Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice
Abstract
1. Introduction
2. Results
2.1. Ech-A Prevents Chronic Heart Failure after MI in Mice
2.2. Ech-A Inhibits LV Remodeling and Oxidative Stress after MI
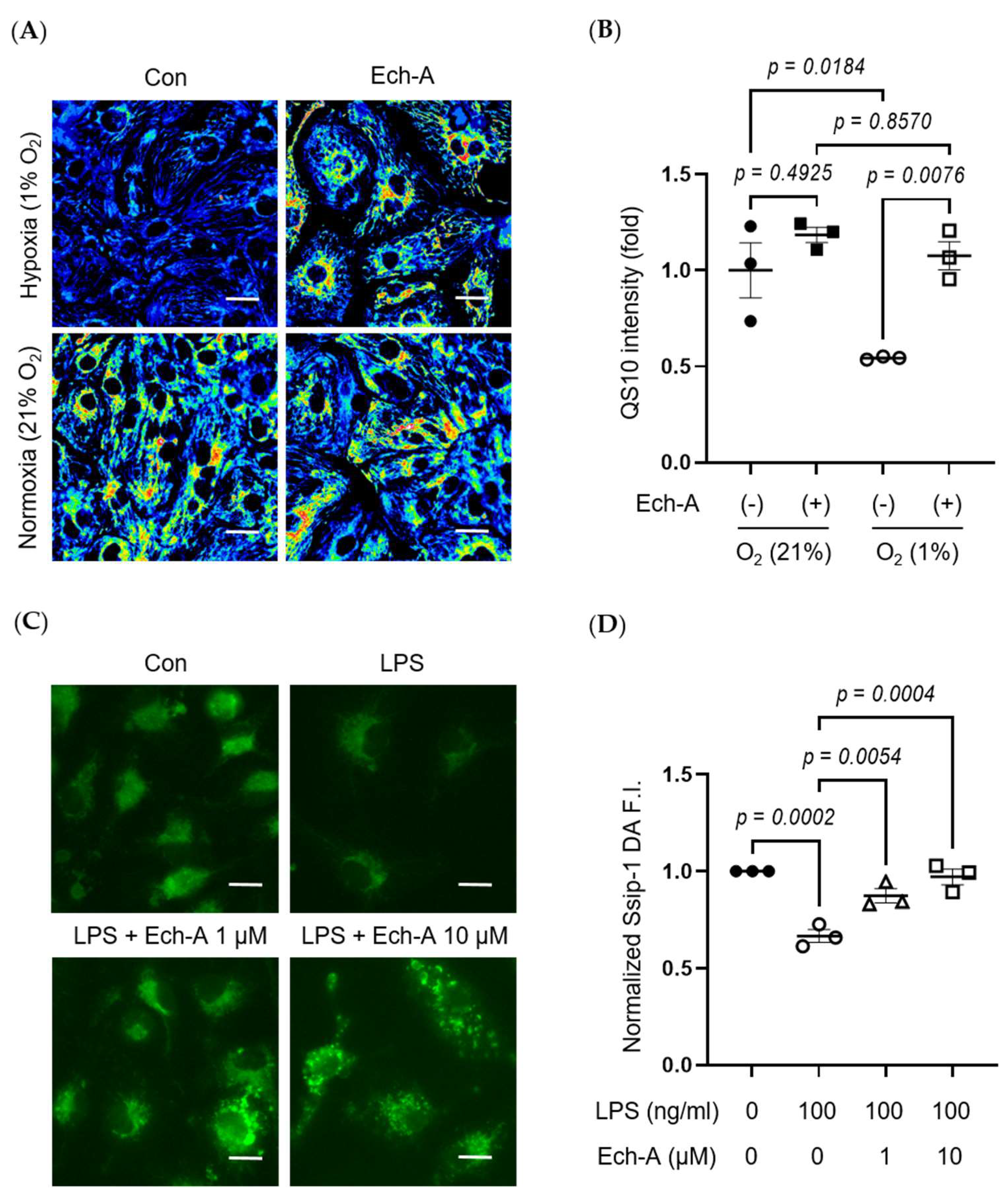
2.3. Ech-A Attenuates RSS Catabolism of the Heart after MI
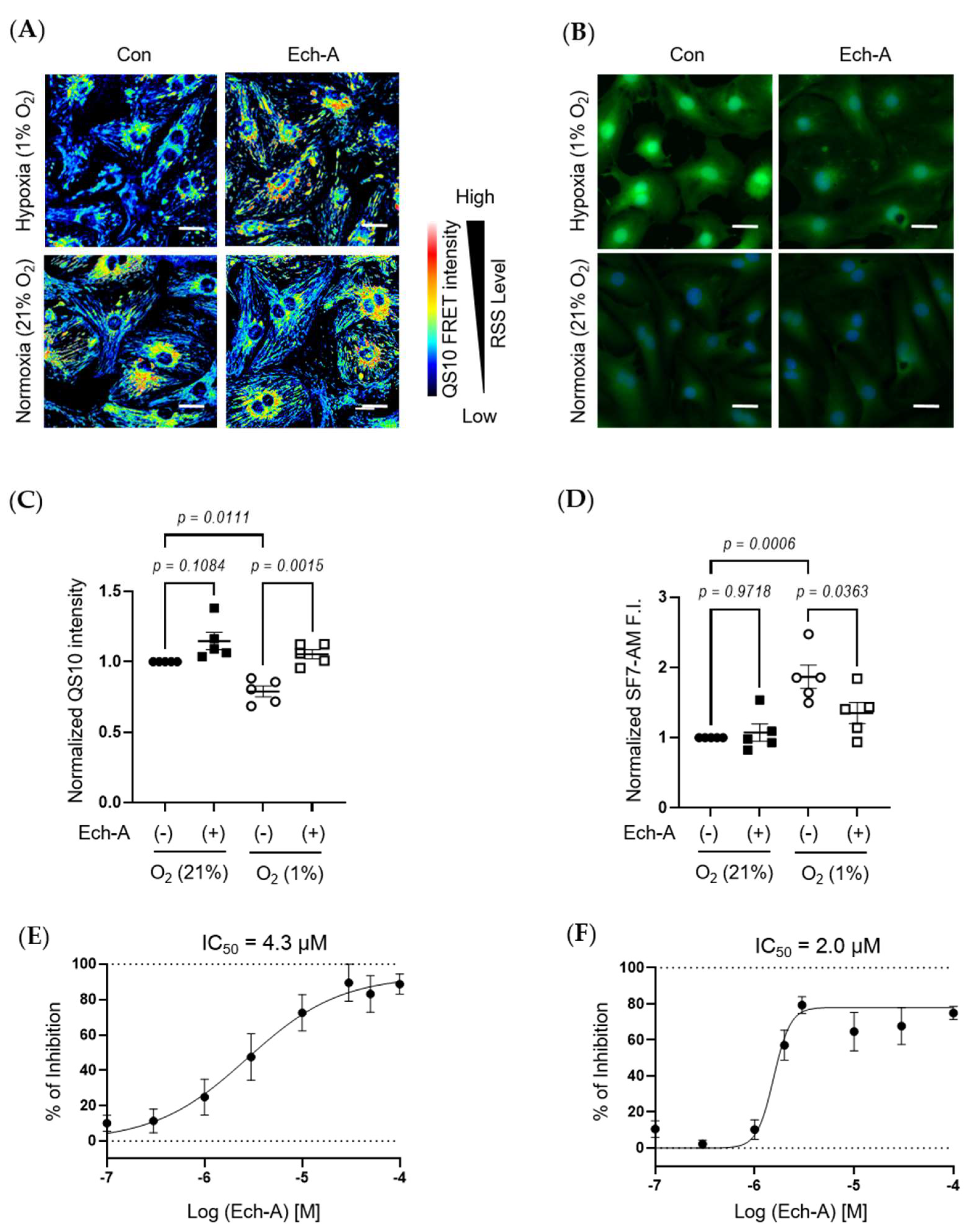
2.4. Ech-A Concentration-Dependently Prevents RSS Catabolism Caused by Hypoxic Stress in Neonatal Rat Cardiomyocytes (NRCMs)
2.5. Ech-A Universally Prevents Cellular RSS Catabolism during Hypoxia and Pseudohypoxia
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. MI Surgery and Transthoracic Echocardiography
4.4. Tissue Analysis
4.5. Cell Culture
4.6. Cell Imaging
4.7. RNA Isolation and Quantitative Real-Time Reverse Transcription PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from ischemic heart disease: Analysis of data from the world health organization and coronary artery disease risk factors from NCD risk factor collaboration. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.B. Historical Perspective on the Pathology of Myocardial Ischemia/Reperfusion Injury. Circ. Res. 2013, 113, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.A.; Gaasch, W.H.; Alexander, J.K. Influence of acute changes in preload, afterload, contractile state and heart rate on ejection and isovolumic indices of myocardial contractility in man. Circulation 1976, 53, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.S.J.; Lee, D.; Rouleau, J.L.; Goldman, S.; Plappert, T.; Braunwald, E.; Pfeffer, M.A. Left Ventricular Remodeling and Ventricular Arrhythmias After Myocardial Infarction. Circulation 2003, 107, 2577–2582. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.-Y.; Nishida, M.; Kumagai, Y.; Alam, M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef]
- Jencks, W.P.; Carriuolo, J. Reactivity of Nucleophilic Reagents toward Esters. J. Am. Chem. Soc. 1960, 82, 1778–1786. [Google Scholar] [CrossRef]
- Edwards, J.O.; Pearson, R.G. The Factors Determining Nucleophilic Reactivities. J. Am. Chem. Soc. 1962, 84, 16–24. [Google Scholar] [CrossRef]
- Possomato-Vieira, R.A.K.; José, S.; Khalil; Kuschner. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Ebert, D.; Greenberg, M.E. Chemical Foundations of Hydrogen Sulfide Biology. Bone 2013, 23, 237–337. [Google Scholar] [CrossRef]
- Nishimura, A.; Shimoda, K.; Tanaka, T.; Toyama, T.; Nishiyama, K.; Shinkai, Y.; Numaga-Tomita, T.; Yamazaki, D.; Kanda, Y.; Akaike, T.; et al. Depolysulfidation of Drp1 induced by low-dose methylmercury exposure increases cardiac vulnerability to hemodynamic overload. Sci. Signal. 2019, 12, eaaw1920. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Chen, X.; Jhee, K.-H.; Kruger, W.D. Production of the Neuromodulator H2S by Cystathionine β-Synthase via the Condensation of Cysteine and Homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef] [PubMed]
- Marutani, E.; Morita, M.; Hirai, S.; Kai, S.; Grange, R.M.H.; Miyazaki, Y.; Nagashima, F.; Traeger, L.; Magliocca, A.; Ida, T.; et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 2021, 12, 3108. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G. Evaluation of Free Radical-Scavenging Activity of Sea Urchin Pigments Using HPTLC with Post-Chromatographic Derivatization. Chromatographia 2013, 76, 1353–1358. [Google Scholar] [CrossRef]
- Prokopov, I.A.; Kovaleva, E.L.; Minaeva, E.D.; Pryakhina, E.A.; Savin, E.V.; Gamayunova, A.V.; Pozharitskaya, O.N.; Makarov, V.G.; Shikov, A.N. Animal-derived medicinal products in Russia: Current nomenclature and specific aspects of quality control. J. Ethnopharmacol. 2019, 240, 111933. [Google Scholar] [CrossRef]
- Kim, H.; Vasileva, E.; Mishchenko, N.; Fedoreyev, S.; Han, J. Multifaceted Clinical Effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Buĭmov, G.A.; Maksimov, I.V.; Perchatkin, V.A.; Repin, A.N.; Afanas’Ev, S.A.; Markov, V.A.; Karpov, R.S. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. arkhiv 2002, 74, 12–16. [Google Scholar]
- Zakirova, A.N.; Ivanova, M.V.; Golubiatnikov, V.B.; Mishchenko, N.P.; Kol’Tsova, E.A.; Fedoreev, S.A.; Krasnovid, N.I.; Lebedev, A.V. Pharmacokinetics and clinical efficacy of histochrome in patients with acute myocardial infarction. Eksp. Klin. Farmakol. 1997, 60, 21–24. [Google Scholar] [PubMed]
- Afanas’Ev, S.A.; Lasukova, T.V.; Chernyavskii, A.M. ATP-sparing effect of histochrome in acute myocardial ischemia in patients with coronary heart disease. Bull. Exp. Biol. Med. 1997, 124, 1217–1219. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Park, J.-H.; Park, B.-W.; Kim, H.; Kim, J.-J.; Sim, W.-S.; Mishchenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A.; Ban, K.; et al. Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death. Antioxidants 2021, 10, 1624. [Google Scholar] [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome a Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar. Drugs 2019, 17, 501. [Google Scholar] [CrossRef]
- Gakhramanov, F.S.; Azerbaijan, Z.A.A. Effect of Natural Antioxidants on Antioxidant Activity and Lipid Peroxidation in Eye Tissue of Rabbits with Chemical Burns. Bull. Exp. Biol. Med. 2005, 140, 289–291. [Google Scholar] [CrossRef]
- Yun, H.R.; Ahn, S.W.; Seol, B.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J.; Ko, K.S.; Rhee, B.D.; et al. Echinochrome A Treatment Alleviates Atopic Dermatitis-like Skin Lesions in NC/Nga Mice via IL-4 and IL-13 Suppression. Mar. Drugs 2021, 19, 622. [Google Scholar] [CrossRef]
- Park, G.-T.; Yoon, J.-W.; Yoo, S.-B.; Song, Y.-C.; Song, P.; Kim, H.-K.; Han, J.; Bae, S.-J.; Ha, K.-T.; Mishchenko, N.; et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Mar. Drugs 2021, 19, 237. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef]
- Sutton, M.G.S.J.; Sharpe, N. Left Ventricular Remodeling After Myocardial Infarction: Pathophysiology and therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef]
- Rosen, M.; Chan, P.; Saleem, M.; Herrmann, N.; Adibfar, A.; Andreazza, A.; Oh, P.I.; Lanctôt, K.L. Longitudinal associations between 4-hydroxynonenal and depression in coronary artery disease patients. Psychiatry Res. 2018, 270, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Dillon, K.M.; Carrazzone, R.J.; Wang, Y.; Powell, C.R.; Matson, J.B. Polymeric persulfide prodrugs: Mitigating oxidative stress through controlled delivery of reactive sulfur species. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, H.; Ida, T.; Sato, K.; Aizawa, N.; Sawa, T.; Tawarayama, H.; Murayama, N.; Fujii, S.; Akaike, T.; Nakazawa, T. Metabolomic profiling of reactive persulfides and polysulfides in the aqueous and vitreous humors. Sci. Rep. 2017, 7, srep41984. [Google Scholar] [CrossRef]
- Takano, Y.; Hanaoka, K.; Shimamoto, K.; Miyamoto, R.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T.; Urano, Y. Development of a reversible fluorescent probe for reactive sulfur species, sulfane sulfur, and its biological application. Chem. Commun. 2017, 53, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. USA 2013, 110, 7131–7135. [Google Scholar] [CrossRef]
- Umezawa, K.; Kamiya, M.; Urano, Y. A Reversible Fluorescent Probe for Real-Time Live-Cell Imaging and Quantification of Endogenous Hydropolysulfides. Angew. Chem. Int. Ed. 2018, 57, 9346–9350. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Cui, X.; Qin, B.; Zhang, J.; Zhang, L.; Zhang, H.; Liu, G.; Wang, W.; Zhang, J. New Insights and Novel Therapeutic Potentials for Macrophages in Myocardial Infarction. Inflammation 2021, 44, 1696–1712. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, hypertension, and cardiac dysfunction novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Kleikers, P.W.M.; Wingler, K.; Hermans, J.J.R.; Diebold, I.; Altenhöfer, S.; Radermacher, K.A.; Janssen, B.; Görlach, A.; Schmidt, H.H.H.W. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J. Mol. Med. 2012, 90, 1391–1406. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Lu, L.-Q.; Jiang, Y.-Q.; Li, N.-S.; Luo, X.-J.; Peng, J.-W.; Peng, J. Allopurinol attenuates oxidative injury in rat hearts suffered ischemia/reperfusion via suppressing the xanthine oxidase/vascular peroxidase 1 pathway. Eur. J. Pharmacol. 2021, 908, 174368. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired Autophagosome Clearance Contributes to Cardiomyocyte Death in Ischemia/Reperfusion Injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.-I.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA Damage and Dysfunction Associated With Oxidative Stress in Failing Hearts After Myocardial Infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.A.; Soliman, A.M.; Fahmy, S.R. Echinochrome pigment as novel therapeutic agent against experimentally—Induced gastric ulcer in rats. Biomed. Pharmacother. 2018, 107, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Sadek, S.A.; Hassanein, S.S.; Soliman, A.M. Hepatoprotective Effect of Echinochrome Pigment in Septic Rats. J. Surg. Res. 2019, 234, 317–324. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Sayed, D.A.; Soliman, A.M.; Almortada, N.Y.; Aal, W.E.A.-E. Protective effect of Echinochrome against intrahepatic cholestasis induced by alpha-naphthylisothiocyanate in rats. Braz. J. Biol. 2020, 80, 102–111. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar]
- Oh, S.-J.; Seo, Y.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Quinzii, C.M.; Luna-Sanchez, M.; Ziosi, M.; Hidalgo-Gutierrez, A.; Kleiner, G.; Lopez, L.C. The Role of Sulfide Oxidation Impairment in the Pathogenesis of Primary CoQ Deficiency. Front. Physiol. 2017, 8, 525. [Google Scholar] [CrossRef]
- Eghbal, M.A.; Pennefather, P.S.; O’Brien, P.J. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 2004, 203, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Furne, J.; Stoltz, G.; Abdel-Rehim, M.; Levitt, M.D.; Szurszewski, J. Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br. J. Pharmacol. 2012, 165, 2178–2190. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H.; et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tsutsuki, H.; Ono, K.; Akaike, T.; Sawa, T. Antioxidative and anti-inflammatory actions of reactive cysteine persulfides. J. Clin. Biochem. Nutr. 2021, 68, 5–8. [Google Scholar] [CrossRef]
- Nishimura, A.; Tanaka, T.; Kato, Y.; Nishiyama, K.; Nishida, M. Cardiac robustness regulated by reactive sulfur species. J. Clin. Biochem. Nutr. 2021, 70, 1–6. [Google Scholar] [CrossRef]
- Nishimura, A.; Shimauchi, T.; Tanaka, T.; Shimoda, K.; Toyama, T.; Kitajima, N.; Ishikawa, T.; Shindo, N.; Numaga-Tomita, T.; Yasuda, S.; et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission–associated myocardial senescence. Sci. Signal. 2018, 11, eaat5185. [Google Scholar] [CrossRef]
- Nishi, K.; Oda, T.; Takabuchi, S.; Oda, S.; Fukuda, K.; Adachi, T.; Semenza, G.L.; Shingu, K.; Hirota, K. LPS Induces Hypoxia-Inducible Factor 1 Activation in Macrophage-Differentiated Cells in a Reactive Oxygen Species–Dependent Manner. Antioxid. Redox Signal. 2008, 10, 983–996. [Google Scholar] [CrossRef]
- Zhang, T.; Ono, K.; Tsutsuki, H.; Ihara, H.; Islam, W.; Akaike, T.; Sawa, T. Enhanced Cellular Polysulfides Negatively Regulate TLR4 Signaling and Mitigate Lethal Endotoxin Shock. Cell Chem. Biol. 2019, 26, 686–698.e4. [Google Scholar] [CrossRef]
- Uba, T.; Matsuo, Y.; Sumi, C.; Shoji, T.; Nishi, K.; Kusunoki, M.; Harada, H.; Kimura, H.; Bono, H.; Hirota, K. Polysulfide inhibits hypoxia-elicited hypoxia-inducible factor activation in a mitochondria-dependent manner. Mitochondrion 2021, 59, 255–266. [Google Scholar] [CrossRef]
- Omatsu-Kanbe, M.; Yoshioka, K.; Fukunaga, R.; Sagawa, H.; Matsuura, H. A simple antegrade perfusion method for isolating viable single cardiomyocytes from neonatal to aged mice. Physiol. Rep. 2018, 6, e13688. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Fujita, T.; Fujimoto, Y.; Nakajima, H.; Takeuchi, T.; Azuma, Y.-T. Fatty acid transport protein 1 enhances the macrophage inflammatory response by coupling with ceramide and c-Jun N-terminal kinase signaling. Int. Immunopharmacol. 2018, 55, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 8686–8691. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Nishimura, A.; Shimoda, K.; Tanaka, T.; Kato, Y.; Shibata, T.; Tanaka, H.; Kurose, H.; Azuma, Y.-T.; Ihara, H.; et al. Redox-dependent internalization of the purinergic P2Y6 receptor limits colitis progression. Sci. Signal. 2022, 15, eabj0644. [Google Scholar] [CrossRef]




| Sham + Vehicle (Saline) | Sham + Ech-A (2.0 mg/kg/day) | MI + Vehicle (Saline) | MI + Ech-A (0.2 mg/kg/day) | MI + Ech-A (0.6 mg/kg/day) | MI + Ech A (2.0 mg/kg/day) | |
|---|---|---|---|---|---|---|
| n | 5 | 5 | 7 | 6 | 6 | 6 |
| EDV (µL) | ||||||
| Week 0 | 41 ± 3 | 41 ± 3 | 39 ± 2 | 40 ± 3 | 42 ± 2 | 35 ± 2 |
| Week 1 | 37 ± 2 | 36 ± 3 | 98 ± 15 ∗ | 76 ± 11 | 80 ± 12 | 93 ± 15 ∗ |
| Week 2 | 38 ± 3 | 35 ± 3 | 119 ± 21 ∗ | 83 ± 15 | 100 ± 19 | 89 ± 11 ∗ |
| Week 3 | 35 ± 1 | 37 ± 4 | 127 ± 21 ∗ | 93 ± 19 | 99 ± 19 | 96 ± 13 ∗ |
| Week 4 | 39 ± 3 | 35 ± 4 | 140 ± 27 ∗ | 88 ± 18 | 99 ± 20 | 96 ± 10 ∗ |
| Week 5 | 37 ± 2 | 38 ± 2 | 149 ± 26 ∗ | 93 ± 18 | 117 ± 28 | 100 ± 10 ∗∗ |
| SV (µL) | ||||||
| Week 0 | 22 ± 1 | 21 ± 2 | 21 ± 1 | 21 ± 2 | 22 ± 2 | 19 ± 1 |
| Week 1 | 22 ± 2 | 19 ± 2 | 19 ± 2 | 20 ± 1 | 18 ± 2 | 21 ± 2 |
| Week 2 | 22 ± 2 | 20 ± 2 | 23 ± 3 | 18 ± 1 | 21 ± 2 | 21 ± 2 |
| Week 3 | 19 ± 1 | 18 ± 2 | 22 ± 3 | 20 ± 2 | 21 ± 2 | 22 ± 2 |
| Week 4 | 21 ± 2 | 18 ± 2 | 19 ± 1 | 18 ± 1 | 20 ± 2 | 23 ± 2 |
| Week 5 | 20 ± 2 | 21 ± 1 | 20 ± 2 | 19 ± 2 | 24 ± 3 | 25 ± 2 |
| CO (mL/min) | ||||||
| Week 0 | 9.9 ± 1.0 | 9.9 ± 0.9 | 8.5 ± 0.8 | 8.9 ± 1.2 | 9.7 ± 1.1 | 7.9 ± 0.4 |
| Week 1 | 10.9 ± 1.7 | 9.7 ± 1.0 | 8.8 ± 0.8 | 10.5 ± 1.2 | 8.6 ± 0.7 | 11.2 ± 1.1 |
| Week 2 | 11.4 ± 1.6 | 10.4 ± 1.1 | 10.9 ± 1.7 | 9.2 ± 1.0 | 9.7 ± 0.9 | 10.3 ± 1.1 |
| Week 3 | 9.9 ± 0.9 | 10.4 ± 1.1 | 10.8 ± 1.5 | 10.5 ± 1.7 | 10.5 ± 1.0 | 11.0 ± 0.9 |
| Week 4 | 10.7 ± 1.4 | 9.4 ± 0.8 | 9.4 ± 0.9 | 9.0 ± 1.0 | 9.7 ± 0.9 | 10.9 ± 1.4 |
| Week 5 | 10.6 ± 1.5 | 10.9 ± 0.2 | 9.6 ± 1.5 | 9.0 ± 1.1 | 11.7 ± 1.9 | 12.7 ± 1.2 |
| LVAWd (mm) | ||||||
| Week 0 | 0.81 ± 0.02 | 0.81 ± 0.06 | 0.76 ± 0.05 | 0.75 ± 0.03 | 0.74 ± 0.05 | 0.72 ± 0.02 |
| Week 1 | 0.80 ± 0.04 | 0.79 ± 0.06 | 0.75 ± 0.06 | 0.64 ± 0.02 | 0.70 ± 0.02 | 0.66 ± 0.04 |
| Week 2 | 0.88 ± 0.07 | 0.93 ± 0.04 | 0.57 ± 0.04 ∗ | 0.64 ± 0.03 | 0.68 ± 0.03 | 0.72 ± 0.12 |
| Week 3 | 0.95 ± 0.05 | 0.87 ± 0.02 | 0.54 ± 0.04 ∗∗ | 0.64 ± 0.05 ∗∗ | 0.65 ± 0.03 ∗∗ | 0.78 ± 0.11 |
| Week 4 | 0.85 ± 0.07 | 0.92 ± 0.07 | 0.54 ± 0.06 ∗ | 0.57 ± 0.03 | 0.65 ± 0.03 | 0.72 ± 0.13 |
| Week 5 | 0.88 ± 0.02 | 0.93 ± 0.05 | 0.46 ± 0.04 ∗∗ | 0.58 ± 0.04 ∗∗ | 0.60 ± 0.06 ∗ | 0.65 ± 0.01 ∗∗## |
| LVPWd (mm) | ||||||
| Week 0 | 0.78 ± 0.04 | 0.87 ± 0.04 | 0.79 ± 0.05 | 0.75 ± 0.03 | 0.83 ± 0.01 | 0.89 ± 0.10 |
| Week 1 | 0.92 ± 0.08 | 0.82 ± 0.05 | 0.93 ± 0.05 | 1.00 ± 0.02 | 0.99 ± 0.10 | 0.84 ± 0.06 |
| Week 2 | 1.06 ± 0.11 | 0.80 ± 0.04 | 0.88 ± 0.05 | 0.86 ± 0.05 | 0.95 ± 0.06 | 0.83 ± 0.03 |
| Week 3 | 0.91 ± 0.09 | 0.89 ± 0.08 | 0.83 ± 0.05 | 0.94 ± 0.05 | 1.02 ± 0.04 | 0.87 ± 0.09 |
| Week 4 | 0.92 ± 0.06 | 0.93 ± 0.09 | 0.84 ± 0.06 | 0.98 ± 0.04 | 0.95 ± 0.05 | 0.92 ± 0.08 |
| Week 5 | 0.98 ± 0.11 | 1.03 ± 0.06 | 0.81 ± 0.06 | 0.97 ± 0.06 | 0.97 ± 0.04 | 1.04 ± 0.07 |
| LVIDd (mm) | ||||||
| Week 0 | 3.93 ± 0.08 | 3.96 ± 0.09 | 3.87 ± 0.13 | 3.95 ± 0.11 | 4.06 ± 0.10 | 3.65 ± 0.15 |
| Week 1 | 3.65 ± 0.09 | 3.77 ± 0.12 | 5.20 ± 0.16 ∗∗ | 4.88 ± 0.22 ∗ | 4.78 ± 0.18 ∗ | 5.35 ± 0.18 ∗∗ |
| Week 2 | 3.60 ± 0.11 | 3.64 ± 0.08 | 5.57 ± 0.20 ∗∗ | 5.08 ± 0.21 ∗ | 5.29 ± 0.18 ∗∗ | 5.19 ± 0.27 ∗ |
| Week 3 | 3.57 ± 0.11 | 3.73 ± 0.05 | 5.85 ± 0.20 ∗∗ | 5.10 ± 0.28 | 5.32 ± 0.25 ∗ | 5.13 ± 0.33 |
| Week 4 | 3.85 ± 0.16 | 3.50 ± 0.14 | 6.02 ± 0.26 ∗∗ | 5.24 ± 0.32 | 5.51 ± 0.27 ∗ | 5.10 ± 0.26 |
| Week 5 | 3.59 ± 0.08 | 3.79 ± 0.06 | 6.10 ± 0.29 ∗∗ | 5.43 ± 0.28 ∗ | 5.60 ± 0.33 ∗ | 5.21 ± 0.21 ∗∗ |
| LVIDs (mm) | ||||||
| Week 0 | 2.61 ± 0.08 | 2.69 ± 0.07 | 2.46 ± 0.13 | 2.63 ± 0.10 | 2.69 ± 0.08 | 2.35 ± 0.20 |
| Week 1 | 2.43 ± 0.07 | 2.52 ± 0.12 | 4.32 ± 0.20 ∗∗ | 3.93 ± 0.24 ∗ | 3.89 ± 0.16 ∗∗ | 4.32 ± 0.20 ∗∗ |
| Week 2 | 2.13 ± 0.07 | 2.38 ± 0.08 | 4.69 ± 0.24 ∗∗ | 4.13 ± 0.23 ∗∗ | 4.30 ± 0.19 ∗∗ | 4.18 ± 0.24 ∗∗ |
| Week 3 | 2.21 ± 0.10 | 2.47 ± 0.06 | 4.96 ± 0.24 ∗∗ | 4.28 ± 0.32 ∗ | 4.41 ± 0.26 ∗∗ | 4.12 ± 0.30 ∗ |
| Week 4 | 2.39 ± 0.15 | 2.23 ± 0.18 | 5.23 ± 0.27 ∗∗ | 4.45 ± 0.35 ∗ | 4.61 ± 0.30 ∗∗ | 3.99 ± 0.21 ∗∗ |
| Week 5 | 2.14 ± 0.10 | 2.44 ± 0.06 | 5.36 ± 0.32 ∗∗ | 4.68 ± 0.30 ∗∗ | 4.70 ± 0.36 ∗ | 4.05 ± 0.20 ∗∗ |
| Sham + Vehicle (Saline) | Sham + Ech-A (2.0 mg/kg/day) | MI + Vehicle (Saline) | MI + Ech-A (0.2 mg/kg/day) | MI + Ech-A (0.6 mg/kg/day) | MI + Ech A (2.0 mg/kg/day) | |
|---|---|---|---|---|---|---|
| n | 5 | 5 | 7 | 6 | 6 | 6 |
| BW (g) | 27.4 ± 0.3 | 27.0 ± 0.4 | 26.9 ± 0.5 | 26.6 ± 0.5 | 26.6 ± 0.6 | 27.0 ± 0.6 |
| HW (mg) | 125 ± 4 | 126 ± 2 | 192 ± 16 ∗∗ | 182 ± 8 ∗ | 189 ± 21 ∗ | 156 ± 6 |
| HW/BW (mg/g) | 4.6 ± 0.2 | 4.7 ± 0.1 | 7.1 ± 0.5 ∗∗ | 6.9 ± 0.3 ∗ | 7.1 ± 0.8 ∗ | 5.8 ± 0.2 |
| HW/TL (g/mm) | 0.70 ± 0.02 | 0.68 ± 0.01 | 1.07 ± 0.09 ∗∗ | 1.01 ± 0.04 ∗ | 1.03 ± 0.11 ∗ | 0.84 ± 0.03 |
| KWl/BW (mg/g) | 5.7 ± 0.2 | 6.6 ± 0.6 ∗ | 6.0 ± 0.2 | 6.0 ± 0.1 | 5.75 ± 0.1 | 5.9 ± 0.2 |
| KWr/BW (mg/g) | 6.0 ± 0.2 | 6.4 ± 0.2 | 6.0 ± 0.2 | 6.3 ± 0.1 | 6.0 ± 0.1 | 5.9 ± 0.2 |
| LivW/BW (mg/g) | 43.1 ± 1.2 | 47.6 ± 2.6 | 42.7 ± 2.6 | 51.0 ± 0.8 | 43.7 ± 2.3 | 44.7 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Nishimura, A.; Ariyoshi, K.; Nishiyama, K.; Kato, Y.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Kim, H.-K.; et al. Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice. Mar. Drugs 2023, 21, 52. https://doi.org/10.3390/md21010052
Tang X, Nishimura A, Ariyoshi K, Nishiyama K, Kato Y, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Kim H-K, et al. Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice. Marine Drugs. 2023; 21(1):52. https://doi.org/10.3390/md21010052
Chicago/Turabian StyleTang, Xiaokang, Akiyuki Nishimura, Kohei Ariyoshi, Kazuhiro Nishiyama, Yuri Kato, Elena A. Vasileva, Natalia P. Mishchenko, Sergey A. Fedoreyev, Valentin A. Stonik, Hyoung-Kyu Kim, and et al. 2023. "Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice" Marine Drugs 21, no. 1: 52. https://doi.org/10.3390/md21010052
APA StyleTang, X., Nishimura, A., Ariyoshi, K., Nishiyama, K., Kato, Y., Vasileva, E. A., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., Kim, H.-K., Han, J., Kanda, Y., Umezawa, K., Urano, Y., Akaike, T., & Nishida, M. (2023). Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice. Marine Drugs, 21(1), 52. https://doi.org/10.3390/md21010052








