Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Enzymolysis Method on Antarctic Krill Oil Yield
2.2. Effect of Enzymolysis Method on the Antarctic Krill Oil Quality
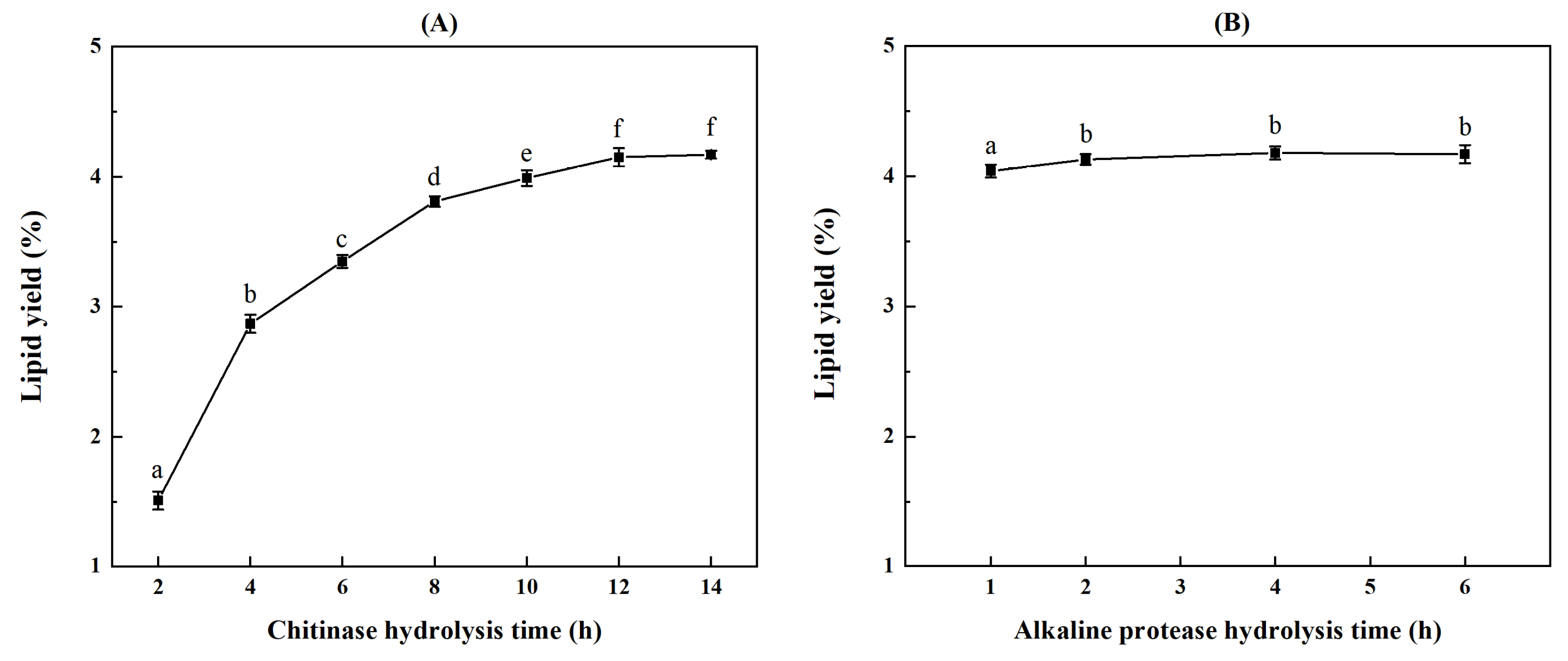
2.3. Screening of Two-Step Enzymolysis Conditions
2.4. The Optimal Quality of Antarctic Krill Oil and Enzymolysate
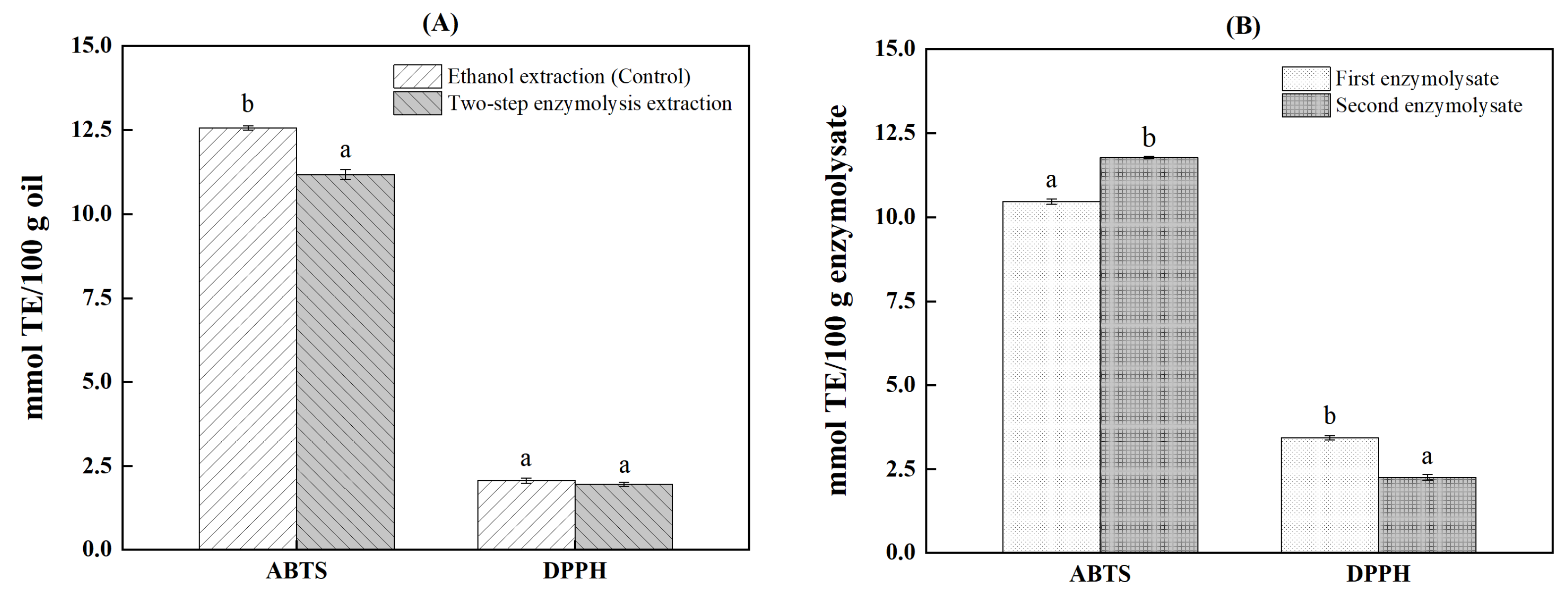
2.5. The Antioxidant Capacity of Antarctic Krill Oil and Enzymolysate
3. Materials and Methods
3.1. Materials
3.2. Enzymolysis
3.3. Extraction of Antarctic Krill Oil
3.4. Optimization of Lipid Yield
3.5. Determination of Lipid Yield and Impurity Content
3.6. Determination of Antioxidant Capacity
3.7. Analytical Methods
3.7.1. Determination of Phospholipid
3.7.2. Determination of Free EPA and DHA
3.7.3. Determination of Astaxanthin
3.7.4. Determination of Protein Content and Composition
3.7.5. Determination of Carbohydrate Content and Composition
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, D.; Mu, H.; Tang, T.; Wang, X.; Wei, W.; Jin, J.; Wang, X.; Jin, Q. Production of three types of krill oils from krill meal by a three-step solvent extraction procedure. Food Chem. 2018, 248, 279–286. [Google Scholar] [CrossRef]
- Grantham, G.J. The Southern Ocean: The utilization of krill. In Southern Ocean Fisheries Survey Programme GLO/SO/7/3; Food and Agriculture Organization: Rome, Italy, 1977; pp. 1–61. [Google Scholar]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp. Biochem. Physiol. Part B 2002, 131, 733–747. [Google Scholar] [CrossRef]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of dietary components from Antarctic krill on atherosclerosis in apoE-deficient mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef]
- Cong, X.; Miao, J.; Zhang, H.; Sun, W.; Xing, L.; Sun, L.; Zu, L.; Gao, Y.; Leng, K. Effects of drying methods on the content, structural isomers, and composition of astaxanthin in Antarctic krill. ACS Omega 2019, 4, 17972–17980. [Google Scholar] [CrossRef]
- Bonaterra, G.; Driscoll, D.; Schwarzbach, H.; Kinscherf, R. Krill oil-in-water emulsion protects against lipopolysaccharide-induced proinflammatory activation of macrophages in vitro. Mar. Drugs 2017, 15, 74. [Google Scholar] [CrossRef]
- Gart, E.; Salic, K.; Morrison, M.; Caspers, M.; van Duyvenvoorde, W.; Heijnk, M.; Giera, M.; Bobeldijk-Pastorova, I.; Keijer, J.; Storsve, A.; et al. Krill oil treatment increases distinct PUFAs and oxylipins in adipose tissue and liver and attenuates obesity-associated inflammation via direct and indirect mechanisms. Nutrients 2021, 13, 2836. [Google Scholar] [CrossRef]
- Guo, P.; Xue, M.; Teng, X.; Wang, Y.; Ren, R.; Han, J.; Zhang, H.; Tian, Y.; Liang, H. Antarctic krill oil ameliorates liver injury in rats exposed to alcohol by regulating bile acids metabolism and gut microbiota. J. Nutr. Biochem. 2022, 107, 109061. [Google Scholar] [CrossRef]
- Kim, H.; Roh, Y.; Yong Park, S.; Lee, C.; Lim, S.; Cho, S.; Lee, H.; Auck Hong, S.; Jin Lee, T.; Chul Myung, S.; et al. In vitro and in vivo anti-tumor efficacy of krill oil against bladder cancer: Involvement of tumor-associated angiogenic vasculature. Food Res. Int. 2022, 156, 111144. [Google Scholar] [CrossRef]
- Teng, X.; Wang, S.; Zeb, L.; Xiu, Z. Effects of carboxymethyl chitosan adsorption on bioactive components of Antarctic krill oil. Food Chem. 2022, 388, 132995. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, X.; Wang, W.; Zhou, Y.; Zhou, X.; Deng, S.; Zheng, B.; Wen, Z. Effect of Antarctic krill phospholipid (KOPL) on high fat diet-induced obesity in mice. Food Res. Int. 2021, 148, 110456. [Google Scholar] [CrossRef]
- Choi, J.; Jang, J.; Son, D.; Im, H.; Kim, J.; Park, J.; Choi, W.; Han, S.; Hong, J. Antarctic krill oil diet protects against lipopolysaccharide-induced oxidative stress, neuroinflammation and cognitive impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef]
- Cui, L.; McClements, D.J.; Decker, E.A. Impact of phosphatidylethanolamine on the antioxidant activity of α-tocopherol and trolox in bulk oil. J. Agr. Food Chem. 2015, 63, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, F.; Wen, M.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. The protective effect of Antarctic krill oil on cognitive function by inhibiting oxidative stress in the brain of senescence-accelerated prone mouse strain 8 (SAMP8) mice. J. Food. Sci. 2018, 83, 543–551. [Google Scholar] [CrossRef]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef]
- Saether, O.; Ellingsen, T.E.; Mohr, V. The distribution of lipid in the tissues of Antarctic krill, Euphausia-superba. Comp. Biochem. Phys. B 1985, 81, 609–614. [Google Scholar] [CrossRef]
- Kim, K.; Lee, D.; Nam, M.; Yoo, H.; Kim, S.; Chun, B.; Lee, Y. Optimization of alcalase for krill byproduct hydrolysis and antioxidative activities by response surface methodology. Prev. Nutr. Food Sci. 2010, 15, 316–321. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Rong, Y.; Yuan, Y.; Ding, Y.; Shi, W.; Wang, Z. Effects of different proteases enzymatic extraction on the lipid yield and quality of Antarctic krill oil. Food Sci. Nutr. 2019, 7, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, F.; Zhang, M.; Liu, J. A green enzymatic extraction optimization and oxidative stability of krill oil from Euphausia superba. Mar. Drugs 2020, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Y.; Ma, M.; Oh, D.; Fu, X. Characterization of chitinase from Exiguobacterium antarcticum and its bioconversion of crayfish shell into chitin oligosaccharides. Food Res. Int. 2022, 158, 111517. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 144. [Google Scholar] [CrossRef]
- Ali-Nehari, A.; Kim, S.; Lee, Y.; Chun, B. Digestive enzymes characterization of krill (Euphausia superba) residues deoiled by supercritical carbon dioxide and organic solvents. J. Ind. Eng. Chem. 2012, 18, 1314–1319. [Google Scholar] [CrossRef]
- Chu, F.; Wang, D.; Liu, T.; Han, H.; Yu, Y.; Yang, Q. An optimized cocktail of chitinolytic enzymes to produce N,N′-diacetylchitobiose and N-acetyl-d-glucosamine from defatted krill by-products. Int. J. Biol. Macromol. 2019, 133, 1029–1034. [Google Scholar] [CrossRef]
- Chen, Y.; Jaczynski, J. Gelation of Protein Recovered from Whole Antarctic Krill (Euphausia superba) by Isoelectric Solubilization/Precipitation as Affected by Functional Additives. J. Agric. Food Chem. 2007, 55, 1814–1822. [Google Scholar] [CrossRef]
- Taskaya, L.; Chen, Y.; Beamer, S.; Tou, J.C.; Jaczynski, J. Compositional characteristics of materials recovered from whole gutted silver carp (Hypophthalmichthys molitrix) using isoelectric solubilization/precipitation. J. Agric. Food Chem. 2009, 57, 4259–4266. [Google Scholar] [CrossRef]
- Köhler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects–a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Cong, P.; Chen, Q.; Li, H.; Fan, Y.; Xu, J.; Wang, J.; Wang, Y.; Xue, C. Lipid degradation during salt-fermented Antarctic krill paste processing and their relationship with lipase and phospholipase activities. Eur. J. Lipid Sci. Technol. 2018, 120, 1700443. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic krill (Euphausia superba) Oil: A comprehensive review of chemical composition, extraction technologies, health benefits, and current applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, Y.; Zhao, W.; Wang, P.; Chi, C.; Wang, B. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Two novel bioactive peptides from Antarctic krill with dual angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef]
- Shen, Q.; Quek, S.Y. Microencapsulation of astaxanthin with blends of milk protein and fiber by spray drying. J. Food Eng. 2014, 123, 165–171. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Park, S.Y.; Han, E.J.; Kim, H.; Kang, D.; Je, J.; Ahn, C.; Ahn, G. Isolation of an antioxidant peptide from krill protein hydrolysates as a novel agent with potential hepatoprotective effects. J. Funct. Foods 2020, 67, 103889. [Google Scholar] [CrossRef]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Jung, S.; Jung, J.; Lin, K.A.; Rinklebe, J.; Baek, K.; Kwon, E.E. Simultaneous productions of biodiesel and biochar from krill. J. Clean. Prod. 2022, 335, 130296. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of refining process on antioxidant capacity, total phenolics and prooxidants contents in rapeseed oils. LWT Food Sci. Technol. 2015, 64, 853–859. [Google Scholar] [CrossRef]
- Zeb, L.; Shafiq, M.; Chi, Z.; Xiu, Z. Separation of microalgal docosahexaenoic acid-rich oils using a microwave-assisted three-phase partitioning system. Sep. Purif. Technol. 2020, 252, 117441. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kalashnikova, T.; Bochkova, M.; Kropaneva, M.; Timganova, V.; Zamorina, S.; Rayev, M. Measuring the concentration of protein nanoparticles synthesized by desolvation method: Comparison of Bradford assay, BCA assay, hydrolysis/UV spectroscopy and gravimetric analysis. Int. J. Pharmaceut. 2021, 599, 120422. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Jain, B.M.; Badve, M.P. A novel process for synthesis of soybean protein hydrolysates and study of its effectiveness as a biostimulant and emulsifier. Chem. Eng. Process. Process Intensif. 2022, 174, 108880. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]



| Active Components | Ethanol Extraction (Control) 1 | Two-Step Enzymolysis Extraction 2 |
|---|---|---|
| PL (mg/g oil) | ||
| PC | 269.60 ± 2.64 a | 219.14 ± 8.59 a |
| PE | 31.75 ± 0.78 b | 26.91 ± 0.11 a |
| FFA (mg/g oil) | ||
| F-EPA | 24.88 ± 0.29 a | 46.58 ± 0.08 b |
| F-DHA | 23.15 ± 0.84 a | 34.38 ± 0.64 b |
| Astaxanthin (mg/g oil) | 0.96 ± 0.02 b | 0.82 ±0.01 a |
| Active Components | First Enzymolysate 1 | Second Enzymolysate 2 | Total |
|---|---|---|---|
| Proteins (mg/g krill) | 82.52 ± 2.08 | ||
| Soluble proteins | 21.32 ± 0.86 b | 13.03 ± 0.20 a | 34.35 ± 0.79 |
| Oligopeptides | 8.05 ± 0.24 b | 5.87 ± 0.17 a | 13.92 ± 0.40 |
| Amino acids | 21.94 ± 0.65 b | 12.30 ± 0.99 a | 34.24 ± 0.89 |
| Carbohydrates (mg/g krill) | 5.79 ± 0.05 | ||
| Polysaccharides | 3.72 ± 0.05 b | 1.43 ± 0.06 a | 5.16 ± 0.04 |
| Reducing sugars | 0.32 ± 0.01 a | 0.31 ± 0.00 a | 0.63 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, X.-N.; Wang, S.-C.; Zeb, L.; Dong, Y.-S.; Xiu, Z.-L. Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate. Mar. Drugs 2023, 21, 47. https://doi.org/10.3390/md21010047
Teng X-N, Wang S-C, Zeb L, Dong Y-S, Xiu Z-L. Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate. Marine Drugs. 2023; 21(1):47. https://doi.org/10.3390/md21010047
Chicago/Turabian StyleTeng, Xin-Nan, Shu-Chang Wang, Liaqat Zeb, Yue-Sheng Dong, and Zhi-Long Xiu. 2023. "Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate" Marine Drugs 21, no. 1: 47. https://doi.org/10.3390/md21010047
APA StyleTeng, X.-N., Wang, S.-C., Zeb, L., Dong, Y.-S., & Xiu, Z.-L. (2023). Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate. Marine Drugs, 21(1), 47. https://doi.org/10.3390/md21010047






