Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Pre-Treatment and Extraction Optimization
2.2. Chemical Characterization
2.2.1. Fucoidans-Related Contents
2.2.2. Co-Extracted Contaminant Contents
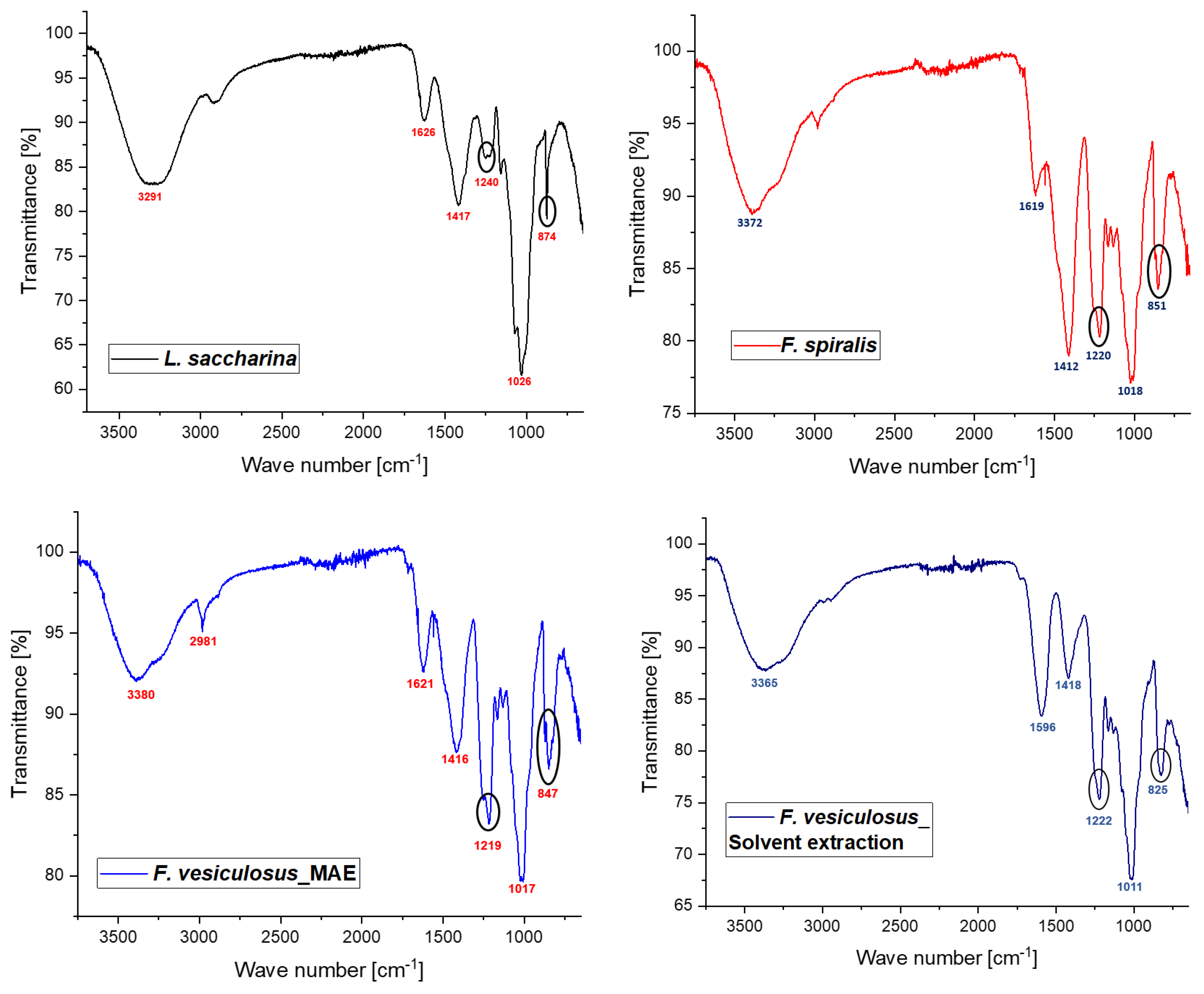
2.2.3. Structural Features
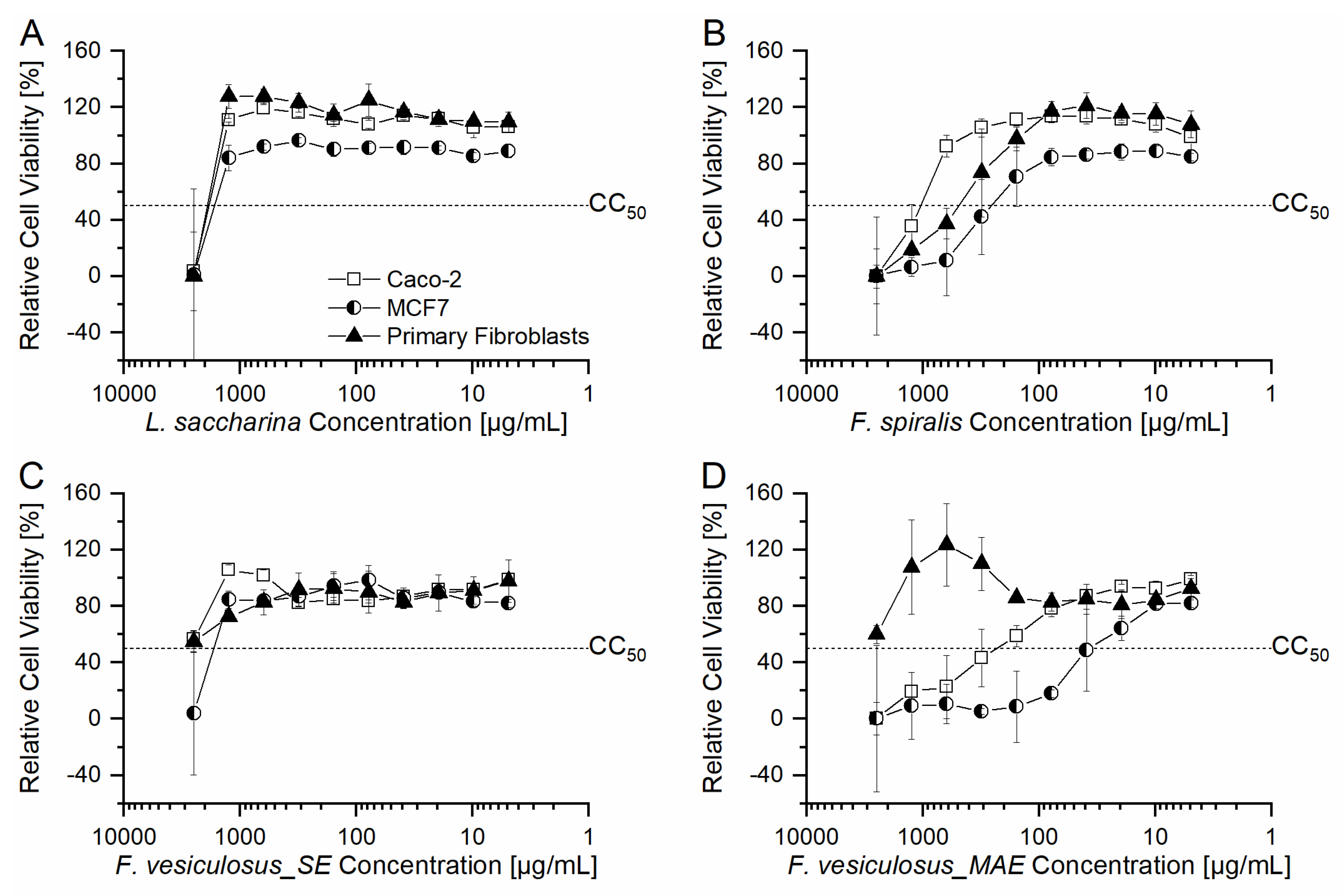
2.3. Evaluation of Cytotoxic Activity
3. Material and Methods
3.1. Algae Harvesting and Drying
3.2. Pre-Treatment Protocol
3.3. Extraction
3.4. Chemical Characterization of Extracted Fucoidans
3.4.1. Sugar Content
3.4.2. Fucoidan Content
3.4.3. Sulfation Degree
3.4.4. Molecular Weight Determination
3.4.5. Monomeric Composition
3.4.6. Interfering Co-Extracted Compounds
3.4.7. Structural Features
3.5. Cytotoxic Activity
3.5.1. Cell Lines and Cell Culture
3.5.2. Cytotoxicity Investigations
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef]
- Zayed, A.; Haggag, Y.; Ezzat, S.M.; Salem, M.A.; Ulber, R. 16-Fucoidans as nanoparticles: Pharmaceutical and biomedical applications. In Polysaccharide Nanoparticles; Venkatesan, J., Kim, S.-K., Anil, S., Rekha, P.D., Eds.; Elsevier: Amesterdam, The Netherlands, 2022; pp. 413–455. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.-I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Neupane, S.; Ptak, S.H.; Römer, R.; Xiong, J.; Ohmes, J.; Seekamp, A.; Fretté, X.; Alban, S.; et al. Influence of Fucoidan Extracts from Different Fucus Species on Adult Stem Cells and Molecular Mediators in In Vitro Models for Bone Formation and Vascularization. Mar. Drugs 2021, 19, 194. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Thomas, O.P.; Pedrosa, R. Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model. Int. J. Mol. Sci. 2017, 18, 292. [Google Scholar] [CrossRef]
- Almeida, B.; Barroso, S.; Ferreira, A.S.D.; Adão, P.; Mendes, S.; Gil, M.M. Seasonal Evaluation of Phlorotannin-Enriched Extracts from Brown Macroalgae Fucus spiralis. Molecules 2021, 26, 4287. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In vitro anti-Inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-Inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Tennakoon, P.; Kim, T.-H.; Kim, S.-C.; Je, J.-Y.; Kim, J.-I.; Lee, B.; Ryu, B.; Kang, H.W.; Kim, H.-W.; et al. Marine biological macromolecules and chemically modified macromolecules; potential anticoagulants. Mar. Drugs 2022, 20, 654. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.M.-d.; Pérez-Rubio, K.G.; Hernández-Corona, D.M.; Cortez-Navarrete, M. Therapeutic effect of fucoidan on metabolic diseases: Experimental data and clinical evidence. J. Med. Food 2022, 25, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, Z.; Feng, X.; Deng, J.; He, C.; Li, R.; Zhao, Y.; Ge, Y.; Zhang, Y.; Song, C.; et al. The emerging evidence for a protective role of fucoidan from Laminaria japonica in chronic kidney disease-triggered cognitive dysfunction. Mar. Drugs 2022, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural characterization and in vivo anti-inflammatory activity of fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- Zahan, M.S.; Hasan, A.; Rahman, M.D.H.; Meem, K.N.; Moni, A.; Hannan, M.A.; Uddin, M.J. Protective effects of fucoidan against kidney diseases: Pharmacological insights and future perspectives. Int. J. Biol. Macromol. 2022, 209, 2119–2129. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Formulation, optimization and in vivo evaluation of fucoidan-based cream with anti-inflammatory properties. Mar. Drugs 2021, 19, 643. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef]
- Zayed, A.; Avila-Peltroche, J.; El-Aasr, M.; Ulber, R. Sulfated galactofucans: An outstanding class of fucoidans with promising bioactivities. Mar. Drugs 2022, 20, 412. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.-Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2010, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Kasai, A.; Arafuka, S.; Koshiba, N.; Takahashi, D.; Toshima, K. Systematic synthesis of low-molecular weight fucoidan derivatives and their effect on cancer cells. Org. Biomol. Chem. 2015, 13, 10556–10568. [Google Scholar] [CrossRef] [PubMed]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Jayashree, S.; Kumar, G.; Sharmili, S.A.; Gopal, M.; Dharmaraj, S.; Chen, W.-H.; Kothari, R.; Manasa, I.; Park, J.H.; et al. Recent developments in biorefining of macroalgae metabolites and their industrial applications—A circular economy approach. Bioresour. Technol. 2022, 359, 127235. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of microwave-assisted extraction of polysaccharides from Ulva pertusa and evaluation of their antioxidant activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Mussatto, S.I. Microwave-Assisted Extraction of Fucoidan from Marine Algae. Methods Mol. Biol. 2015, 1308, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Pedisić, S.; Zorić, Z.; Jurin, M.; Roje, M.; Čož-Rakovac, R.; Dragović-Uzelac, V. Microwave Assisted Extraction and Pressurized Liquid Extraction of Sulfated Polysaccharides from Fucus virsoides and Cystoseira barbata. Foods 2021, 10, 1481. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Ehrig, K.; Alban, S. Sulfated galactofucan from the brown alga Saccharina latissima—variability of yield, structural composition and bioactivity. Mar. Drugs 2014, 13, 76–101. [Google Scholar] [CrossRef]
- Foo, S.C.; Khoo, K.S.; Ooi, C.W.; Show, P.L.; Khong, N.M.H.; Yusoff, F.M. Meeting Sustainable Development Goals: Alternative Extraction Processes for Fucoxanthin in Algae. Front. Bioeng. Biotechnol. 2020, 8, 546067. [Google Scholar] [CrossRef]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef]
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process. Biochem. 2019, 81, 182–187. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Hahn, T.; Zayed, A.; Kovacheva, M.; Stadtmüller, R.; Lang, S.; Muffler, K.; Ulber, R. Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae. Eng. Life Sci. 2016, 16, 78–87. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process. Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Latha, C. Microwave-assisted extraction of embelin from Embelia ribes. Biotechnol. Lett. 2007, 29, 319–322. [Google Scholar] [CrossRef]
- Zayed, A. Bioactive Compounds from Marine Sources. Ph.D. Thesis, TU Kaiserslautern, Kaiserslautern, Germany, 2018. [Google Scholar]
- Hahn, T.; Schulz, M.; Stadtmüller, R.; Zayed, A.; Muffler, K.; Lang, S.; Ulber, R. Cationic Dye for the Specific Determination of Sulfated Polysaccharides. Anal. Lett. 2016, 49, 1948–1962. [Google Scholar] [CrossRef]
- Croci, D.O.; Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Piccoli, A.; Totani, L.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Grachev, A.A.; et al. Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS ONE 2011, 6, e17283. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-assisted fucoidan extraction from brown macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Imbs, T.I.; Ermakova, S.P. Can Fucoidans of Brown Algae Be Considered as Antioxidants? Russ. J. Mar. Biol. 2021, 47, 157–161. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Al Monla, R.; Dassouki, Z.; Sari-Chmayssem, N.; Mawlawi, H.; Gali-Muhtasib, H. Fucoidan and alginate from the brown algae Colpomenia sinuosa and their combination with vitamin C trigger apoptosis in colon cancer. Molecules 2022, 27, 358. [Google Scholar] [CrossRef] [PubMed]
- Ptak, S.H.; Sanchez, L.; Fretté, X.; Kurouski, D. Complementarity of Raman and Infrared spectroscopy for rapid characterization of fucoidan extracts. Plant Methods 2021, 17, 130. [Google Scholar] [CrossRef]
- Kim, H.; Lee, A.; Jung, W.-K.; Jeon, T.J. Effects of fucoidan on cell morphology and migration in osteoblasts. Food Sci. Biotechnol. 2015, 24, 699–704. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, T.J. Fucoidan induces cell aggregation and apoptosis in osteosarcoma MG-63 Cells. Anim. Cells Syst. 2016, 20, 186–192. [Google Scholar] [CrossRef]
- Gupta, D.; Silva, M.; Radziun, K.; Martinez, D.C.; Hill, C.J.; Marshall, J.; Hearnden, V.; Puertas-Mejia, M.A.; Reilly, G.C. Fucoidan inhibition of osteosarcoma cells is species and molecular weight dependent. Mar. Drugs 2020, 18, 104. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Boo, H.-J.; Kwon, J.-M.; Koh, Y.-S.; Hyun, J.-W.; Park, D.-B.; Yoo, E.-S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef]
- Zhurishkina, E.V.; Stepanov, S.I.; Shvetsova, S.V.; Kulminskaya, A.A.; Lapina, I.M. A comparison of the effect of fucoidan from alga Fucus vesiculosus and its fractions obtained by anion-exchange chromatography on HeLa G-63, Hep G2, and Chang liver cells. Cell Tissue Biol. 2017, 11, 242–249. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourão, P.A. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef]
- Graiff, A.; Dankworth, M.; Wahl, M.; Karsten, U.; Bartsch, I. Seasonal variations of Fucus vesiculosus fertility under ocean acidification and warming in the western Baltic Sea. Bot. Mar. 2017, 60, 239–255. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How Does LC/MS Compare to UV in Coffee Authentication and Determination of Antioxidant Effects? Brazilian and Middle Eastern Coffee as Case Studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Condition ≠ | Chemical Character | |||
|---|---|---|---|---|
| Extraction Yield (% w/w) | Fucoidan Content (mg/mg Extract) | Sugar Content (mg Glucose Equivalent/mg Extract) | Sulfate Content (mg/mg Extract) | |
| 1 | 6.61 ± 0.040 | 0.643 ± 0.03 | 0.181 ± 0.08 | 0.092 ± 0.030 |
| 2 | 4.56 ± 0.006 | 0.770 ± 0.05 | 0.159 ± 0.22 | 0.042 ± 0.004 |
| 3 | 0.86 ± 0.004 | 0.459 ± 0.03 | 0.110 ± 0.05 | 0.043 ± 0.001 |
| 4 | 1.26 ± 0.000 | 0.351 ± 0.05 | 0.084 ± 0.02 | 0.069 ± 0.002 |
| 5 | 12.25 ± 0.011 | 0.595 ± 0.07 | 0.166 ± 0.09 | 0.062 ± 0.016 |
| Fucoidan Type | Chemical Character | |||||
|---|---|---|---|---|---|---|
| Sugar Content (mg Glucose Equivalent/mg Extract) | Fucoidan Content Based on TB Assay | Sulfation Degree | Total Phenolic Content (µg Gallic Acid Equivalent (GAE)/mg Extract) | Protein Content (µg Bovine Serum Albumin Equivalent/mg Extract) | ||
| (mg F. vesiculosus Fucoidan Equivalent/mg Extract) | (mg U. pinnatifida Fucoidan Equivalent/mg Extract) | |||||
| L. saccharina_MAE | 0.47 ± 0.015 | 0.09 ± 0.06 | 0.11 ± 0.047 | 0.13 | <1.0 ± 0.00 | <5.0 ± 0.00 |
| F. spiralis_MAE | 0.12 ± 0.029 | 0.52 ± 0.035 | 0.43 ± 0.026 | 0.73 | ||
| F. vesiculosus_MAE | 0.13 ± 0.015 | 0.65 ± 0.12 | --- * | 0.75 | 1.64 ± 0.23 | |
| F. vesiculosus_SE | 0.21 [47] | 0.73 [47] | --- * | 0.67 [47] | <1.0 ± 0.00 | |
| Fucoidan | CC50 [µg/mL] | ||
|---|---|---|---|
| Primary Fibroblasts | MCF7 | Caco-2 | |
| F. vesiculosus_SE | >2500.0 | 1784.2 ± 33.6 | >2500.0 |
| F. vesiculosus_MAE | >2500.0 | 45.9 ± 22.0 | 265.0 ± 94.9 |
| F. spiralis | 483.1 ± 73.7 | 266.7 ± 64.9 | 1091.6 ± 61.9 |
| L. saccharina | 2008.4 ± 44.9 | 1758.0 ± 68.1 | 1959.9 ± 11.1 |
| Conditions | Biomass: Solvent Ratio (g:mL) | Power (W) | Time (min) |
|---|---|---|---|
| 1 | 1:25 | 560 | 1.0 |
| 2 | 1:25 | 560 | 2.0 |
| 3 | 1:10 | 560 | 1.0 |
| 4 | 1:10 | 240 | 1.0 |
| 5 | 1:25 | 240 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, A.; Finkelmeier, D.; Hahn, T.; Rebers, L.; Shanmugam, A.; Burger-Kentischer, A.; Ulber, R. Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds. Mar. Drugs 2023, 21, 48. https://doi.org/10.3390/md21010048
Zayed A, Finkelmeier D, Hahn T, Rebers L, Shanmugam A, Burger-Kentischer A, Ulber R. Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds. Marine Drugs. 2023; 21(1):48. https://doi.org/10.3390/md21010048
Chicago/Turabian StyleZayed, Ahmed, Doris Finkelmeier, Thomas Hahn, Lisa Rebers, Anusriha Shanmugam, Anke Burger-Kentischer, and Roland Ulber. 2023. "Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds" Marine Drugs 21, no. 1: 48. https://doi.org/10.3390/md21010048
APA StyleZayed, A., Finkelmeier, D., Hahn, T., Rebers, L., Shanmugam, A., Burger-Kentischer, A., & Ulber, R. (2023). Characterization and Cytotoxic Activity of Microwave-Assisted Extracted Crude Fucoidans from Different Brown Seaweeds. Marine Drugs, 21(1), 48. https://doi.org/10.3390/md21010048








