Fluorescently Labeled α-Conotoxin TxID, a New Probe for α3β4 Neuronal Nicotinic Acetylcholine Receptors
Abstract
:1. Introduction
2. Results
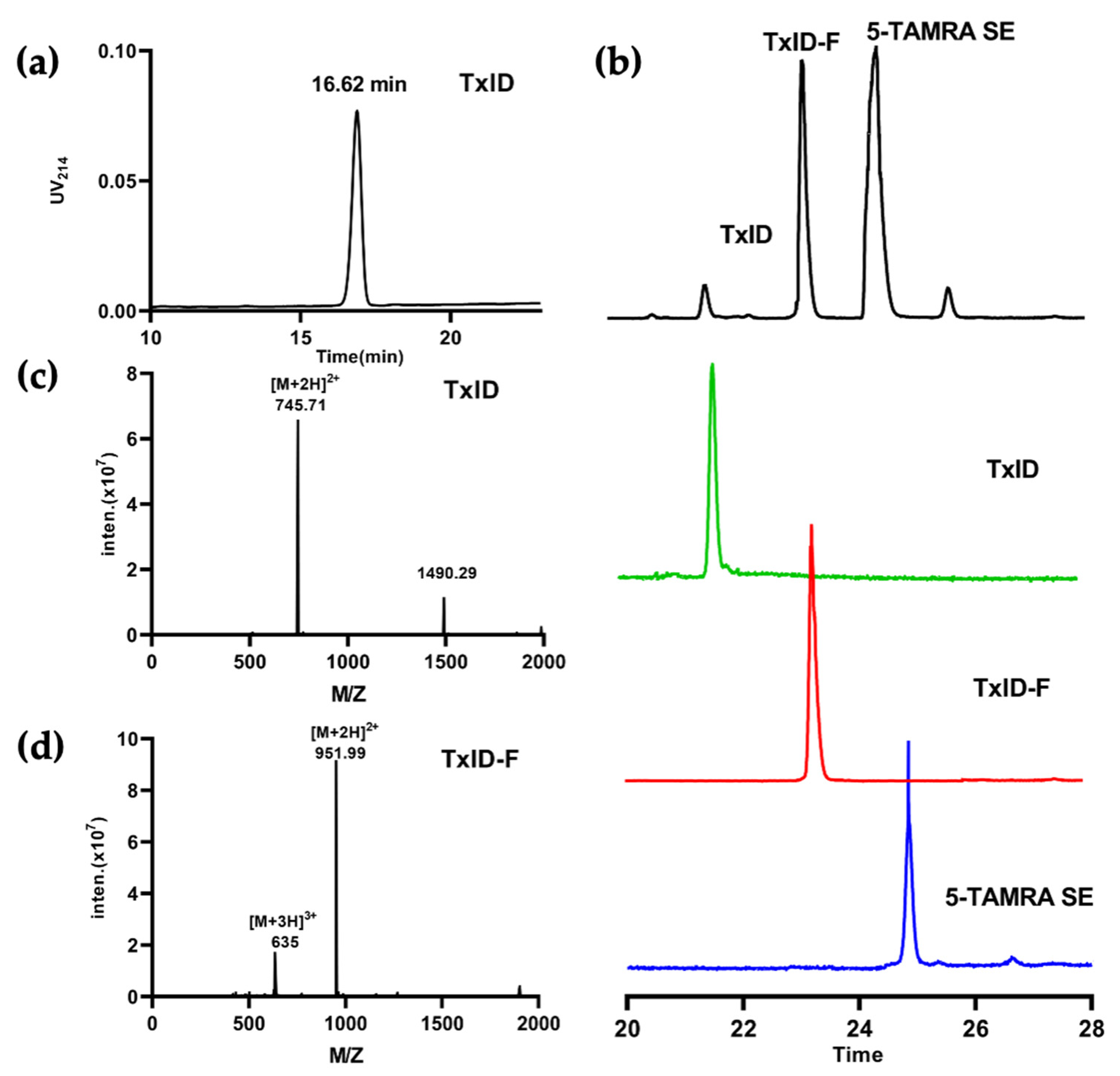
2.1. α-CTx TxID Linear Peptide Oxidative Folding
2.2. Fluorescence Spectrum and Circular Dichroism Analysis of TxID-F
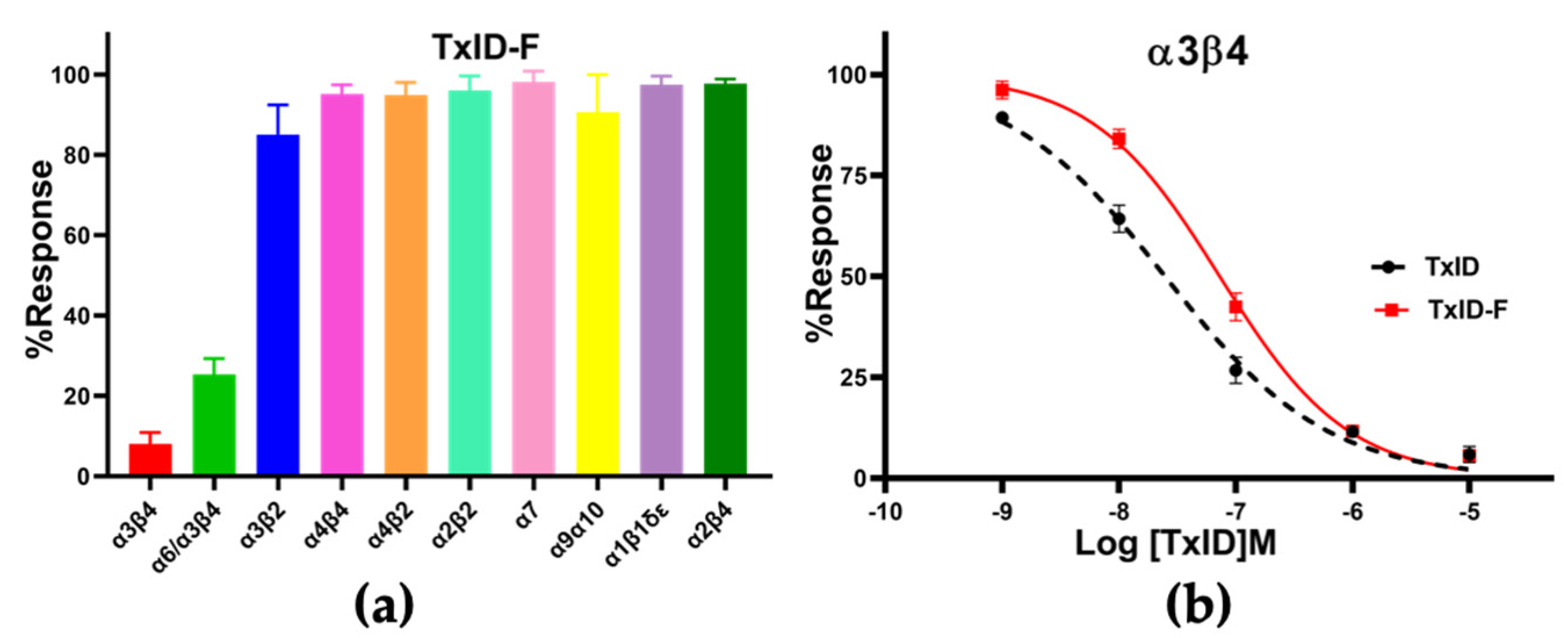
2.3. Pharmacological Activity of TxID-F
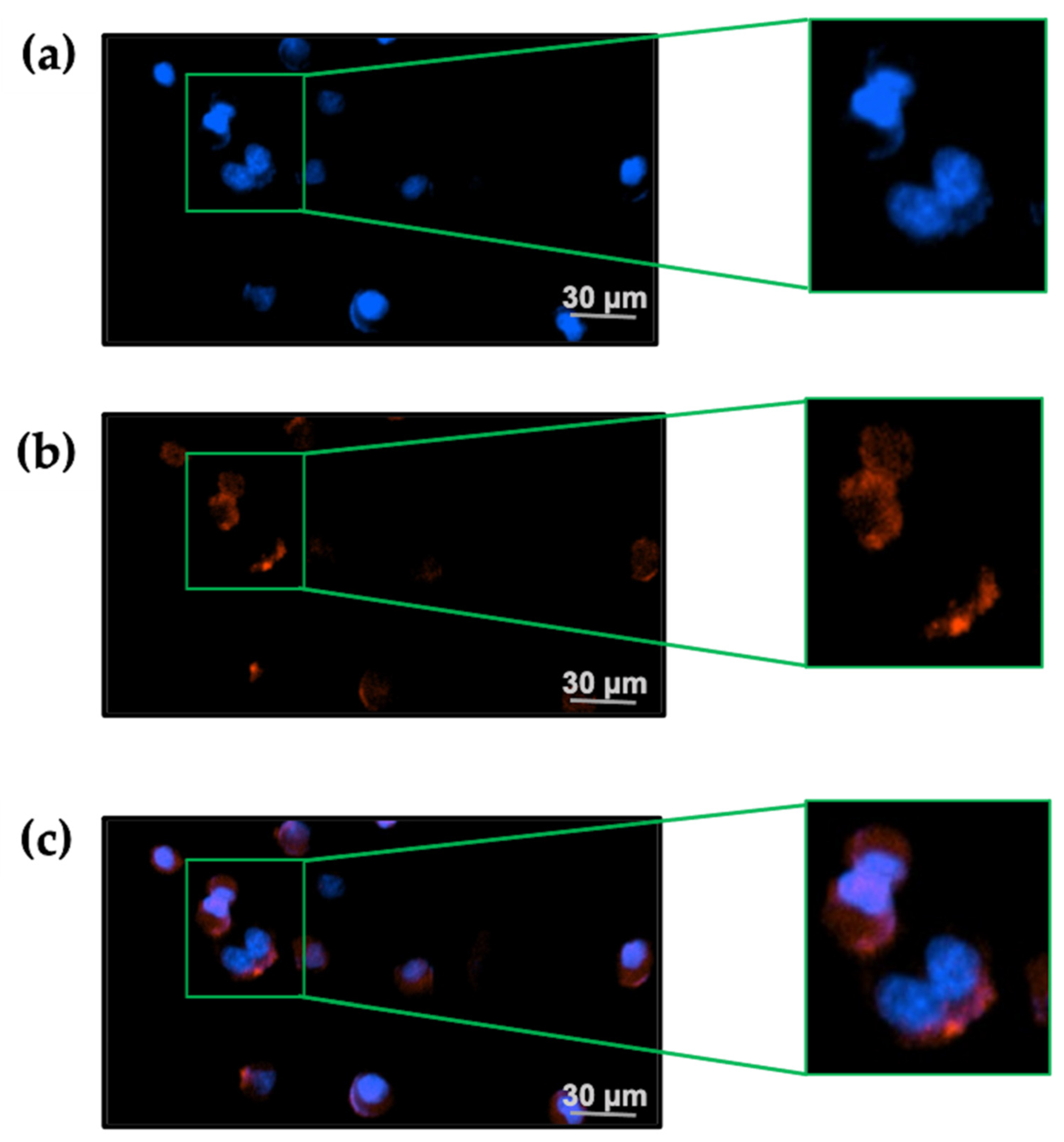
2.4. TxID-F Detection by the RAW264.7 Cell Line
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. TxID Linear Peptide Synthesis and Oxidative Folding
4.3. Exploration of the Method of Labeling TxID with 5-TAMRA SE
4.4. Circular Dichroism and Fluorescence Spectra
4.5. Activity Assay of the Fluorescent Analogue
4.6. Detection by Flow Cytometry
4.7. Fluorescence Imaging
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhilyakov, N.; Arkhipov, A.; Malomouzh, A.; Samigullin, D. Activation of Neuronal Nicotinic Receptors Inhibits Acetylcholine Release in the Neuromuscular Junction by Increasing Ca2+ Flux through Cav1 Channels. Int. J. Mol. Sci. 2021, 22, 9031. [Google Scholar] [CrossRef] [PubMed]
- Yoshikami, D.; Bagabaldo, Z.; Olivera, B.M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann. N. Y. Acad. Sci. 2010, 560, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, J.; Haufe, Y.; Evans, E.; Amar, M.; Dutertre, S. Backbone Cyclization Turns a Venom Peptide into a Stable and Equipotent Ligand at Both Muscle and Neuronal Nicotinic Receptors. J. Med. Chem. 2020, 63, 12682–12692. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, M.; Cai, R.; Ling, L.; Hackett, T.A.; Caspary, D.M. Nicotinic Receptor Subunit Distribution in Auditory Cortex: Impact of Aging on Receptor Number and Function. J. Neurosci. 2020, 40, 5724–5739. [Google Scholar] [CrossRef]
- Mashimo, M.; Moriwaki, Y.; Misawa, H.; Kawashima, K.; Fujii, T. Regulation of Immune Functions by Non-Neuronal Acetylcholine (ACh) via Muscarinic and Nicotinic ACh Receptors. Int. J. Mol. Sci. 2021, 22, 6818. [Google Scholar] [CrossRef] [PubMed]
- Sine, S.M.; Bren, N.; Quiram, P.A. Molecular dissection of subunit interfaces in the nicotinic acetylcholine receptor. J. Physiol. 1998, 92, 101–105. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Bonsi, P.; Centonze, D.; Gubellini, P.; Ca, L.P. Targeting striatal cholinergic interneurons in Parkinson’s disease: Focus on metabotropic glutamate receptors. Neuropharmacology 2003, 45, 45–56. [Google Scholar] [CrossRef]
- Becchetti, A.; Grandi, L.C.; Colombo, G.; Meneghini, S.; Amadeo, A. Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology. Brain Sci. 2020, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Deng, Y.X.; Gao, Y.M.; Dong, Y.T.; Wang, F.; Guan, Z.Z.; Hong, W.; Qi, X.L. Activation of α7 nAChR by PNU-282987 improves synaptic and cognitive functions through restoring the expression of synaptic-associated proteins and the CaM-CaMKII-CREB signaling pathway. Aging 2020, 12, 543–570. [Google Scholar] [CrossRef]
- Brunzell, D.H.; McIntosh, J.M. Alpha 7 Nicotinic Acetylcholine Receptors Modulate Motivation to Self-Administer Nicotine: Implications for Smoking and Schizophrenia. Neuropsychopharmacology 2012, 37, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Ma, X.L. α5-nAChR modulates nicotine-induced cell migration and invasion in A549 lung cancer cells. Exp. Toxicol. Pathol. 2015, 67, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Nordberg, A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000, 61, 75–111. [Google Scholar] [CrossRef]
- Moccia, F.; Frost, C.; Berra-Romani, R.; Tanzi, F.; Adams, D.J. Expression and function of neuronal nicotinic ach receptors in rat microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2019, 286, 486–491. [Google Scholar] [CrossRef] [PubMed]
- McCallum, S.E.; Cowe, M.A.; Lewis, S.W.; Glick, S.D. alpha3beta4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology 2012, 63, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.J.; Sanjakdar, S.S.; Muldoon, P.P.; McIntosh, J.M.; Damaj, M.I. The alpha3beta4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the alpha5 subunit in the mouse. Neuropharmacology 2013, 70, 228–235. [Google Scholar] [CrossRef]
- Salas, R.; Cook, K.D.; Bassetto, L.; De Biasi, M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology 2004, 47, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Eggan, B.L.; McCallum, S.E. alpha3beta4 nicotinic receptors in the medial habenula and substance P transmission in the interpeduncular nucleus modulate nicotine sensitization. Behav. Brain Res. 2017, 316, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; You, S.; Xiong, J.; Qiao, Y.M.; Luo, S.L. α-Conotoxin TxID and [S9K] TxID, α3β4 nAChR Antagonists, Attenuate Expression and Reinstatement of Nicotine-Induced Conditioned Place Preference in Mice. Mar. Drugs 2020, 18, 646. [Google Scholar] [CrossRef] [PubMed]
- Sharples, C.; Jones, I.W.; Millar, N.; Karig, G.; Gallagher, T.; Wonnacott, S. Characterisation of UB-165 analogues at the alpha3beta4 nicotinic acetylcholine receptor. Soc. Neurosci. Abstr. 2001, 27, 1276. [Google Scholar]
- Glick, S.D.; Sell, E.M.; McCallum, S.E.; Maisonneuve, I.M. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur. J. Pharmacol. 2011, 669, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef]
- Chang, Y.P.; Banerjee, J.; Dowell, C.; Wu, J.; Gyanda, R.; Houghten, R.A. Discovery of a potent and selective α3β4 nicotinic acetylcholine receptor antagonist from an α-conotoxin synthetic combinatorial library. J. Med. Chem. 2014, 57, 3511–3521. [Google Scholar] [CrossRef]
- Zheng, M.; Tae, H.S.; Xue, L.; Jiang, T.; Yu, R.L. Mechanism of interactions between α-conotoxin RegIIA and carbohydrates at the human α3β4 nicotinic acetylcholine receptor. Mar. Life Sci. Technol. 2021, 4, 98–105. [Google Scholar] [CrossRef]
- Guddat, L.W.; Martin, J.A.; Shan, L.; Edmundson, A.B.; Gray, W.R. Three-dimensional structure of the alpha-conotoxin GI at 1.2 A resolution. Biochemistry 1996, 35, 11329–11335. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhangsun, D.T.; Zhu, X.P.; Yong, W.; Hu, Y.Y.; Christensen, S. Characterization of a Novel Alpha-Conotoxin TxID from Conus textile that Potently Blocks rat Alpha3beta4 Nicotinic Acetylcholine Receptors. J. Med. Chem. 2013, 288, 894–902. [Google Scholar]
- Ulens, C.; Hogg, R.C.; Celie, P.H.; Bertrand, D.; Tsetlin, V.; Smit, A.B. Structural determinants of selective α-conotoxin binding to a nicotinic acetylcholine receptor homolog AChBP. Proc. Natl. Acad. Sci. USA 2006, 103, 3615–3620. [Google Scholar] [CrossRef]
- Vishwanath, V.A.; Mcintosh, J.M. Synthesis of fluorescent analogs of α-conotoxin MII. Bioconj. Chem. 2006, 17, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Tan, Y.; Zhangsun, D.T.; Zhu, X.P.; Luo, S.L. Design, Synthesis, and Activity of an α-Conotoxin LtIA Fluorescent Analogue. ACS Chem. Neurosci. 2021, 12, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Fllin, J.; Belov, V.N.; Kunetsky, R.; Medda, R.; Hell, S.W. Photochromic Rhodamines Provide Nanoscopy with Optical Sectioning. Angew. Chem. Int. Ed. Engl. 2010, 46, 6266–6270. [Google Scholar]
- Fisher, F.; Zhang, Y.; Vincent, P.F.; Gajewiak, J.; Gordon, T.J.; Glowatzki, E. Cy3-RgIA-5727 Labels and Inhibits α9-Containing nAChRs of Cochlear Hair Cells. Front. Cell. Neurosci. 2021, 15, 697560. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.H.; Olsen, S.; Bigelow, N.C.; Chen, J.C. Detection of fetal red cells in fetomaternal hemorrhage using a fetal hemoglobin monoclonal antibody by flow cytometry. Transfusion 2010, 38, 749–756. [Google Scholar] [CrossRef]
- Hone, A.J.; Whiteaker, P.; Christensen, S.; Xiao, Y.; Meyer, E.L.; Michaelmcintosh, J. A novel fluorescent α-conotoxin for the study of α7 nicotinic acetylcholine receptors. J. Neurochem. 2009, 111, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; Nevin, S.T.; Inserra, M.; Lewis, R.J.; Alewood, P.F. On-Resin Strategy to Label α-Conotoxins: Cy5-RgIA, a Potent α9α10 Nicotinic Acetylcholine Receptor Imaging Probe. Aust. J. Chem. 2020, 73, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chu, Z.L.; Shan, H.; Zhangsun, D.T.; Zhu, X.P. Inflammation Regulation via an Agonist and Antagonists of α7 Nicotinic Acetylcholine Receptors in RAW264.7 Macrophages. Mar. Drugs 2022, 20, 200. [Google Scholar] [CrossRef]
- Franco, A.; Heighinian, M.; Mejia, M.; Mccall, J.; Nag, S.; Akondi, K. Discovery, Characterization, and Functional Implications of Conotoxins from Cone Snails Species of the Americas. Toxicon 2012, 60, 148. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhangsun, D.T.; Schroeder, C.I.; Zhu, X.; Hu, Y.; Wu, Y. A novel α4/7-conotoxin LvIA from that selectively blocks α3β2 vs. α6/α3β2β3 nicotinic acetylcholine receptors. FASEB J. 2014, 28, 1842–1853. [Google Scholar] [CrossRef]
- Nicke, A.; Loughnan, M.L.; Millard, E.L.; Alewood, P.F.; Adams, D.J.; Daly, N.L.; Craik, D.J.; Lewis, R.J. Isolation, Structure, and Activity of GID, a Novel α4/7-Conotoxin with an Extended N-terminal Sequence. J. Biol. Chem. 2002, 278, 3137–3144. [Google Scholar] [CrossRef]
- Hone, A.J.; Whiteaker, P.; Mohn, J.L.; Jacob, M.H.; McIntosh, J.M. Alexa Fluor 546-ArIB [V11L;V16A] is a potent ligand for selectively labeling α7 nicotinic acetylcholine receptors. J. Neurochem. 2010, 114, 994–1006. [Google Scholar] [CrossRef]
- Sun, W.C.; Gee, K.R.; Klaubert, D.H.; Haugland, R.P. Synthesis of Fluorinated Fluoresceins. J. Org. Chem. 1997, 62, 6469–6475. [Google Scholar] [CrossRef]
- Jones, O.T.; Kunze, D.L.; Angelides, K.J. Localization and Mobility of o-Conotoxin-Sensitive Ca2+ Channels in Hippocampal CAI Neurons. Science 1989, 244, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhangsun, D.T.; Zhu, X.P.; Kaas, Q.; Zhangsun, M.Q.; Peta, J.H.; David, J.C.; Michael, M.; Luo, S.L. α-Conotoxin [S9A] TxID Potently Discriminates between α3β4 and α6/α3β4 Nicotinic Acetylcholine Receptors. J. Med. Chem. 2017, 60, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Zhangsun, D.T.; Zhu, X.P.; Kaas, Q.; Wu, Y.; Craik, D.J.; McIntosh, J.M.; Luo, S.L. αO-Conotoxin GeXIVA disulfide bond isomers exhibit differential sensitivity for various nicotinic acetylcholine receptors but retain potency and selectivity for the human α9α10 subtype. Neuropharmacology 2017, 127, 243–252. [Google Scholar] [CrossRef] [PubMed]



| Peptide | IC50 (nM) | Hill Slope |
|---|---|---|
| TxID | 25 (20–32) | 0.6 (0.5–0.7) |
| TxID-F | 73 (60–89) | 0.8 (0.7–0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Zhu, X.; Yang, Y.; Tan, Y.; Luo, S.; Zhangsun, D. Fluorescently Labeled α-Conotoxin TxID, a New Probe for α3β4 Neuronal Nicotinic Acetylcholine Receptors. Mar. Drugs 2022, 20, 511. https://doi.org/10.3390/md20080511
Huang M, Zhu X, Yang Y, Tan Y, Luo S, Zhangsun D. Fluorescently Labeled α-Conotoxin TxID, a New Probe for α3β4 Neuronal Nicotinic Acetylcholine Receptors. Marine Drugs. 2022; 20(8):511. https://doi.org/10.3390/md20080511
Chicago/Turabian StyleHuang, Meiling, Xiaopeng Zhu, Yishuai Yang, Yao Tan, Sulan Luo, and Dongting Zhangsun. 2022. "Fluorescently Labeled α-Conotoxin TxID, a New Probe for α3β4 Neuronal Nicotinic Acetylcholine Receptors" Marine Drugs 20, no. 8: 511. https://doi.org/10.3390/md20080511





