Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis
Abstract
1. Introduction
2. Benzyl Alcohol/Salicyladehyde-Type Secondary Cometabolites
2.1. Varitriol, Varioxirane, Andytriol, and Xanthones
2.1.1. Isolation
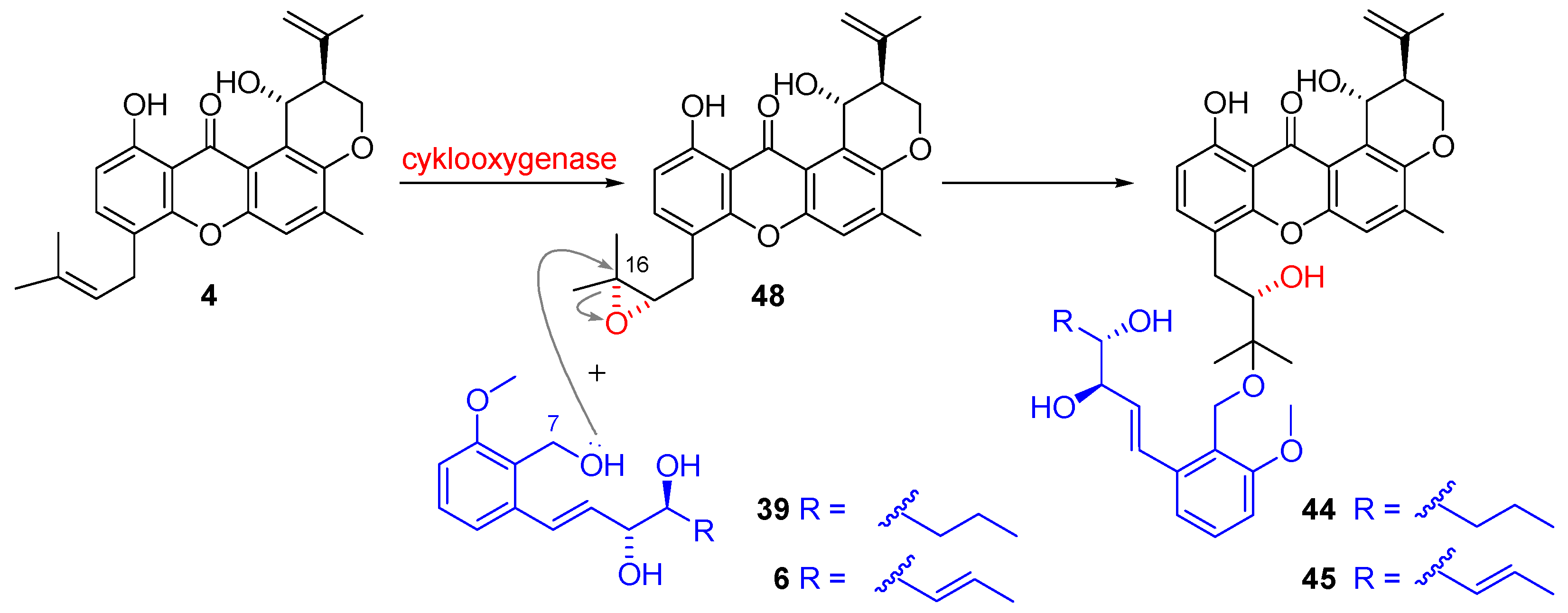
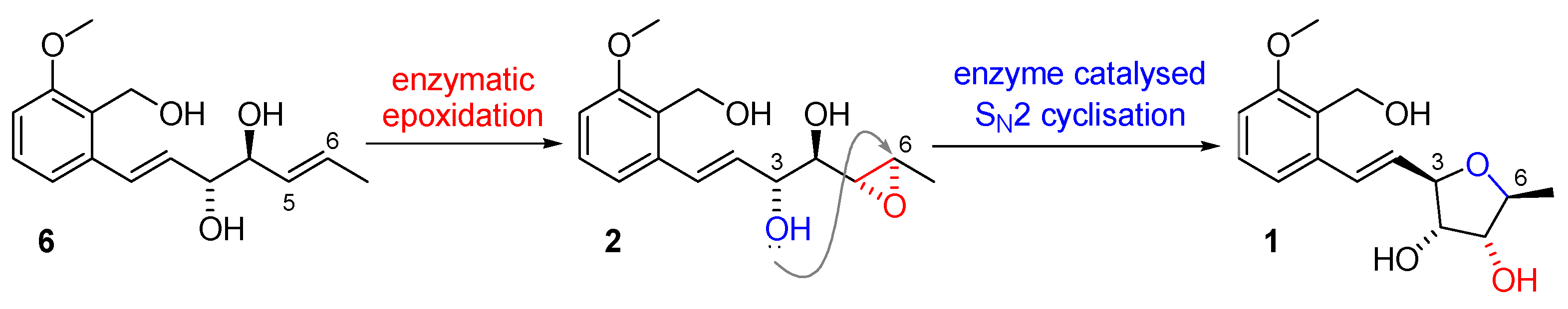
2.1.2. Biosynthesis
2.1.3. Bioactivity
2.1.4. Synthesis
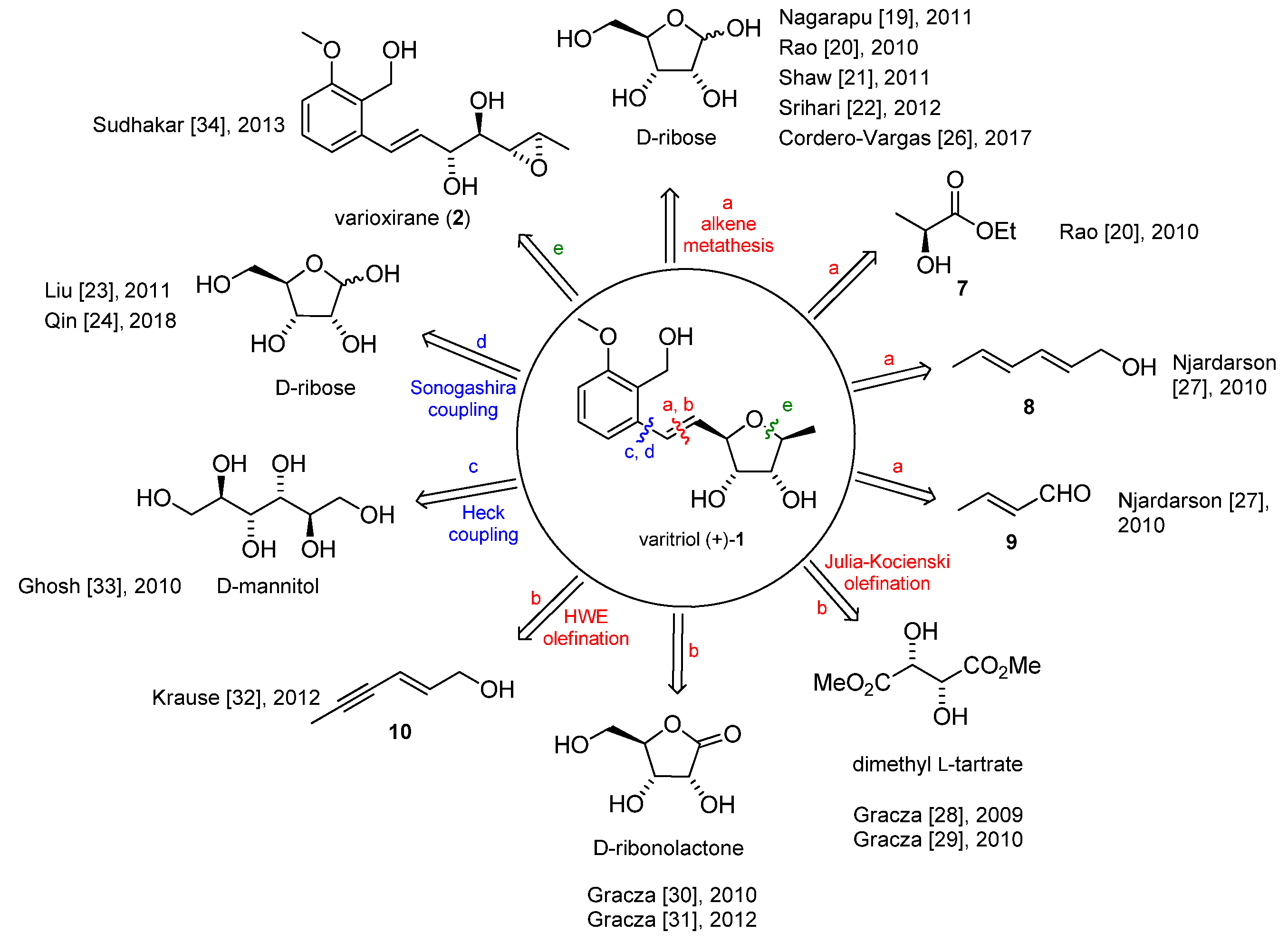
Varitriol
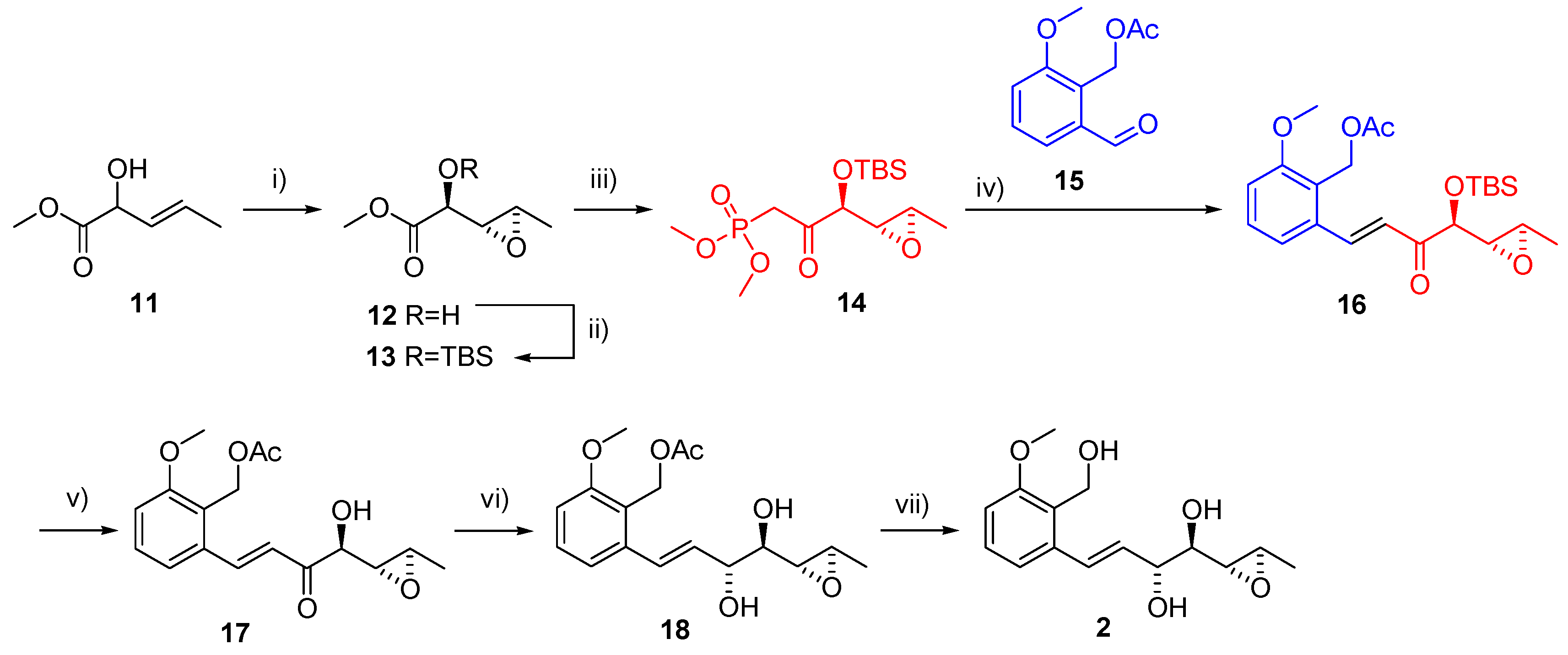
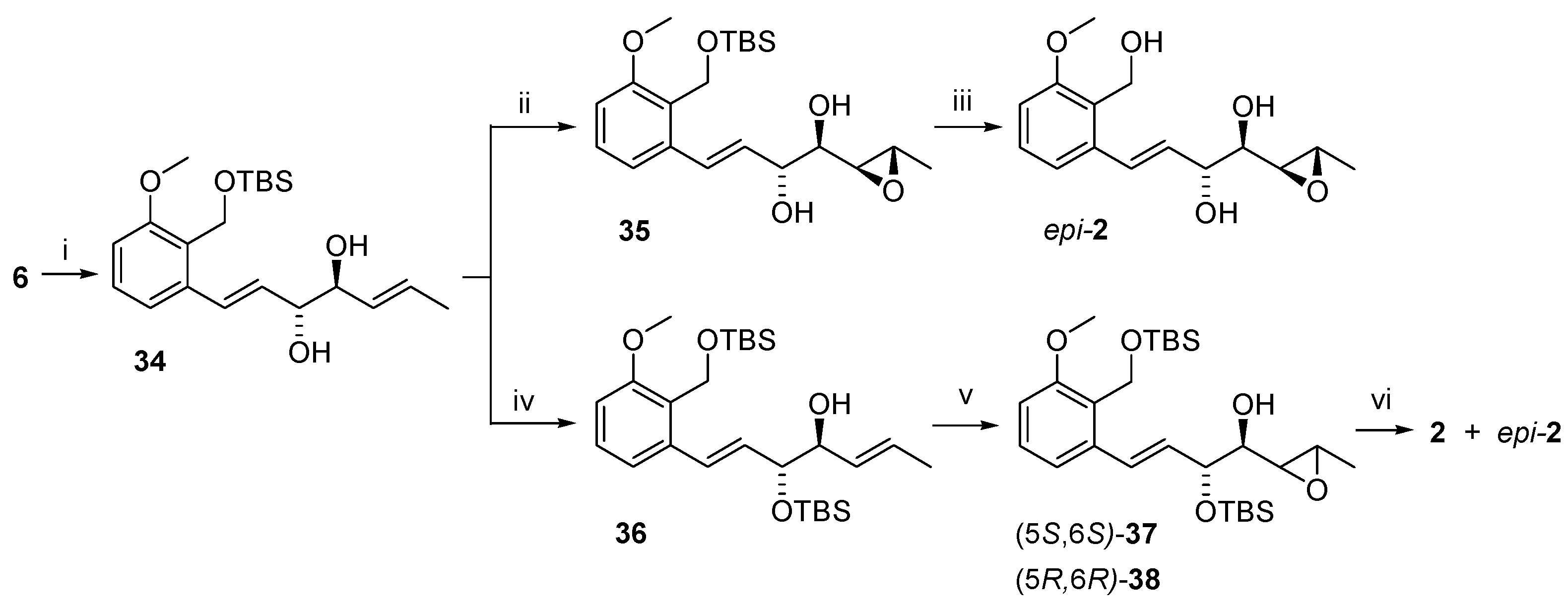
Varioxirane and Andytriol
2.2. Varioxiranols
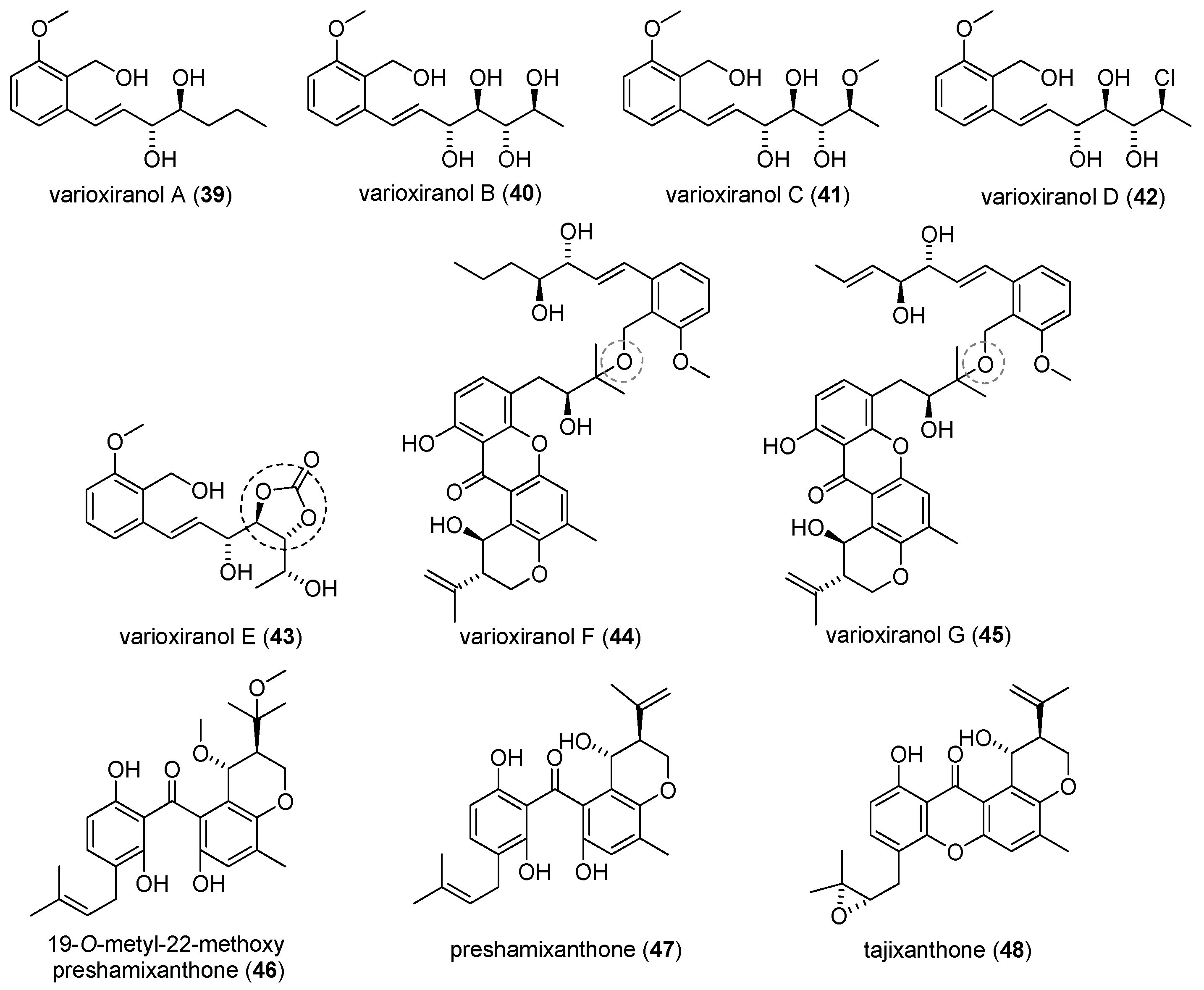
2.2.1. Isolation
2.2.2. Biosynthesis
2.2.3. Bioactivity
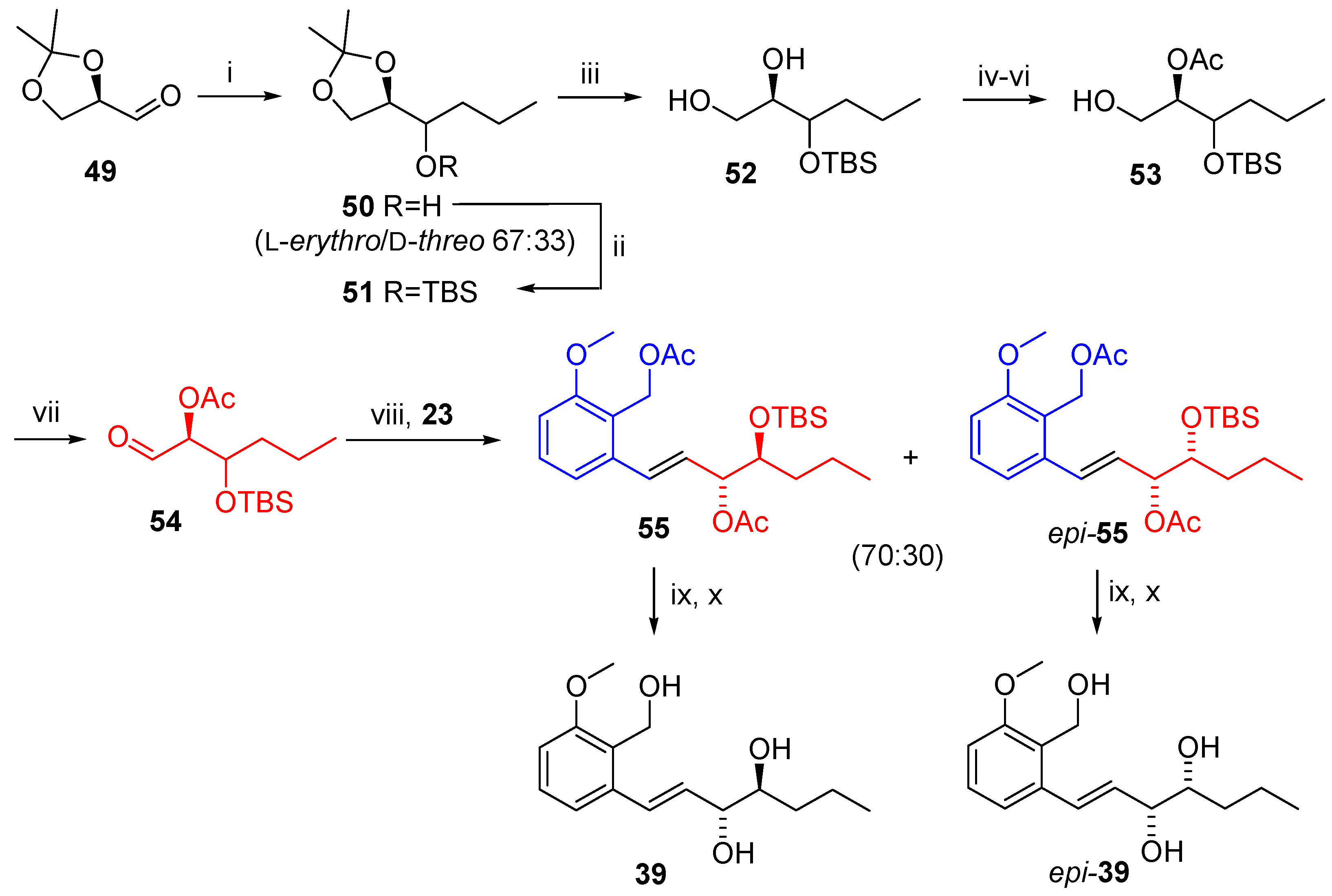
2.2.4. Synthesis of Varioxiranol A and 4-Epi-Varioxiranol A
2.3. Varioxiranediol
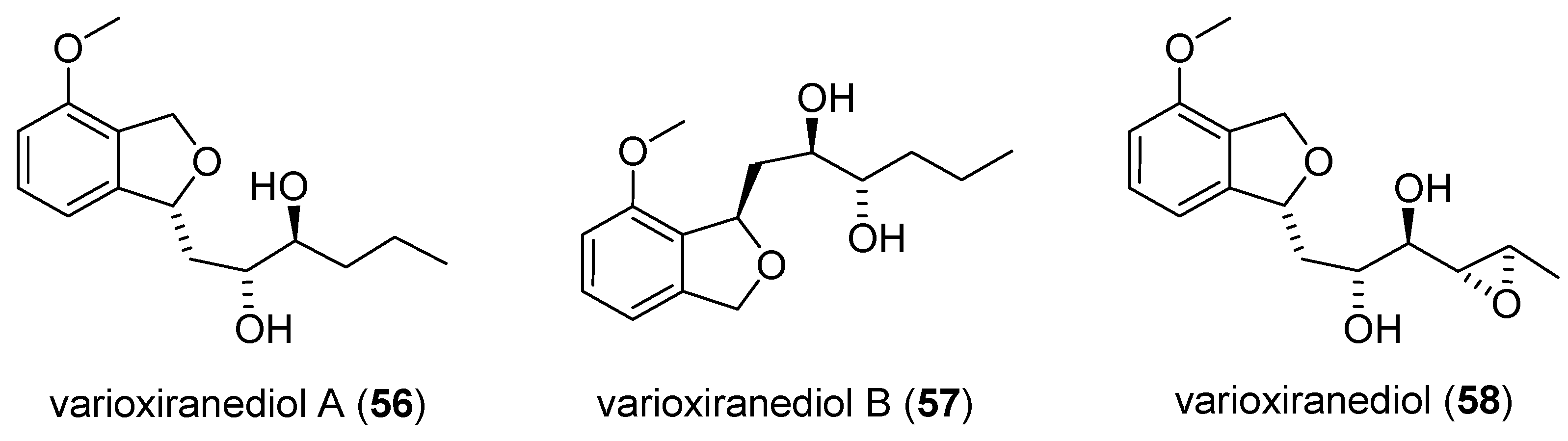
2.3.1. Isolation
2.3.2. Bioactivity
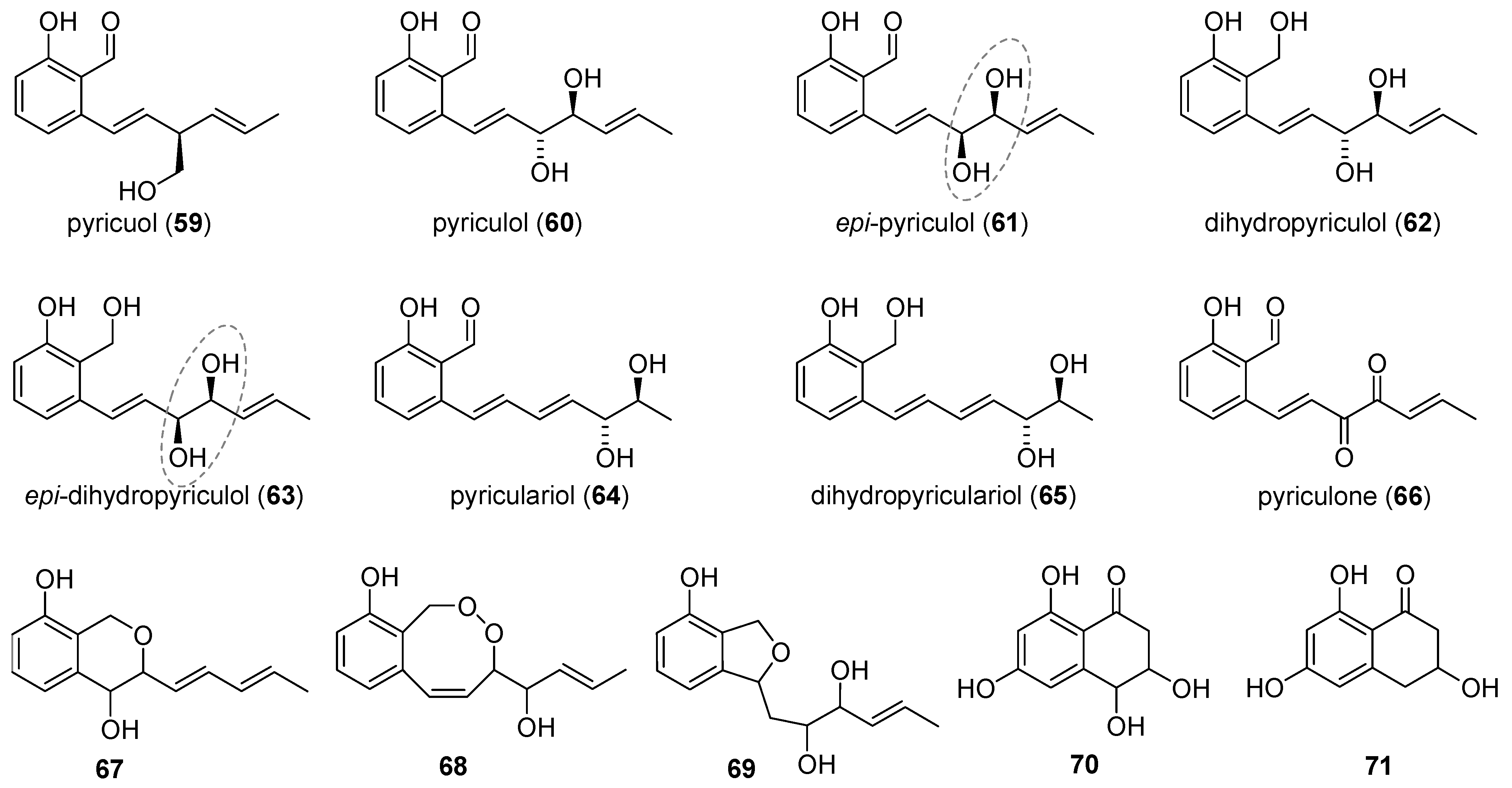
2.4. Pyricuol and Pyriculols
2.4.1. Isolation
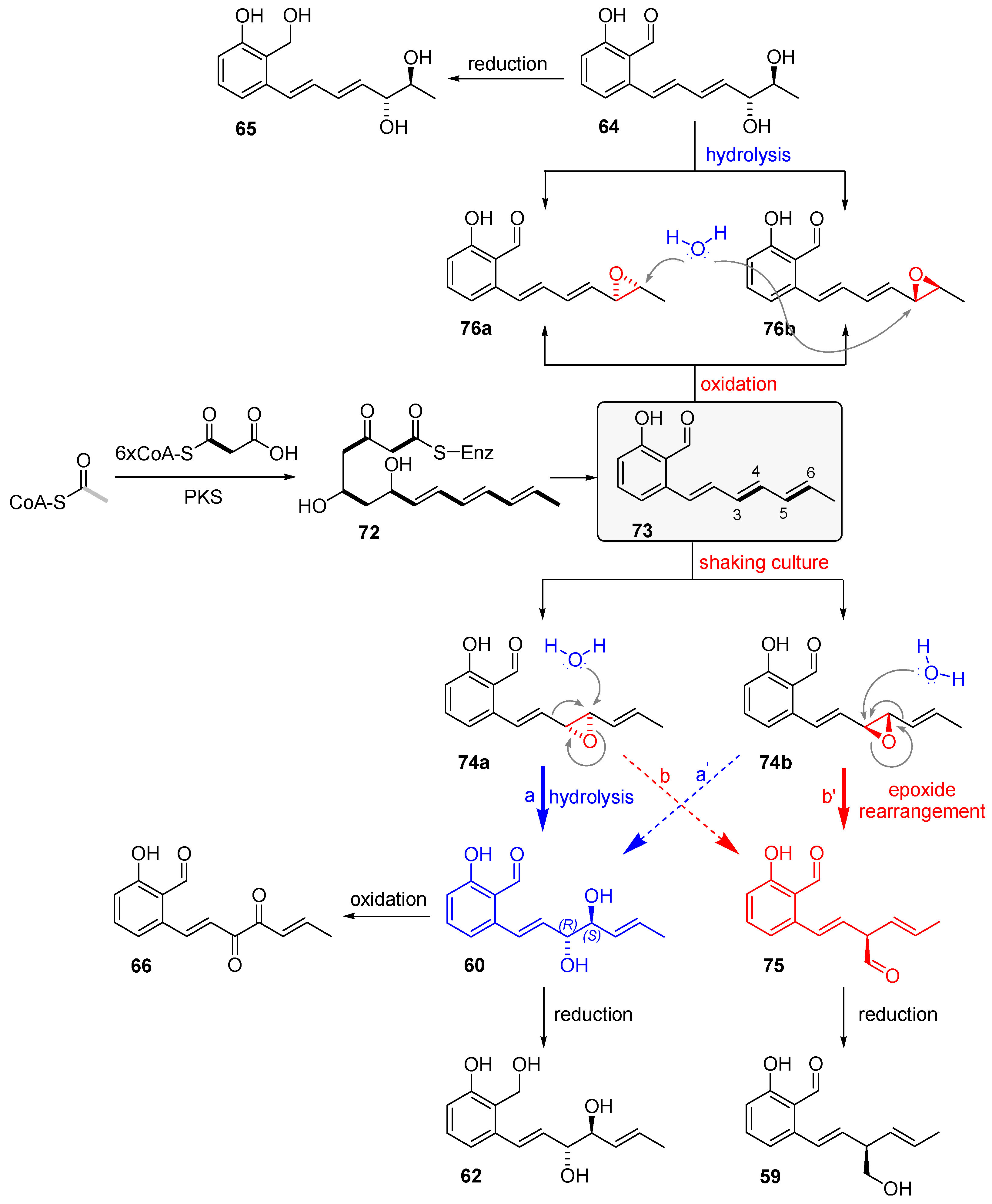
2.4.2. Biosynthesis
2.4.3. Bioactivity
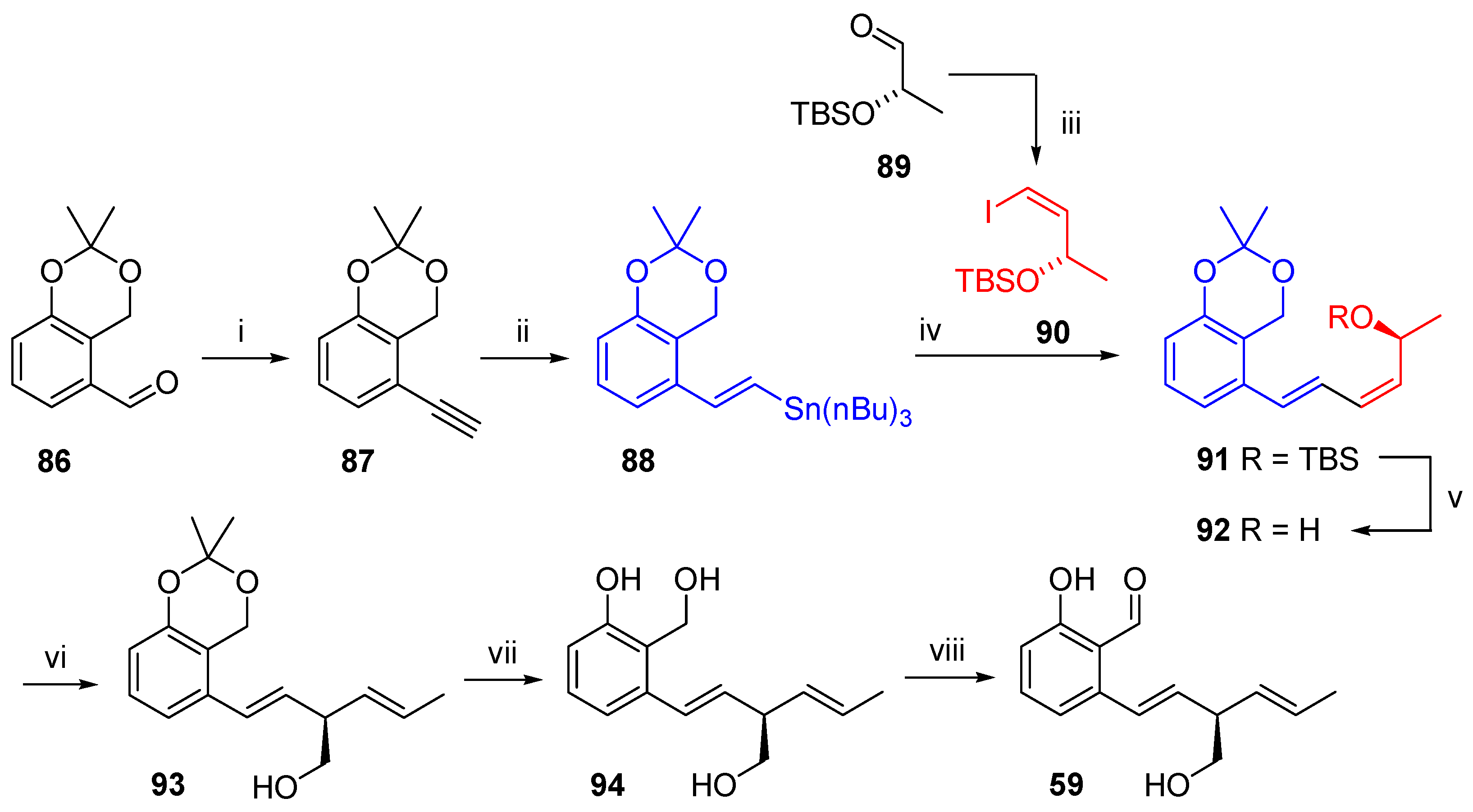
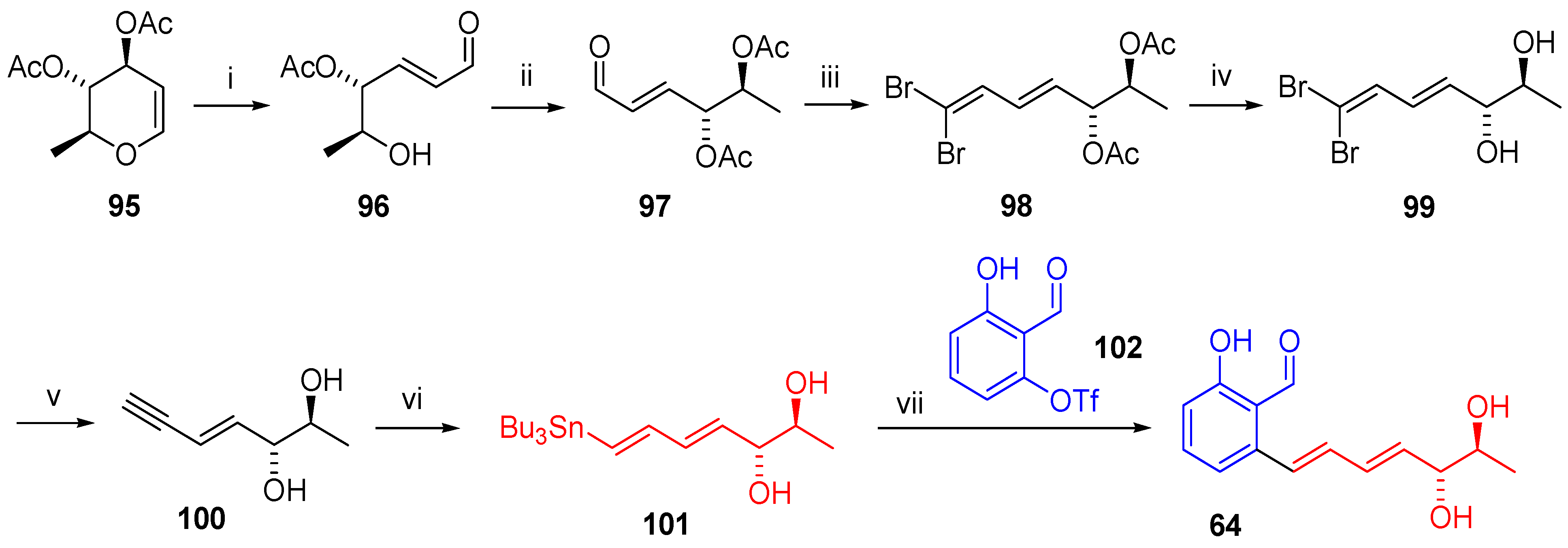
2.4.4. Synthesis
Pyriculol
Pyricuol
Pyriculariol
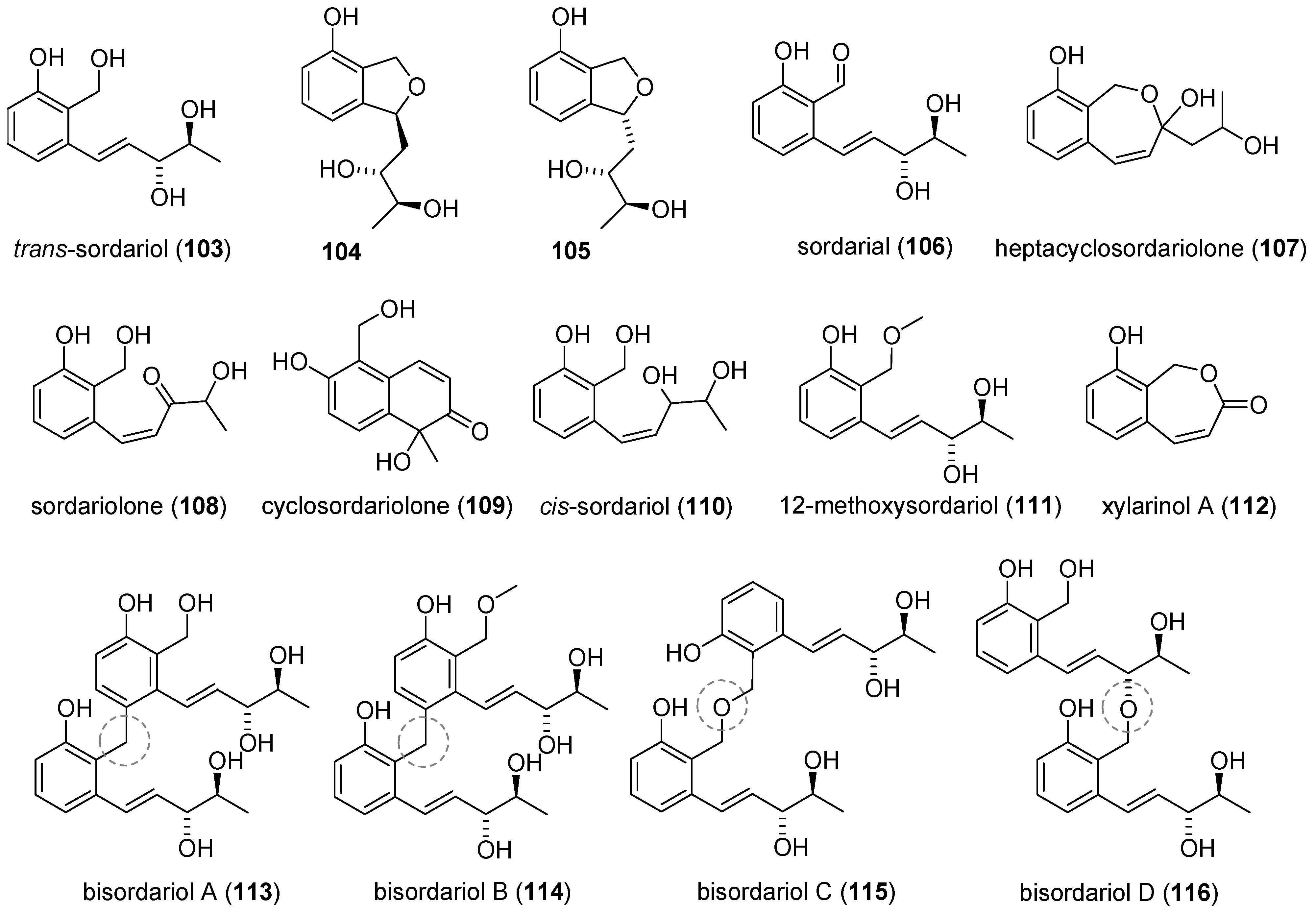
2.5. Sordarials
2.5.1. Isolation and Identification of Metabolites
2.5.2. Biosynthesis
2.5.3. Bioactivity
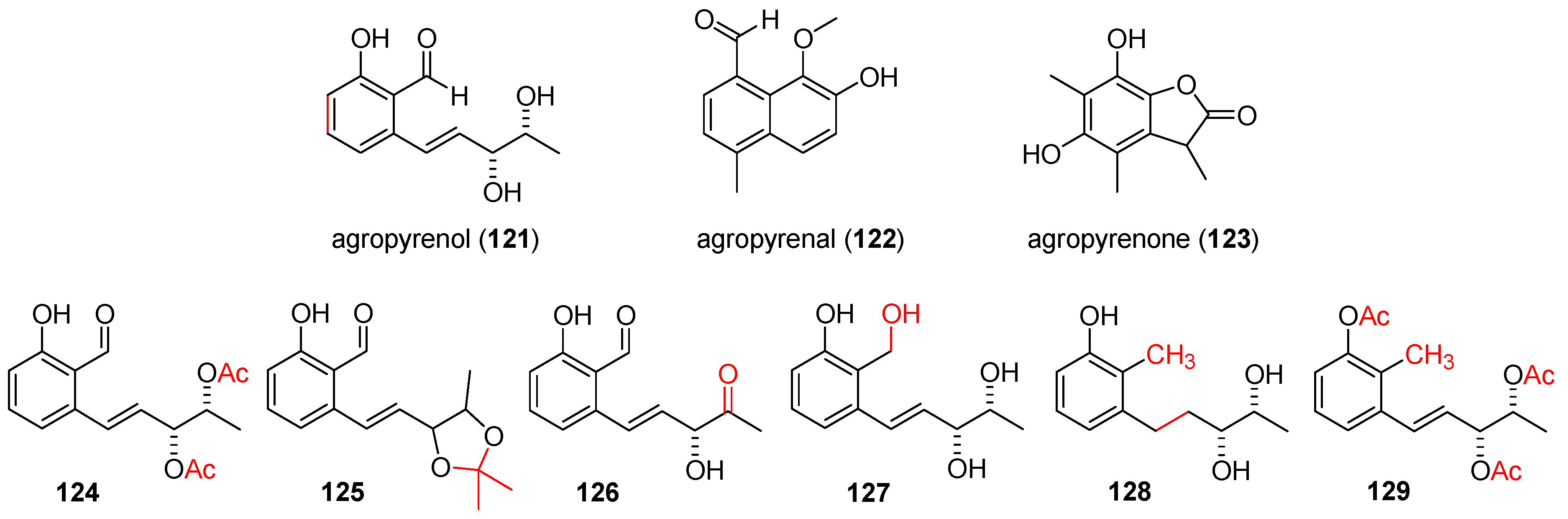
2.6. Agropyrenols, Agropyrenals, and Agropyrenone
2.6.1. Isolation and Identification
2.6.2. Bioactivity
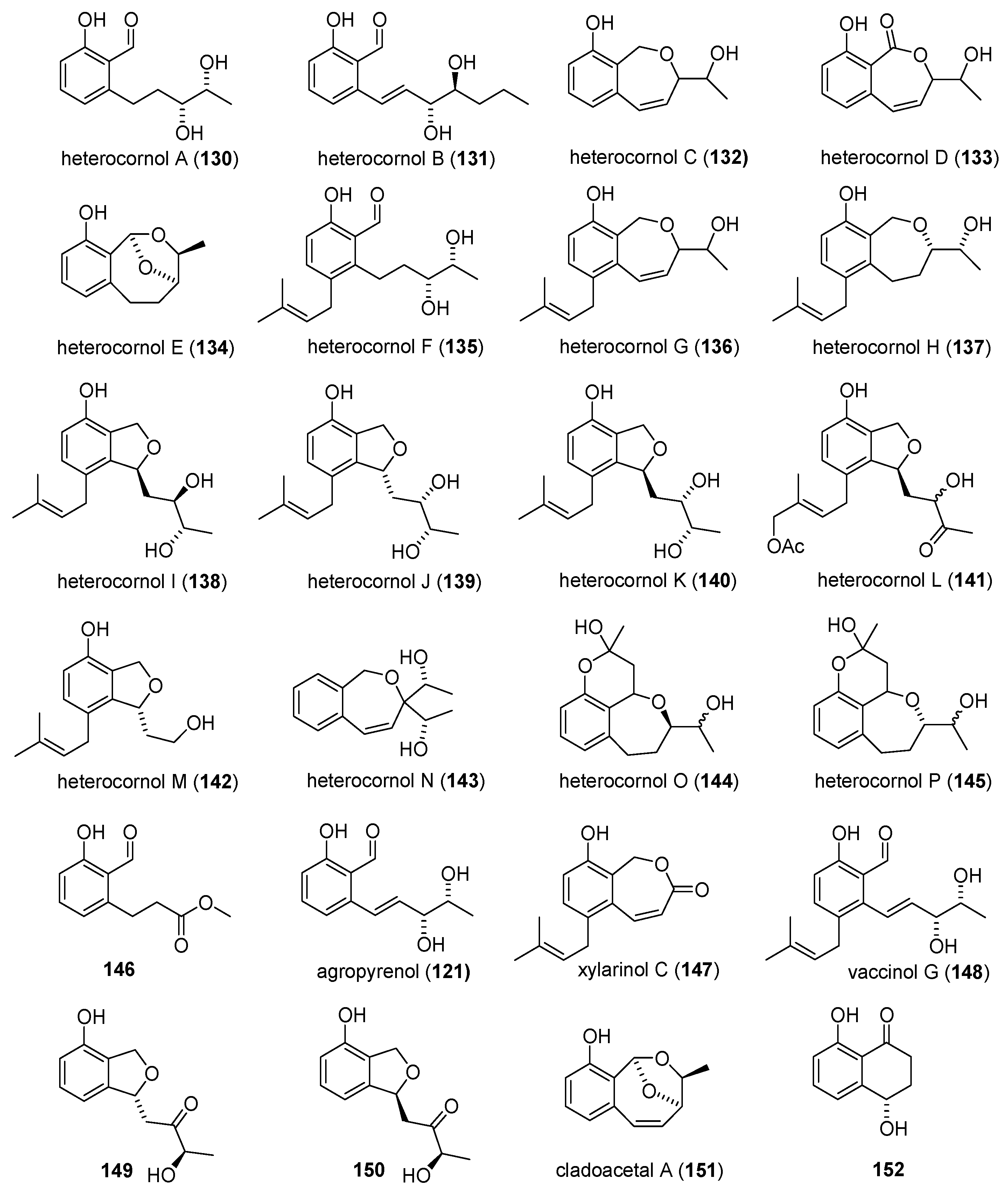
2.7. Heterocornols
2.7.1. Isolation and Identification
2.7.2. Biosynthesis
2.7.3. Bioactivity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baerson, S.R.; Rimando, A.M. Polyketides: Biosynthesis, Biological Activity, and Genetic Engineering; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Cummings, M.; Breitling, R.; Takano, E. Steps towards the synthetic biology of polyketide biosynthesis. FEMS Microbiol. Lett. 2014, 351, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Dechert-Schmitt, A.-M.R.; Schmitt, D.C.; Gao, X.; Itoh, T.; Krische, M.J. Polyketide construction via hydrohydroxyalkylation and related alcohol C–H functionalizations: Reinventing the chemistry of carbonyl addition. Nat. Prod. Rep. 2014, 31, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Berkeley, M.J. Reviews: Introduction to Cryptogamic Botany. J. Cell Sci. 1858, s1-6, 176–183. [Google Scholar] [CrossRef]
- Pitt, J.I.; Samson, R.A.; Frisvad, J.C. List of Accepted Species and Their Synonyms in the Family Trichocomaceae. In Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification; Samson, R.A., Pitt, J.I., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 9–49. [Google Scholar]
- Frisvad, J.C.; Samson, R.A. Emericella venezuelensis, a New Species with Stellate Ascospores Producing Sterigmatocystin and Aflatoxin B1. Syst. Appl. Microbiol. 2004, 27, 672–680. [Google Scholar] [CrossRef]
- Christensen, M.; States, J.S. Aspergillus nidulans Group: Aspergillus navahoensis, and a Revised Synoptic Key. Mycologia 1982, 74, 226. [Google Scholar] [CrossRef]
- Peterson, S.W. Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification; Samson, R.A., Pitt, J.I., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 323–355. [Google Scholar]
- Houbraken, J.; Due, M.; Varga, J.; Meijer, M.; Frisvad, J.; Samson, R. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 2007, 59, 107–128. [Google Scholar] [CrossRef]
- Malloch, D.; Cain, R.F. New species and combinations of cleistothecial Ascomycetes. Can. J. Bot. 1972, 50, 61–72. [Google Scholar] [CrossRef]
- Malmstrøm, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar] [CrossRef]
- Dunn, A.W.; Entwistle, I.D.; Johnstone, R.A. Terrein and other metabolites of Phoma species. Phytochemistry 1975, 14, 2081–2082. [Google Scholar] [CrossRef]
- Dunn, A.W.; Johnstone, A.W. Fungal Metabolites. Part 8. Isolation of 2-methoxy-6-(3,4-dihydroxyhepta-1,3-dienyl)benzyl alcohol. J. Chem. Soc. Perkin Trans. 1979, 1, 2122–2123. [Google Scholar] [CrossRef]
- Markovič, M.; Lopatka, P.; Koóš, P.; Gracza, T. First total synthesis of natural andytriol and a biomimetic approach to varioxiranes. Tetrahedron 2015, 71, 8407–8415. [Google Scholar] [CrossRef]
- Clemens, R.T.; Jennings, M.P. An efficient total synthesis and absolute configuration determination of varitriol. Chem. Commun. 2006, 25, 2720–2721. [Google Scholar] [CrossRef] [PubMed]
- Majik, M.; Tilvi, S.; Parvatkar, P. Recent Developments Towards the Synthesis of Varitriol: An Antitumour Agent from Marine Derived Fungus Emericella Variecolor. Curr. Org. Synth. 2014, 11, 268–287. [Google Scholar] [CrossRef][Green Version]
- McAllister, G.D.; Robinson, J.E.; Taylor, R.J. The synthesis of (−)-varitriol and (−)-3′-epi-varitriol via a Ramberg–Bäcklund route. Tetrahedron 2007, 63, 12123–12130. [Google Scholar] [CrossRef]
- Kumar, V.; Shaw, A.K. First total synthesis of (+)-varitriol. J. Org. Chem. 2008, 73, 7526–7531. [Google Scholar] [CrossRef]
- Nagarapu, L.; Paparaju, V.; Satyender, A.; Bantu, R. Total synthesis of (+)-varitriol via a symmetrical furanose diol as the key intermediate. Tetrahedron Lett. 2011, 52, 7075–7078. [Google Scholar] [CrossRef]
- Srinivas, B.; Sridhar, R.; Rao, K.R. Stereoselective total synthesis of (+)-varitriol. Tetrahedron 2010, 66, 8527–8535. [Google Scholar] [CrossRef]
- Ghosal, P.; Sharma, D.; Kumar, B.; Meena, S.; Sinha, S.; Shaw, A.K. Diastereoselective one-pot Wittig olefination–Michael addition and olefincross metathesis strategy for total synthesis of cytotoxic natural product (+)-varitriol and its higher analogues. Org. Biomol. Chem. 2011, 9, 7372–7383. [Google Scholar] [CrossRef]
- Vamshikrishna, K.; Srihari, P. Total synthesis of (+)-varitriol and (+)-6′-epi-varitriol. Tetrahedron 2012, 68, 1540–1546. [Google Scholar] [CrossRef]
- Zeng, J.; Vedachalam, S.; Xiang, S.; Liu, X.-W. Direct C-glycosylation of organotrifluoroborates with glycosyl fluorides and its application to the total synthesis of (+)-varitriol. Org. Lett. 2011, 13, 42–45. [Google Scholar] [CrossRef]
- He, H.; Qin, H.B. ZnBr2-catalyzed direct C-glycosylation of glycosyl acetates with terminal alkynes. Org. Chem. Front. 2018, 5, 1962–1966. [Google Scholar] [CrossRef]
- Mahadevegowda, S.H.; Khan, F.A. Synthesis of the tetrahydrofuran unit of varitriol and γ-butyrolactones from 5-oxabicyclo[2.1.1]hexane derivative via oxidative cleavage reactions. Tetrahedron Lett. 2014, 55, 2266–2269. [Google Scholar] [CrossRef]
- Sánchez-Eleuterio, A.; García-Santos, W.H.; Díaz-Salazar, H.; Hernández-Rodríguez, M.; Cordero-Vargas, A. Stereocontrolled Nucleophilic Addition to Five-Membered Oxocarbenium Ions Directed by the Protecting Groups. Application to the Total Synthesis of (+)-Varitriol and of Two Diastereoisomers Thereof. J. Org. Chem. 2017, 82, 8464–84754. [Google Scholar] [CrossRef]
- Brichacek, M.; Batory, L.A.; McGrath, N.A.; Njardarson, J.T. The strategic marriage of method and motif. Total synthesis of varitriol. Tetrahedron 2010, 66, 4832–4840. [Google Scholar] [CrossRef]
- Palík, M.; Karlubíková, O.; Lásiková, A.; Kožíšek, J.; Gracza, T. Total Synthesis of (+)-Varitriol. Eur. J. Org. Chem. 2009, 73, 7526–7531. [Google Scholar] [CrossRef]
- Palík, M.; Karlubíková, O.; Lackovičová, D.; Lásiková, A.; Gracza, T. Formal synthesis of (+)-varitriol. Application of Pd(II)/Cu(II)-catalysed bicyclisation of unsaturated polyols. Tetrahedron 2010, 66, 5244–5249. [Google Scholar] [CrossRef]
- Gracza, T.; Karlubíková, O.; Palík, M.; Lásiková, A. An Efficient Total Synthesis of (+)-Varitriol from d-Ribonolactone. Synthesis 2010, 2010, 3449–3452. [Google Scholar] [CrossRef]
- Caletková, O.; Lásiková, A.; Hajdúch, M.; Džubák, P.; Gracza, T. Synthesis and antitumour activity of varitriol and its analogues. Arkivoc 2012, 6, 365–383. [Google Scholar] [CrossRef]
- Sun, T.; Deutsch, C.; Krause, N. Combined coinage metal catalysis in natural product synthesis: Total synthesis of (+)-varitriol and seven analogs. Org. Biomol. Chem. 2012, 10, 5965–5970. [Google Scholar] [CrossRef]
- Ghosh, S.; Pradhan, T.K. Stereoselective total synthesis of (+)-varitriol, (−)-varitriol, 5′-epi-(+)-varitriol, and 4′-epi-(−)-varitriol from d-mannitol. J. Org. Chem. 2010, 75, 2107–2110. [Google Scholar] [CrossRef]
- Sudhakar, G.; Raghavaiah, J. Total synthesis of varitriol, varioxirane, and enantiomer of the proposed biosynthetic precursor. J. Org. Chem. 2013, 78, 8840–8846. [Google Scholar] [CrossRef]
- Dalence-Guzmán, M.F.; Berglund, M.; Skogvall, S.; Sterner, O. SAR studies of capsazepinoid bronchodilators. Part 1: The importance of the catechol moiety and aspects of the B-ring structure. Bioorg. Med. Chem. 2008, 16, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Dillard, R.D.; Carr, F.P.; McCullough, D.; Haisch, K.D.; Rinkema, L.E.; Fleisch, J.H. Leukotriene receptor antagonists. 2. The [[(tetrazol-5-ylaryl)oxy]methyl]acetophenone derivatives. J. Med. Chem. 1987, 30, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, Y.; Ohashi, K.; Sakamota, Y.; Hirakawa, S.; Kamikawa, T.; Kubo, I. Syntheses of anacardic acids and ginkgoic acid. Tetrahedron 1987, 43, 3387–33940. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, C.; Long, H.; Chen, R.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Varioxiranols A–G and 19-O-Methyl-22-methoxypre-shamixanthone, PKS and Hybrid PKS-Derived Metabolites from a Sponge-Associated Emericella variecolor Fungus. J. Nat. Prod. 2015, 78, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Ebbole, D.J. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007, 45, 437–456. [Google Scholar] [CrossRef]
- Wu, X.; Fang, L.-Z.; Liu, F.-L.; Pang, X.-J.; Qin, H.-L.; Zhao, T.; Xu, L.-L.; Yang, D.-F.; Yang, X.-L. New prenylxanthones, polyketide hemiterpenoid pigments from the endophytic fungus Emericella sp. XL029 and their anti-agricultural pathogenic fungal and antibacterial activities. RSC Adv. 2017, 7, 31115–31122. [Google Scholar] [CrossRef]
- Lásiková, A.; Doháňošová, J.; Štiblariková, M.; Parák, M.; Moncol, J.; Gracza, T. First Total Synthesis of Varioxiranol A. Molecules 2019, 24, 862. [Google Scholar] [CrossRef]
- Liangsakul, J.; Pornpakakul, S.; Sangvichien, E.; Muangsin, N.; Sihanonth, P. Emervaridione and varioxiranediol, two new metabolites from the endophytic fungus, Emericella variecolor. Tetrahedron Lett. 2011, 52, 6427–6430. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, Q.; Huang, J.; Li, X.N.; Zhu, H.; Liu, J.; Wang, J.; Xue, J.; Zhang, Y. Spiroaspertrione A, a bridged spirocyclic meroterpenoid, as a potent potentiator of oxacillin against methicillin-resistant Staphylococcus aureus from Aspergillus sp. TJ23. J. Nat. Prod. 2017, 80, 2399–2405. [Google Scholar] [CrossRef]
- Kim, J.-C.; Min, J.-Y.; Kim, H.-T.; Cho, K.-Y.; Yu, S.-H. Pyricuol, a New Phytotoxin from Magnaporthe grisea. Biosci. Biotechnol. Biochem. 1998, 62, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Muro, H.; Sasaki, K.; Woe, S.; Okuda, S. Isolations of phytotoxic substances produced by Pyricularia oryzae cavara. Tetrahedron Lett. 1973, 37, 3537–3542. [Google Scholar] [CrossRef]
- Kono, Y.; Sekido, S.; Yamaguchi, I.; Kondo, H.; Suzuki, Y.; Neto, G.C.; Sakurai, A.; Yaegashi, H. Structures of two novel pyriculol-related compounds and identification of naturally produced epipyriculol from Pyricularia oryzae. Agric. BioI. Chem. 1991, 55, 2785–2791. [Google Scholar] [CrossRef]
- Zhao, Z.; Ying, Y.; Hung, Y.S.; Tang, Y. Genome mining reveals neurospora crassa can produce the salicylaldehyde sordarial. J. Nat. Prod. 2019, 82, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sasaki, A.; Cao, H.Q.; Yamada, T.; Igarashi, M.; Komine, I.; Nakahigashi, H.; Minami, N.; Kuwahara, S.; Nukina, M.; et al. Synthesis and biotransformation of plausible biosynthetic intermediates of salicylaldehyde-type phytotoxins of rice blast fungus, Magnaporthe grisea. Eur. J. Org. Chem. 2011, 31, 6276–6280. [Google Scholar] [CrossRef]
- Nukina, M.; Otsuki, T.; Kuniyasu, N. Microbial transformation of 1- and 2-phenyl-1-propenes by the fungus Pyricularia oryzae Cavara. Biosci. Biotechnol. Biochem. 1994, 58, 2293–2294. [Google Scholar] [CrossRef][Green Version]
- Bouillant, M.L.; Favre-Bonvin, J.; Salin, N.; Bernillon, J. Sordariol and related compounds, hexaketides in the fungus Sordaria macrospora. Phytochemistry 1988, 27, 1517–1519. [Google Scholar] [CrossRef]
- Bouillant, M.L.; Bernilion, J.; Favre-Bonvin, J.; Salin, N. New hexaketides related to sordariol in Sordaria macrospora. Z. Naturforsch. C 1989, 44, 719–723. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugiyama, T.; Watanabe, M.; Yamashita, K. Synthesis of optically active pyriculol, a phytotoxic metabolite produced by Pyricularia oryzae Cavara. Agric. Biol. Chem. 1986, 50, 2159–21600. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Sugiyama, T.; Watanabe, M.; Murayama, T.; Yamashita, K. Synthesis and absolute configuration of pyriculol. Agric. Biol. Chem. 1987, 51, 1121–1127. [Google Scholar] [CrossRef]
- Kiyota, H.; Ueda, R.; Oritani, T.; Kuwahara, S. First Synthesis of (±)-Pyricuol, a Plant Pathogen Isolated from Rice Blast Disease Fungus Magnaporthe grisea. Synlett 2003, 2003, 0219–0220. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kiyota, H.; Ueda, R.; Kuwahara, S. Synthesis to determine the absolute configuration of (−)-pyricuol, a phytotoxin isolated from rice blast disease fungus Magnaporthe grisea. Tetrahedron Lett. 2005, 46, 7107–7109. [Google Scholar] [CrossRef]
- Kiyota, H. Synthesis of naturally derived bioactive compounds of agricultural interest. Biosci. Biotechnol. Biochem. 2006, 70, 317–324. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, Y.; Sasaki, A.; Ueda, R.; Suzuki, Y.; Kuwahara, S.; Kiyota, H. Synthesis and plant growth inhibitory activity of both enantiomers of pyricuol, a phytotoxin isolated from rice blast disease fungus Magnaporthe grisea. Tetrahedron 2009, 65, 6115–6122. [Google Scholar] [CrossRef]
- Sasaki, A.; Tanaka, K.; Sato, Y.; Kuwahara, S.; Kiyota, H. First synthesis and absolute configuration of (−)-pyriculariol, a phytotoxin isolated from rice blast fungus, Magnaporthe grisea. Use of microwave irradiation to control Stille coupling reaction products. Tetrahedron Lett. 2009, 50, 4637–4638. [Google Scholar] [CrossRef]
- Sugiyama, T.; Watanabe, M.; Sassa, T.; Yamashita, K. Syntheses of monilidiol and dechloromonilidiol, phytotoxic octaketides of Monilinia fructicola. Agric. Biol. Chem. 1983, 47, 2411–2413. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Sugiyama, T.; Watanabe, M.; Murayama, T.; Yamashita, K. Syntheses of all four stereoisomers of pyriculol. Agric. Biol. Chem. 1987, 51, 2161–2166. [Google Scholar] [CrossRef]
- Ohira, S. Methanolysis of dimethyl (1-diazo-2-oxopropyl) phosphonate: Generation of dimethyl (diazomethyl) phosphonate and reaction with carbonyl compounds. Synth. Commun. 1989, 19, 561–564. [Google Scholar] [CrossRef]
- Müller, S.; Liepold, B.; Roth, G.J.; Bestmann, H.J. An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes. Synlett 1996, 1996, 521–522. [Google Scholar] [CrossRef]
- Massad, S.K.; Hawkins, L.D.; Baker, D.C. A series of (2S)-2-O-protected-2-hydroxypropanals (L-lactaldehydes) suitable for use as optically active intermediates. J. Org. Chem. 1983, 48, 5180–5182. [Google Scholar] [CrossRef]
- Molander, G.A.; Dehmel, F. Formal Total Synthesis of Oximidine II via a Suzuki-Type Cross-Coupling Macrocyclization Employing Potassium Organotrifluoroborates. J. Am. Chem. Soc. 2004, 126, 10313–10318. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Luo, J.; Yang, M.; Kong, L. Antioxidant sordariol dimers from Sordaria macrospora and the absolute configuration determinations of their two simultaneous linear 1,2-diols. Tetrahedron Lett. 2016, 57, 2754–2757. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Nagano, J.; Natori, H.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from three ascomycetes, Gelasinospora heterospora, G. multiforis, and G. longispora. Chem. Pharm. Bull. 1999, 47, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Cimmino, A.; Vurro, M.; Berestetskiy, A.; Troise, C.; Zonno, M.C.; Motta, A.; Evidente, A. Agropyrenol and agropyrenal, phytotoxins from Ascochyta agropyrina var. nana, a fungal pathogen of Elitrigia repens. Phytochemistry 2012, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Dong, K.; Wang, X.; Zhong, J.; Mu, Y.; Liu, Y.; Huang, X. Polyketide derivatives from a marine-sponge-associated fungus Pestalotiopsis heterocornis. Phytochemistry 2017, 142, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.S.; Lin, S.C. Total syntheses of cladoacetals A and B: Confirmation of absolute configurations. J. Org. Chem. 2012, 77, 6139–6146. [Google Scholar] [CrossRef]
- Lee, I.-K.; Jang, Y.-W.; Kim, Y.-S.; Yu, S.H.; Lee, K.J.; Park, S.-M.; Oh, B.-T.; Chae, J.-C.; Yun, B.-S. Xylarinols A and B, two new 2-benzoxepin derivatives from the fruiting bodies of Xylaria polymorpha. J. Antibiot. 2009, 62, 163–165. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Lu, X.; Xu, F.; Wan, J.; Lin, X.; Zhou, X.; Liao, S.; Yang, B.; Tu, Z.; et al. Eight new polyketide metabolites from the fungus Pestalotiopsis vaccinii endogenous with the mangrove plant Kandelia candel (L.) Druce. Tetrahedron 2014, 70, 9695–9701. [Google Scholar] [CrossRef]
- Kesting, J.R.; Olsen, L.; Staerk, D.; Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Jaroszewski, J.W. Production of Unusual Dispiro Metabolites in Pestalotiopsis virgatula Endophyte Cultures: HPLC-SPE-NMR, Electronic Circular Dichroism, and Time-Dependent Density-Functional Computation Study. J. Nat. Prod. 2011, 74, 2206–2215. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, X.; Hu, M.; Niu, H.; Song, C.; Chen, S.; Liu, Y.; Zhang, D. Cytotoxic Polyketides from the Marine Sponge-Derived Fungus Pestalotiopsis heterocornis XWS03F09. Molecules 2019, 24, 2655. [Google Scholar] [CrossRef]






| Microorganism | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 121 | 124 | 125 | 126 | 127 | 128 | 129 | |
| Cirsium arvense a | 3 | 3 | 4 | 2 | 2 | 0 | 0 |
| Chenopodium albuma | 4 | 4 | 4 | 0 | 2 | 0 | 0 |
| Mercurialis annua a | 4 | 2 | 4 | 0 | 0 | 0 | 0 |
| Setaria viridis a | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Sonchus oleraceus a | 2 | 2 | 4 | 0 | 2 | 2 | 0 |
| Setaria viridis b | 40.8 (0.3) | 28.8 (1.1) | 22.1 (1.7) | 30.5 (2.2) | 54.9 (0.1) | 43.5 (0.9) | 54.2 (0.4) |
| Lycopersicon esculentum c | 23.8 (11.9) | 15.3 (7.1) | 5.2 (2.7) | 49.0 (21.0) | 38.5 (14.0) | 37.3 (9.9) | 33.1 (9.7) |
| Lemna minor d | 6.4 (0.4) | 5.3 (0.4) | 2.5 (0.4) | 7.3 (1.0) | 7.1 (0.8) | 8.5 (0.4) | 8.8 (0.3) |
| Lemna minor e | 23.8 (2.8) | 23.5 (1.8) | 14.4 (4.1) | 24.4 (3.5) | 21.4 (2.3) | 23.0 (1.6) | 22.8 (1.9) |
| Artemia salina f | 0 | 0 | 88 (4) | 0 | 0 | 23 (2) | 0 |
| Geotrichum candidum | - | - | + | - | - | - | - |
| Bacillus subtilis | - | - | + | - | - | - | - |
| Escherichia coli | - | - | - | - | - | - | - |
| Compound Source | Biological Activity | Cell Line or Microorganism | Biological Result | Ref. |
|---|---|---|---|---|
| Varitriol (1) Emericella variecolor M75-2 (marine) | Cytotoxic activity | RXF 393 renal cancer | GI50 = 1.63 × 10−7 M | [11] |
| TD-47 breast cancer | GI50 = 2.10 × 10−7 M | |||
| SNB CNS cancer | GI50 = 2.44 × 10−7 M | |||
| Varixanthone (3) Emericella variecolor M75-2 (marine) | Antimicrobial activity | Enterococcus faecalis | MIC = 50.0 μg·mL−1 | [11] |
| Bacillus subtilis | MIC = 12.5 μg·mL−1 | |||
| Escherichia coli | MIC = 12.5 μg·mL−1 | |||
| Proteus sp. | MIC = 12.5 μg·mL−1 | |||
| Varioxiranediol A (56) Emericella variecolor TJ29 (plant-derived) | Antibacterial activity | MRSA | MIC = 32 μg·mL−1 | [43] |
| E. faecalis | MIC = 32 μg·mL−1 | |||
| ESBL-E. coli | MIC = 4 μg·mL−1 | |||
| P. aeruginosa | MIC = 4 μg·mL−1 | |||
| K. pneumoniae | MIC = 16 μg·mL−1 | |||
| Varioxiranediol B (57) Emericella variecolor TJ29 (plant-derived) | Antibacterial activity | E. faecalis | MIC = 32 μg·mL−1 | [43] |
| ESBL-E. coli | MIC = 8 μg·mL−1 | |||
| P. aeruginosa | MIC = 32 μg·mL−1 | |||
| Varioxirane (2) Emericella variecolor TJ29 (plant-derived) | Antibacterial activity | MRSA | MIC = 64 μg·mL−1 | [43] |
| ESBL-E. coli | MIC = 32 μg·mL−1 | |||
| P. aeruginosa | MIC = 32 μg·mL−1 | |||
| Heterocornol A (130) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 32.9 μM | [68] |
| H-460 lung carcinoma | IC50 = 37.3 μM | |||
| PC-3 prostate cancer | IC50 = 54.8 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 26.4 μM | |||
| Heterocornol B (131) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 48.1 μM | [68] |
| H-460 lung carcinoma | IC50 = 27.6 μM | |||
| PC-3 prostate cancer | IC50 = 31.5 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 21.7 μM | |||
| Heterocornol C (132) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 28.2 μM | [68] |
| H-460 large-cell lung carcinoma | IC50 = 18.7 μM | |||
| PC-3 prostate cancer | IC50 = 59.3 μM | |||
| SMMC-7721 human hepatocellular carcinoma | IC50 = 34.8 μM | |||
| Compound 146 Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 80.2 μM | [68] |
| H-460 lung carcinoma | IC50 = 87.3 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 49.6 μM | |||
| Agropyrenol (121) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 77.4 μM | [68] |
| H-460 lung carcinoma | IC50 = 24.5 μM | |||
| PC-3 prostate cancer | IC50 = 58.5 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 52.0 μM | |||
| Heterocornol F (135) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 47.1 μM | [68] |
| H-460 lung carcinoma | IC50 = 52.1 μM | |||
| PC-3 prostate cancer | IC50 = 70.6 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 50.9 μM | |||
| Heterocornol G (136) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 15.0 μM | [68] |
| H-460 lung carcinoma | IC50 = 53.3 μM | |||
| PC-3 prostate cancer | IC50 = 21.3 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 20.2 μM | |||
| Heterocornol H (137) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 23.2 μM | [68] |
| H-460 lung carcinoma | IC50 = 79.7 μM | |||
| PC-3 prostate cancer | IC50 = 83.5 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 42.3 μM | |||
| Vaccinol G (148) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 51.2 μM | [68] |
| H-460 lung carcinoma | IC50 = 57.9 μM | |||
| PC-3 prostate cancer | IC50 = 35.0 μM | |||
| SMMC-7721 hepatocellular carcinoma | IC50 = 44:4 μM | |||
| Heterocornol M (142) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 61.1 μM | [68] |
| Hep G2 liver cancer | IC50 = 20.4 μM | |||
| Heterocornol O/P (144 / 145) Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 35.0 μM | [68] |
| Ichikawa | IC50 = 54.3 μM | |||
| Hep G2 liver cancer | IC50 = 42.0 μM | |||
| 7860 kidney cancer | IC50 = 22.1 μM | |||
| Compound 149 Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 82.1 μM | [68] |
| Ichikawa | IC50 = 65.3 μM | |||
| Hep G2 liver cancer | IC50 = 94.2 μM | |||
| Compound 150 Pestalotiopsis heterocornis (marine) | Cytotoxic activity against human cancer cell lines | BGC–823 gastric carcinoma | IC50 = 78.1 μM | [68] |
| Ichikawa | IC50 = 58.5 μM | |||
| Hep G2 liver cancer | IC50 = 85.4 μM |
| Compound | Ref. | Compound | Ref. |
|---|---|---|---|
 Varitriol ((+)-1) | [18,19,20,21,22,26,27,28,29] [25,33,34,35] | 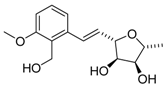 ent-Varitriol ((-)-1) | [15] [17] [32] [19] |
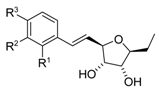 Analogues of varitriol 1a–h 1a R1=CH2OH, R2=OMe, R3=H 1b R1=CH2OMe, R2=OMe, R3=H 1c R1=OMe, R2=OEt, R3=H 1d R1=OEt, R2=OEt, R3=H 1e R1=OMe, R2=OMe, R3=OMe 1f R1=Cl, R2=Cl, R3=H 1g R1,R2=OCH2O, R3=H 1h R1=OMe, R2=OMe, R3=H | [18] | 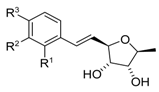 Analogues of varitriol 1i–s 1i R1=CH2OAc, R2=OMe, R3=H 1j R1=H, R2=H, R3=H 1k R1=OMe, R2=H, R3=H 1l R1=H, R2=H, R3=OMe 1m R1=OMe, R2=H, R3=OMe 1n R1=CF3, R2=H, R3=H 1o R1=H, R2= CF3, R3=H 1p R1=H, R2=H, R3=Br 1r R1=F, R2=H, R3=H 1s R1=H, R2= H, R3=F | [30] |
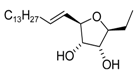 Analogue of varitriol 1t | [18] | 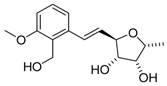 4-epi-Varitriol (4-epi-1) | [22] |
 Analogues of 2,3-diepi-varitriol 2,3-diepi-1a R1=CH2OH, R2=OMe 2,3-diepi-1b R1=CH2OH, R2=H 2,3-diepi-1c R1=H, R2=OMe 2,3-diepi-1d R1=H, R2=H | [31] | 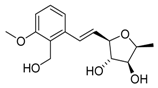 3-epi-Varitriol (3-epi-1) | [32] |
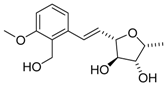 3-epi-ent-Varitriol (3-epi-ent-1) | [32] | 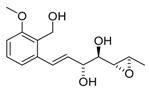 Varioxirane (2) | [33] [14] |
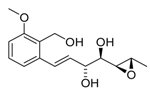 5,6-diepi-Varioxirane (5,6-diepi-2) | [14] | 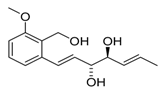 Andytriol (6) | [14] |
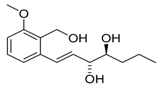 Varioxiranol A (39) | [41] | 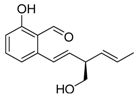 Pyricuol (59) | [54] |
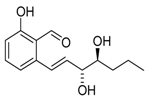 Pyriculol (60) | [52] | 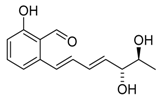 Pyriculariol (64) | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štiblariková, M.; Lásiková, A.; Gracza, T. Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis. Mar. Drugs 2023, 21, 19. https://doi.org/10.3390/md21010019
Štiblariková M, Lásiková A, Gracza T. Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis. Marine Drugs. 2023; 21(1):19. https://doi.org/10.3390/md21010019
Chicago/Turabian StyleŠtiblariková, Mária, Angelika Lásiková, and Tibor Gracza. 2023. "Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis" Marine Drugs 21, no. 1: 19. https://doi.org/10.3390/md21010019
APA StyleŠtiblariková, M., Lásiková, A., & Gracza, T. (2023). Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis. Marine Drugs, 21(1), 19. https://doi.org/10.3390/md21010019