qiushi Cao, Mengke Yang, Yue Gao, Shuiping Fu, Wenhao Du, David J. Adams, Tao Jiang, Han-Shen Tae, and Rilei Yu. 2022. "Single-Disulfide Conopeptide Czon1107, an Allosteric Antagonist of the Human α3β4 Nicotinic Acetylcholine Receptor" Marine Drugs 20, no. 8: 497. https://doi.org/10.3390/md20080497






